1. Introduction
An ischemic stroke represents the onset of a high-mortality disease and enhances social burden and causes economic disability for countries and communities. In the clinic, therapy for an ischemic stroke involves only an injection of tissue plasminogen activator (tPA), whereby the tPA dissolves any clots and restores blood supply [
1]. On the other hand, the restoration of cerebral blood supply by tPA causes neuroinflammation and oxidative stress [
2,
3], and previous studies have reported that cerebral ischemia/reperfusion (I/R) induced excessive production of aggregating and misfolded proteins [
4,
5]. Thus, reperfusion in the brain exacerbates ischemic injury with neurological dysfunctions [
6,
7]. However, effective methods for the prevention of cerebral I/R injury have not been established in the clinic.
In a previous study, Jin et al. reported that the inhibition of inflammation-related signaling pathways could reduce I/R injury and increase functional outcome in preclinical studies of ischemic stroke [
8]. Moreover, Shinohara et al. found that the administration of cilostazol (CIL) was more effective in the prevention of the recurrence of ischemic stroke, and its long-term safety and effectiveness were better than that of aspirin in patients [
9]. In addition, previous reports have shown that CIL exerted a protective effect by promoting blood–brain barrier integrity [
10,
11] as well as improved the status of neurological dysfunctions following cerebral I/R injury [
12]. We also reported that the oral or intravenous administration of CIL solid nanoparticles (CIL-NPs) prevented I/R-induced brain injury in a middle cerebral artery occlusion (MCAO)/reperfusion model mouse [
13,
14]. However, the administration method of CIL is limited in patients with a disturbed consciousness, whereby medical staff, such as doctors, need to inject it intravenously. Therefore, this requires the design of effective and safe formulations, allowing an evaluation of their therapeutic effect for ischemic stroke.
Application via the skin can be implemented for patients who have swallowing problems or are unconscious, and this method of administration is simple and convenient. Although drug administration through the skin permits sustained delivery [
15,
16], the main challenge associated with transdermal drug delivery (TDD) systems is low drug absorption, since the stratum corneum functions as a barrier. Therefore, the improvement of skin permeation using physical or chemical methods is necessary for percutaneous absorption [
17]. Decades ago, it was thought that nanoparticles (NPs) could not penetrate intact skin. On the other hand, it was recently reported that NPs permeate deep into the skin depending on the type of material, surface charge, and size of the NPs [
18]. Thus, nanotechnology, involving nanocarriers, nanocrystals, liposomes, polymeric NPs, solid NPs, nanoemulsions, and dendrimers, is rapidly evolving for TDD [
18,
19,
20,
21]. Our previous reports also showed that gels containing NPs of ketoprofen, indomethacin, and raloxifene showed high skin penetration via energy-dependent endocytosis, such as clathrin-dependent endocytosis (CME), caveolae-dependent endocytosis (CavME), and macropinocytosis [
19,
20,
21]. For these reasons, TDD using solid NPs could address the abovementioned issues. Taken together, we attempted to design a gel using solid CIL-NPs (CIL-Ngel), and then investigated the relationships between energy-dependent endocytosis and skin penetration of the CIL-Ngel in this study. In addition, we evaluated whether the CIL-Ngel attenuated I/R-induced brain injury in an MCAO/reperfusion mouse model.
2. Materials and Methods
2.1. Animals
Wistar rats and Institute of Cancer Research (ICR) mice were used in this study. The 7 week old Wistar rats were purchased from Kiwa Laboratory Animals Co., Ltd. (Wakayama, Japan), and the 5 week old ICR mice (male) were obtained from Shimizu Laboratory Supplies Co., Ltd. (Kyoto, Japan). These animals were allowed free access to a CE-2 commercial diet (Clea Japan Inc., Tokyo, Japan) and water, and they were housed under standard conditions. All experiments using animals were performed in accordance with the guidelines of Kindai University, and the procedures described herein were approved on 1 April 2013 (project identification code KAPS-25-001) and 1 April 2019 (project identification code KAPS-31-010) by Kindai University.
2.2. Chemicals
CIL powder (CIL-MPs) was provided by Otsuka Pharmaceutical Co., Ltd. (Tokushima, Japan), and carboxypolymethylene (carbopol, Carbopol® 934) was purchased from Serva (Heidelberg, Germany). 2-Hydroxyproplyl-β-cyclodextrin (HPβCD) and methylcellulose (MC) were kindly donated by Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan) and Nihon Shokuhin Kako Co., Ltd. (Tokyo, Japan), respectively. Nystatin (CavME inhibitor) and docusate sodium salt were obtained from Sigma-Aldrich Japan (Tokyo, Japan). An MFTM membrane filter (450 nm pore size) was purchased from Merck Millipore (Tokyo, Japan). Dynasore (CME inhibitor) and rottlerin (micropinocytosis inhibitor) were provided by Nacalai Tesque (Kyoto, Japan), and l-menthol, indomethacin, dimethyl sulfoxide (DMSO), isoflurane, 2,3,5-triphenyl tetrazolium chloride (TTC), and cytochalasin D (phagocytosis inhibitor) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals used were of the highest purity commercially available.
2.3. Preparation of Gel Based on CIL-NPs
CIL powder and MC were mixed and then milled using an agate mortar at 4 °C for 1 h. The milled powder (0.5 g CIL and 0.5 g MC) was suspended in a 100 mL solution containing 0.005 g of docusate sodium salt and 5 g of HPβCD, and the dispersion was stirred in a 1.5 mL tube with zirconia beads (diameter, 0.1 mm). After that, the mixture was crushed using the Micro Smash MS-100R at 5500 rpm for 30 s for a total of 30 times at 4 °C (TOMY Digital Biology Co., LTD., Tokyo, Japan). Furthermore, the dispersion containing milled CIL (CIL-NPs) was incorporated into the carbopol gel with
l-menthol, and the gel containing CIL-NPs was designated CIL-Ngel. On the other hand, the gel containing CIL-MPs (CIL-Mgel) was prepared by incorporating the CIL powder and additives (MC and HPβCD) into Carbopol
® 934 gel with
l-menthol. In this study, the compositions of CIL-Mgel and CIL-Ngel were as follows: 0.5% CIL, 0.5% MC, 0.005% docusate sodium salt, 5% HPβCD, 2%
l-menthol, and 3% carbopol (
w/
w); their pH was adjusted to 6.8 [
19,
20,
21]. The 3% carbopol gel containing 0.5% MC, 0.005% docusate sodium salt, 5% HPβCD, and 2% L-menthol was used as vehicle.
2.4. Measurement of CIL Concentration
The CIL content was determined using an HPLC LC-20AT system (Shimadzu Corp., Kyoto, Japan) with an Inertsil
® ODS-3 column (GL Science Co., Inc., Tokyo, Japan). Fifty microliters of sample and 50 µL of methanol containing 0.25 µg of indomethacin (internal standard) were mixed, and then 10 µL of the mixture was injected into the HPLC LC-20AT system. In the HPLC method, acetonitrile/methanol/water (35/15/50,
v/
v/
v) at flow rate of 0.25 mL/min was used as the mobile phase, and CIL was detected at 254 nm at 35 °C [
22].
2.5. Characteristics of CIL Gel
Soluble CIL and CIL-NPs in the gels were separated by centrifugation at 100,000× g using a Beckman OptimaTM MAX-XP Ultracentrifuge (Beckman Coulter, Osaka, Japan), and the solubility was determined on the basis of the concentration of soluble-CIL measured via the HPLC method described above. An atomic force microscopy (AFM) image of the CIL-NPs was generated using an SPM-9700 (Shimadzu Corp., Kyoto, Japan), and the particle-size distribution of CIL was measured using both a NANOSIGHT LM10 (QuantumDesign Japan, Tokyo, Japan) and an SALD-7100 (Shimadzu Corp., Kyoto, Japan). In this study, the refractive index used to analyze CIL particles was set at 1.60–0.10i in the SALD-7100. The viscosity of the CIL gel was analyzed using a Brookfield digital viscometer (Brookfield Engineering Laboratories, Inc., Middleboro, MA, USA), and the number of CIL-NPs was measured using the NANOSIGHT LM10. The viscosity in the NANOSIGHT LM10 was set to 1.27 mPa·s. In the evaluation of CIL gels, they were stored in the dark at 20 °C for 30 days, and the size, number, and shape of CIL-NPs were measured according to the above-described procedures.
2.6. Drug Release from CIL Gel
An O-ring flange (1.6 cm inner diameter (i.d.)) was placed on the MFTM membrane filter, along with a Franz diffusion cell. The reservoir chamber in the Franz diffusion cell was filled with 12.2 mL of 0.85% NaCl/10 mM phosphate buffer (pH 7.4), which was thermoregulated at 37 °C. In the experiment, the CIL gels (0.3 g) were applied into the O-ring flange on the MFTM membrane filter, and 100 µL of the sample was withdrawn from the reservoir chamber over time for 24 h [
19,
20,
21]. The CIL concentration in the samples was measured using HPLC methods, and the size distribution and number of CIL-NPs in the samples were determined using the NANOSIGHT LM10.
2.7. Transdermal Penetration of CIL Gel
The hair on the abdominal area of the 7 week old Wistar rats was removed on the day prior to the experiment [
19,
20,
21]. For the experiment, the rats were euthanized by injection with a lethal dose of pentobarbital. Then, the abdominal area was removed and collected before setting the sample on the Franz diffusion cell filled with 12.2 mL of 0.85% NaCl/10 mM phosphate buffer (pH 7.4). Next, 0.3 g of CIL gel was applied onto the O-ring flange (1.6 cm i.d.) on the skin at 4 °C (cold condition) and 37 °C (normal condition), followed by withdrawing 100 µL of solution from the reservoir chamber (sample) over time for 24 h. The cold condition was implemented to inhibit energy-dependent endocytosis [
23]. Furthermore, energy-dependent endocytosis in the skin was also prevented by treatment with pharmacological inhibitors in 0.5% DMSO. The inhibitors of CavME (54 µM nystatin) [
24], CME (40 µM dynasore) [
25], micropinocytosis (2 µM rottlerin) [
26], and phagocytosis (10 µM cytochalasin D) [
24] were used in this study, and these inhibitors were used to pretreat the removed skin 1 h before treatment with the CIL gels at 37 °C. The CIL concentration in the samples was measured using HPLC methods, and the size distribution and number of CIL-NPs in the samples were determined using the NANOSIGHT LM10. The areas under the penetrated CIL concentration–time curves (
AUC0–24h) were measured using the trapezoidal method.
2.8. Assay of Plasma CIL Concentrations
The hair on the abdominal area of the 7 week old Wistar rats was removed on the day prior to the experiment. For the experiment, CIL was orally (3 mg/kg) or transdermally (0.3 g CIL gel) administered to fasted Wistar rats once a day at 10:00 a.m. for 1 (single administration) or 3 days (repetitive administration). The CIL powder was used in the oral administration (traditional administration), whereas the CIL gel was applied for transdermal administration. Then, 100 µL of blood was drawn from the jugular vein after CIL administration, which was centrifuged at 20,400× g for 20 min (4 °C). Next, the CIL levels in the supernatant were measured using the HPLC method described above. The areas under the plasma CIL concentration–time curves (AUC0–66h) were measured using the trapezoidal method.
2.9. Induction of Focal Cerebral Ischemia/Reperfusion
The 5 week old ICR mice (41 mice) were anesthetized with 2.5% isoflurane from BS-400T (Brain Science Idea Co., Ltd., Osaka, Japan) at 37 °C, and a silicone-coated 8-0 nylon monofilament (Natsume Seisakusyo Co., Ltd., Tokyo, Japan) was inserted into the left middle cerebral artery, which was maintained for 6 h at 37 °C using a heating pad. Then, the middle cerebral artery blood flow was restored by withdrawing the nylon monofilament under anesthesia with isoflurane (3:00 p.m.) [
27]. Subsequently, neurological functional were observed 4 h after the reperfusion (7:00 p.m.), and the 8 mice with no neurological symptoms (
n = 1) or unconscious (
n = 7) were excluded. After that, the 33 mice with the I/R injury induced by MCAO were separated into 5 groups (non-treated group (
n = 5, neurological score 2.2 ± 0.2), group orally treated with vehicle (
n = 5, neurological score 2.2 ± 0.2), group orally treated with CIL powder (
n = 9, neurological score 2.3 ± 0.2), group transdermally treated with vehicle (
n = 5, neurological score 2.4 ± 0.2), group transdermally treated with CIL-Ngel (
n = 9, neurological score 2.4 ± 0.2)). The CIL was orally (3 mg/kg) or transdermally (0.3 g CIL gel) administered once a day (7:00 p.m., repetitive administration) and maintained for 66 h (the reperfusion was performed 70 h). Next, the brain was removed at 1:00 p.m. and three slices were cut out for analysis: from the bregma (2 mm, area
bregma), 1 mm anterior to the bregma (2 mm, area
anterior), and 1 mm posterior to the bregma (2 mm, area
posterior), using brain matrices (Brain ScienceIdea Co., Ltd., Osaka, Japan). The noninfarct and infarct areas in the slices were dyed with 1.5% TTC for 15 min and monitored under a digital camera. The infarct area was analyzed with ImageJ, and the area (mm
2) was expressed as the average area for each part of brain measured from both anterior and posterior sides of that part of brain. The infarct volume (mm
3) was calculated using Equation (1) [
13].
In this study, the neurological functional was determined on a four-score as follows: 0 = no neurological symptoms; 1 point = inability to completely extend the right front paw; 2 = rotating while crawling and falling to the right side; 3 = inability to walk without assistance; and 4 = unconscious. The mice scoring 0 and 4 were excluded.
2.10. CIL Contents in Brain
Focal cerebral I/R injury was induced by MCAO on the left side as described above. Three days after reperfusion, CIL was orally (3 mg/kg) or transdermally (0.3 g CIL gel) administered once a day (7:00 p.m.) and maintained for 3 days. Then, the mice were euthanized by injection with a lethal dose of pentobarbital at 1:00 p.m. Next, the brains were collected and sliced 1 mm anterior and 1 mm posterior to the center of the bregma using brain matrices [
13]. The sliced brains were homogenized in methanol and centrifuged at 20,400×
g for 20 min (4 °C). Lastly, the CIL levels in the supernatant were measured using the HPLC method described above.
2.11. Statistical Analysis
All values are expressed as the mean ± standard error (SE). The significant differences between mean values (p < 0.05) were analyzed according to Student’s t-test and Dunnett’s multiple comparisons.
4. Discussion
CIL, 6-[4-(1-cyclohexyl-1
H-tetrazol-5yl)butoxy]-3, 4-dihydro-2(1
H)-quiolinone, can decrease the degree of neuronal cell death following transient cerebral ischemia [
28], and it provides a neuroprotective effect against brain injury induced by I/R [
29,
30]. It was suggested that CIL inhibits the inflammatory reaction of microglia or tumor necrosis factor alpha after ischemic insult, resulting in a neuroprotective effect [
28]. Therefore, CIL is expected to be effective for treating transient cerebral ischemia. In this study, we designed a CIL-Ngel (gel based on solid CIL-NPs) and showed that the CIL-Ngel was better for the therapy of I/R-induced brain injury.
The skin is the largest multilayered organ of the body, and TDD permits sustained delivery [
15,
16]. In particular, the skin route is useful for unconscious patients, and it provides assurance of more consistent serum drug levels [
15,
16]. On the other hand, the skin plays a role as a biologic protector, preventing the entry of foreign substances into the body; thus, improvement of the low absorption is a major challenge associated with transdermal drug administration. In order to overcome this hurdle, various techniques for cutaneous penetration enhancement using chemical, physical, and NP approaches have been proposed [
18,
19,
20,
21]. We also reported that 2%
l-menthol increased the skin penetration of solid NPs, whereby indomethacin and raloxifene NPs presented high percutaneous absorption in combination with
l-menthol [
20,
21]. Moreover, energy-dependent endocytosis is related to the enhanced skin penetration of NPs [
19,
20,
21]. Taken together, we prepared a transdermal system using solid CIL-NPs in this study.
Firstly, we attempted to prepare a carbopol gel containing solid CIL-NPs following our previous reports [
19,
20,
21]. The bead mill treatment decreased the particle size of CIL to 50–180 nm (
Figure 1), with 93.8% of the total CIL in the gel uniformly present as solid CIL-NPs (
Figure 2). In addition, the CIL particles in the gel were stable and retained their nanosize 1 month after preparation (
Figure 3). Furthermore, the CIL-Ngel released CIL-NPs during the in vitro release examination using a Franz diffusion cell set in an MFTM membrane filter (
Figure 4). These results validated the practicality of the CIL-Ngel.
Next, the skin penetration of the CIL-Ngel was demonstrated (
Figure 5A). It was reported that NPs can permeate deep into the skin depending on the type of material, surface charge, and size of the NPs [
18]. In this study, transdermal penetration was also enhanced following the preparation of NPs with respect to the CIL-Mgel. On the other hand, it is important to elucidate the mechanism underlying the high skin penetration of CIL-NPs in the CIL-Ngel. The precise mechanism for the transdermal penetration of NPs is not clearly defined; however, recent reports showed that the activity of endocytosis increases during the cellular uptake of NPs [
19,
20,
21]. Therefore, we investigated the relationships between endocytosis and transdermal penetration in the CIL-Ngel. He et al. reported that the cold condition (4 °C) was able to inhibit the activity of energy-dependent endocytosis [
23]. In this study, we demonstrated the effect of endocytosis inhibition on the transdermal penetration of CIL under the cold condition [
23] using pharmacological inhibitors [
24,
25,
26]. The inhibition of energy-dependent endocytosis under the cold condition (4 °C) inhibited the transdermal penetration of CIL (
Figure 5B,C), with nystatin (CavME inhibitor) and dynasore (CME inhibitor) significantly attenuating the transdermal penetration of CIL in the CIL-Ngel (
Figure 5D,E). From these results, it was suggested that both CavME and CME promoted the transdermal penetration of CIL.
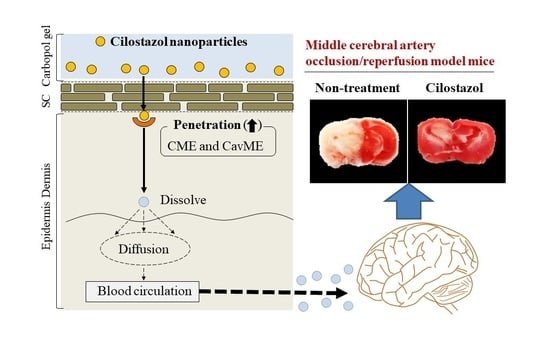
Furthermore, we evaluated whether the CIL was delivered via percutaneous absorption to the infarct site of the brain in mice with I/R-induced brain injury. The retention time of plasma CIL was longer in the CIL-Ngel, and the CIL in plasma and the brain was significantly higher in comparison with the oral administration of CIL powder (56.4%) (
Figure 6A,B,D). On the other hand, the CIL concentration in non-ischemia site was higher than that in ischemia site (
Figure 6D). These results suggested that the blood flow may be limited under the the ischemia, resulting in the delivery of CIL in ischemia site was not lower than that of non-ischemia site. It was important to measure whether treatment with the CIL-Ngel prevented ischemic stroke. Therefore, we also investigated the therapeutic effect of the CIL-Ngel on ischemic stroke in MCAO/reperfusion model mice (
Figure 7). The CIL-Ngel significantly attenuated ischemic stroke. Furthermore,
AUC0–66h was similar between orally and transdermally treated mice (
Figure 6C); however, the infarct area in MCAO/reperfusion model mice treated with the CIL-Ngel was higher than that following the oral administration of CIL powder. Taken together, we hypothesize that the CIL-NPs released from the CIL-Ngel penetrated through the system via CavME and CME and dissolved in the skin, since no CIL-NPs were detected in the reservoir chamber following the treatment with CIL-Ngel in the in vitro skin penetration study using a Franz diffusion cell. The dissolved CIL was absorbed into the blood and delivered to the brain regardless of the presence of infarct. Moreover, the CIL-Ngel provided assurance of more consistent plasma CIL levels in comparison with that following the oral administration of CIL powder, resulting in a strong inhibition of the onset of ischemic stroke in MCAO/reperfusion model mice. Yoneyama et al. reported that CIL promoted neuronal repair following cerebral ischemic injury [
12], while Horai et al. showed that CIL exerted a protective effect by promoting blood-brain barrier integrity [
11]. Our results in this study support these previous reports, showing that the sustained release of CIL may be effective in the treatment of ischemic stroke in MCAO/reperfusion model mice.
Further studies are needed to clarify the relationships between percutaneous absorption and endocytosis pathways in the CIL-Ngel. In addition, it is important to establish effective therapy for ischemic stroke. In future work, we plan to investigate the therapeutic effect of a combination of CIL and tPA.