In Situ Atomic-Scale Observation of Silver Oxidation Triggered by Electron Beam Irradiation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
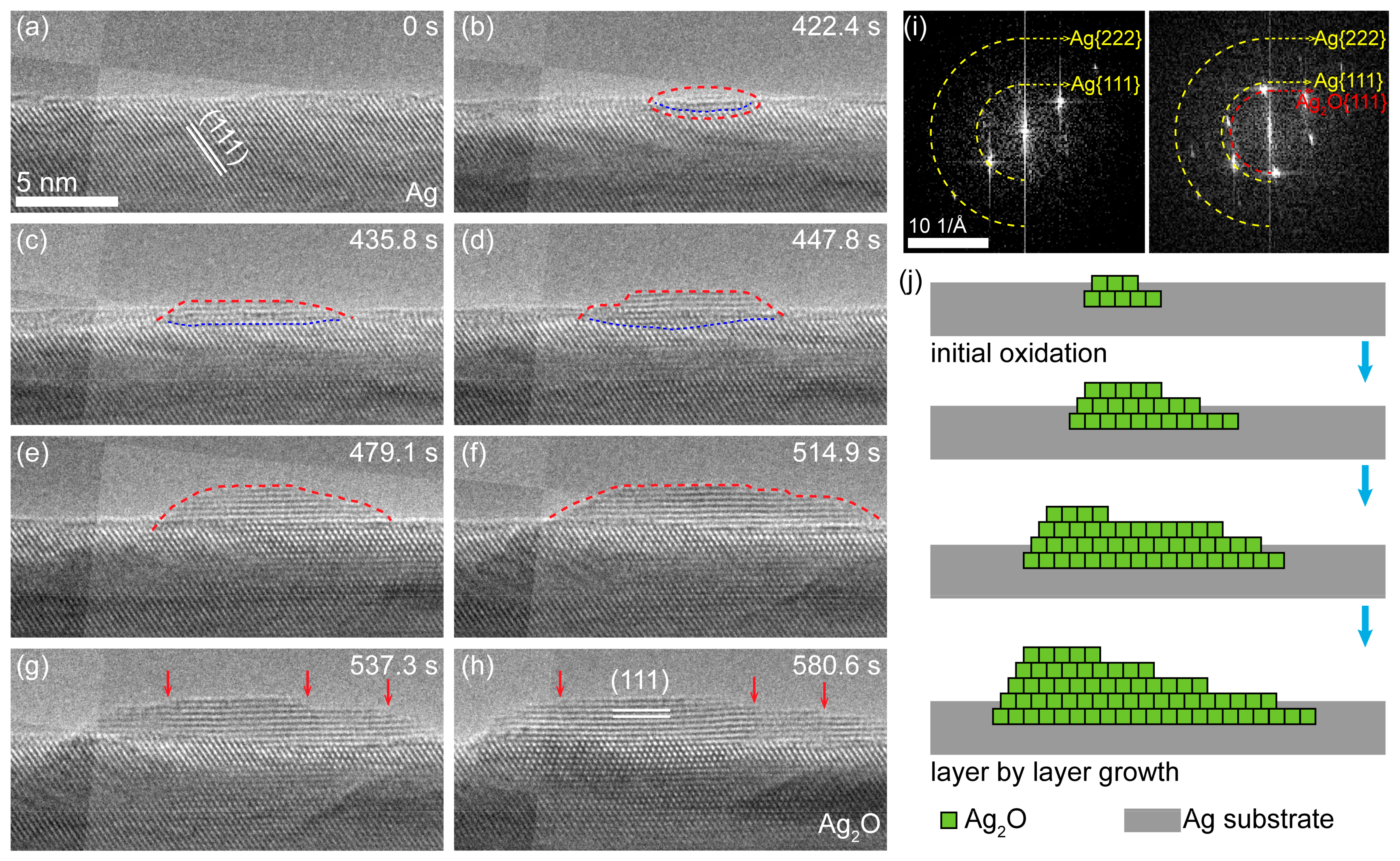
3.1. In Situ Oxidation Process
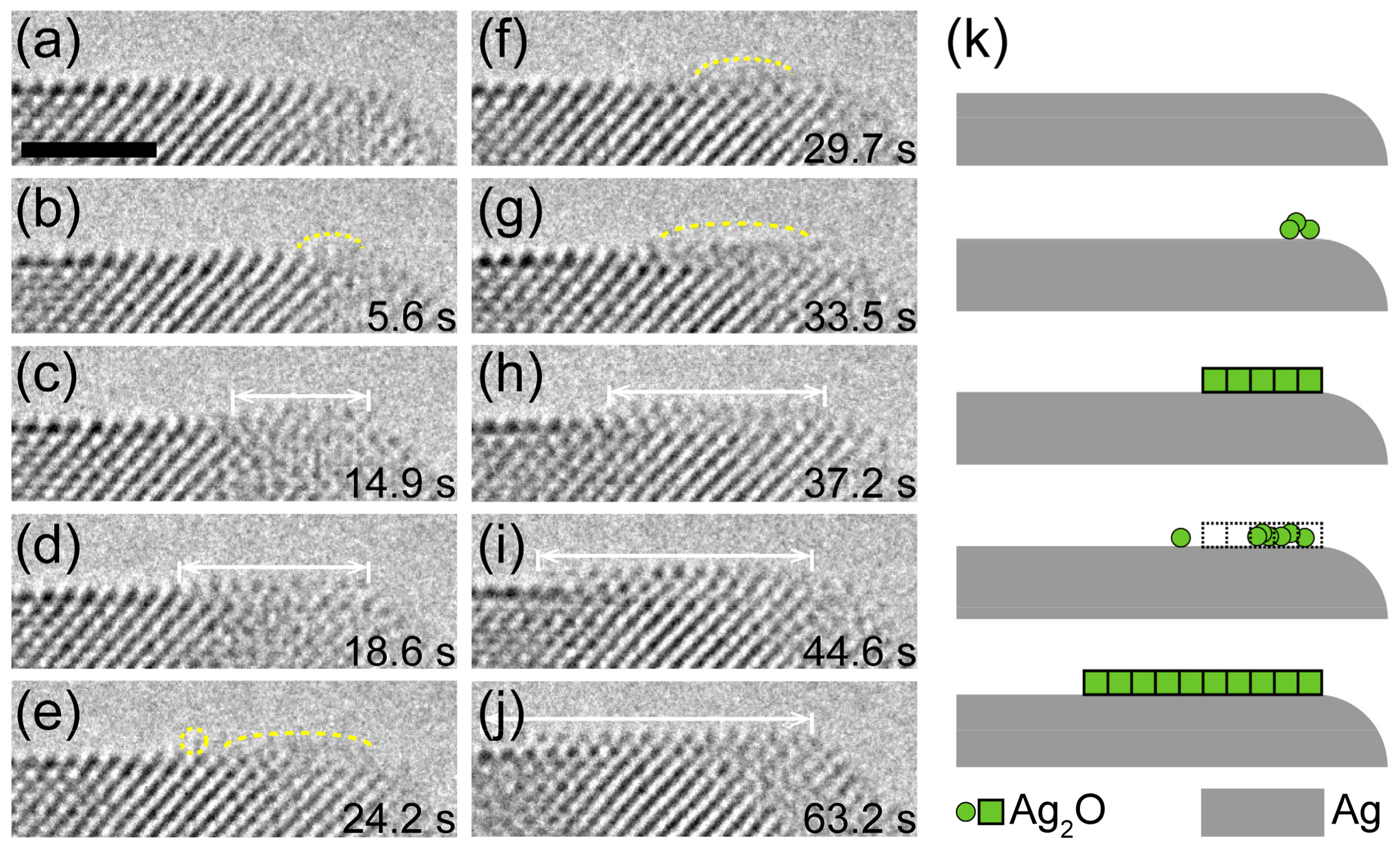
3.2. Mass Transport in Crystal Growth
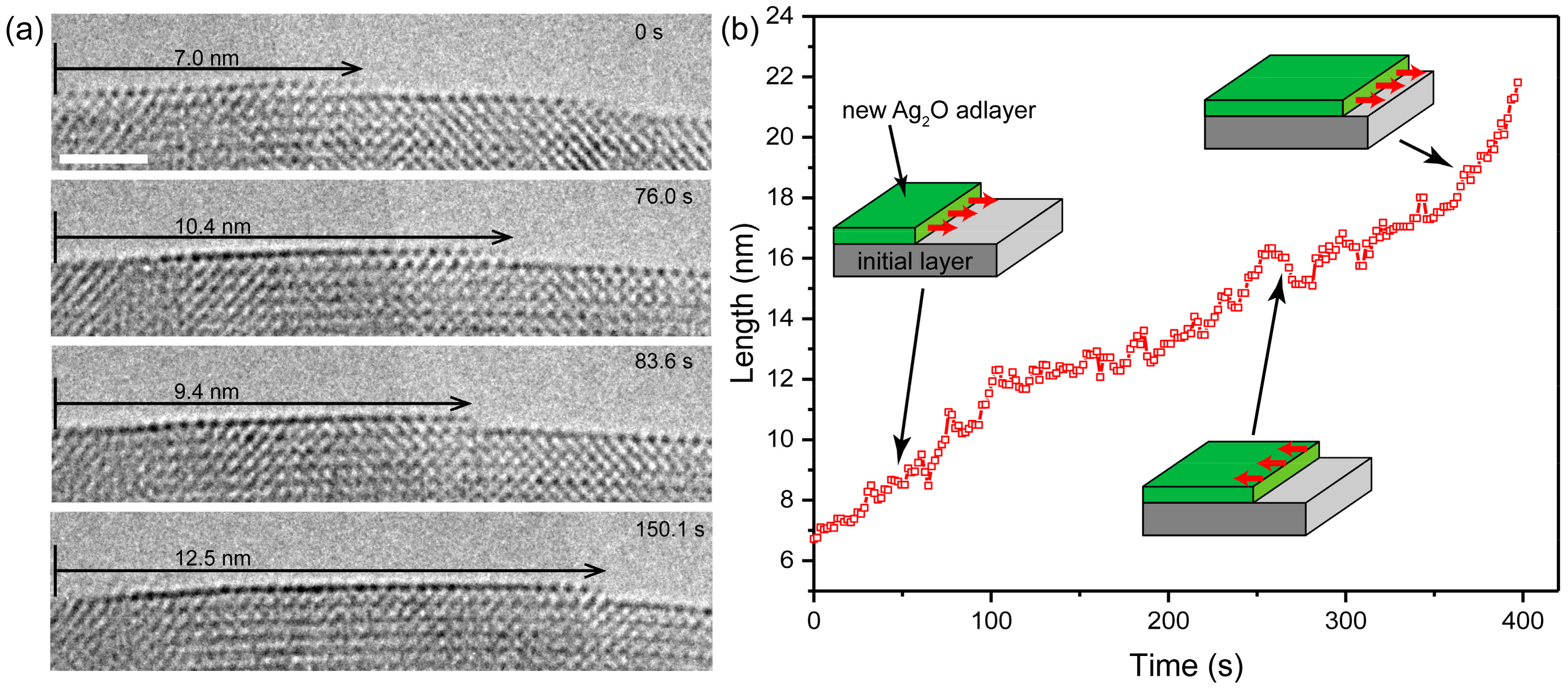
3.3. Layer-by-Layer Growth on {110} Planes
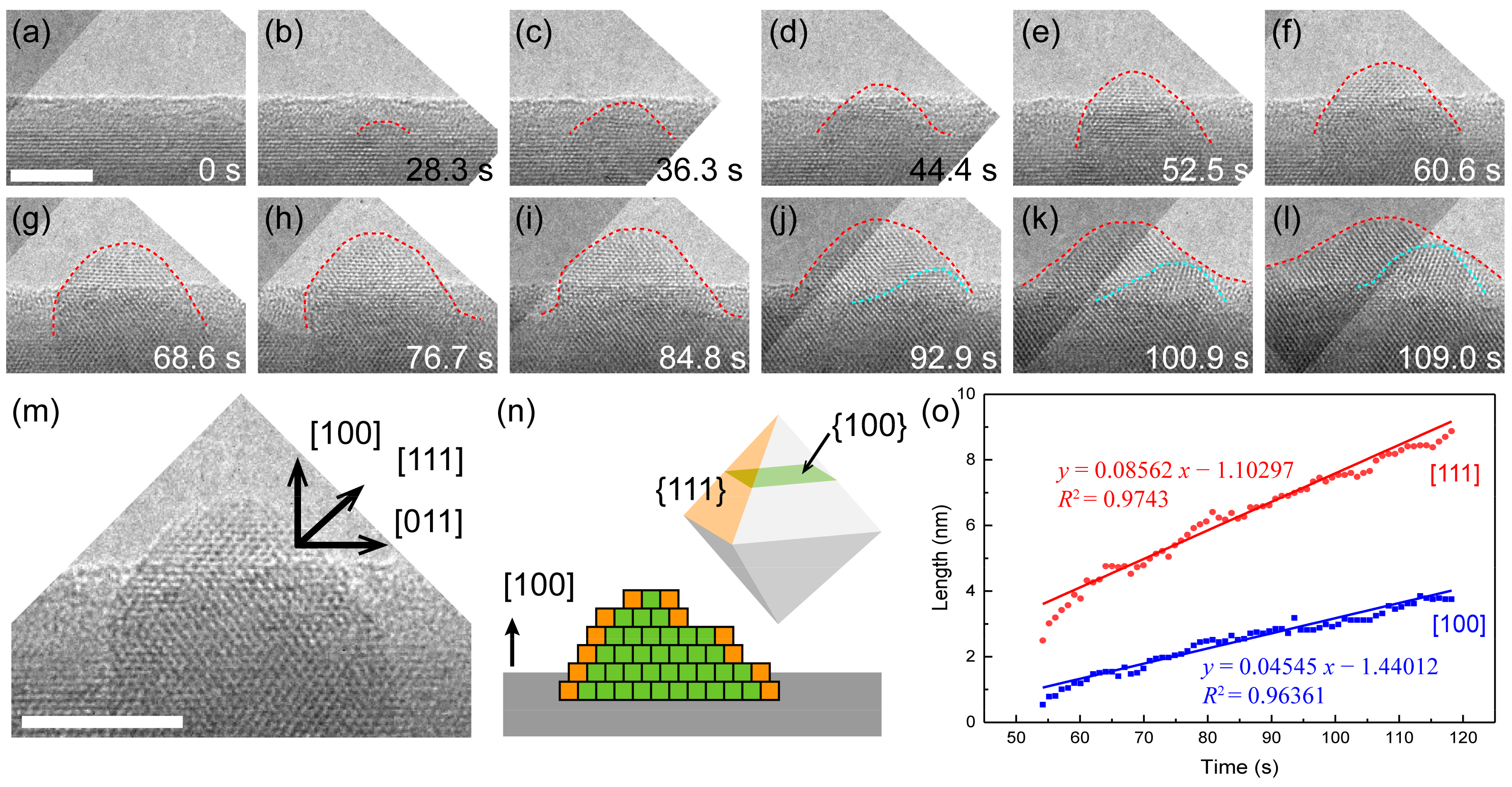
3.4. Growth Kinetics and Its Effect on Oxide Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhan, M.K. Chemical and morphological changes on silver surfaces produced by microwave generated atomic oxygen. J. Vac. Sci. Technol. A 1994, 12, 699–706. [Google Scholar] [CrossRef]
- Miller, G.P.; Pettigrew, P.J.; Raikar, G.N.; Gregory, J.C. A simple, inexpensive, hyperthermal atomic oxygen sensor. Rev. Sci. Instrum. 1997, 68, 3557–3562. [Google Scholar] [CrossRef]
- Pettersson, L.; Snyder, P. Preparation and characterization of oxidized silver thin films. Thin Solid Film. 1995, 270, 69–72. [Google Scholar] [CrossRef]
- Reddy, M.R. Effect of low earth orbit atomic oxygen on spacecraft materials. J. Mater. Sci. 1995, 30, 281–307. [Google Scholar] [CrossRef]
- Moore, W.M.; Codella, P.J. Oxidation of silver films by atomic oxygen. J. Phys. Chem. 1988, 92, 4421–4426. [Google Scholar] [CrossRef]
- Hedin, A.E. MSIS-86 Thermospheric Model. J. Geophys. Res. Space Phys. 1987, 92, 4649–4662. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.C. Complex oxide structures formed by oxidation of Ag(100) and Ag(111) by hyperthermal atomic oxygen. Mater. High. Temp. 2003, 20, 601–606. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Gusakov, A.G.; Voropaev, A.G.; Vecher, A.A.; Kozyrski, E.N.; Raspopov, S.A. Oxidation of Silver by Atomic Oxygen. Oxid. Met. 2004, 61, 39–48. [Google Scholar] [CrossRef]
- Harris, I.; Chambers, A.; Roberts, G. Preliminary results of an atomic oxygen spaceflight experiment. Mater. Lett. 1997, 31, 321–328. [Google Scholar] [CrossRef]
- Chambers, A.; Harris, I.; Roberts, G. Reactions of spacecraft materials with fast atomic oxygen. Mater. Lett. 1996, 26, 121–131. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vemula, P.K.; Ajayan, P.M.; John, G.C. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 2008, 7, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Peyser, L.A.; Roos, D.; Winterbourn, C.C. Photoactivated Fluorescence from Individual Silver Nanoclusters. Science 2001, 291, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zheng, H.; Jia, S.; Chan, M.K.Y.; Rajh, T.; Wang, J.; Wen, J. Atomistic manipulation of reversible oxidation and reduction in Ag with an electron beam. Nanoscale 2019, 11, 10756–10762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.-M.; Robertson, I.; Jenkins, M.; Hutchison, J.; Doole, R. In situ TEM observation of the nucleation and growth of silver oxide nanoparticles. Micron 2005, 36, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Masson, A.; Bréchignac, C. Oxygen and Silver Clusters: Transition from Chemisorption to Oxidation. Phys. Rev. Lett. 2003, 91, 243401. [Google Scholar] [CrossRef]
- Oakes, D.B.; Krech, R.H.; Upschulte, B.L.; Caledonia, G.E. Oxidation of polycrystalline silver films by hyperthermal oxygen atoms. J. Appl. Phys. 1995, 77, 2166–2172. [Google Scholar] [CrossRef]
- Sun, L.; Noh, K.W.; Wen, J.-G.; Dillon, S.J. In Situ Transmission Electron Microscopy Observation of Silver Oxidation in Ionized/Atomic Gas. Langmuir 2011, 27, 14201–14206. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics? Angew. Chem. Int. Ed. 2008, 48, 60–103. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Large-Scale Synthesis of Uniform Silver Nanowires Through a Soft, Self-Seeding, Polyol Process. Adv. Mater. 2002, 14. [Google Scholar] [CrossRef]
- Madasu, M.; Hsieh, P.-L.; Chen, Y.-J.; Huang, M.H. Formation of Silver Rhombic Dodecahedra, Octahedra, and Cubes through Pseudomorphic Conversion of Ag2O Crystals with Nitroarene Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 38039–38045. [Google Scholar] [CrossRef]
- Lyu, L.-M.; Wang, W.-C.; Huang, M.H. Synthesis of Ag2O Nanocrystals with Systematic Shape Evolution from Cubic to Hexapod Structures and Their Surface Properties. Chem. A Eur. J. 2010, 16, 14167–14174. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ma, X.; Huang, B.; Cheng, H.; Wang, Z.; Zhan, J.; Qin, X.; Zhang, X.; Dai, Y. Controlled synthesis of Ag2O microcrystals with facet-dependent photocatalytic activities. J. Mater. Chem. 2012, 22, 21189–21194. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, L.; Wang, S.; Luo, L. Direct visualization of dynamic atomistic processes of Cu2O crystal growth through gas-solid reaction. Nano Energy 2020, 70, 104527. [Google Scholar] [CrossRef]
- Lyu, L.-M.; Huang, M.H. Investigation of Relative Stability of Different Facets of Ag2O Nanocrystals through Face-Selective Etching. J. Phys. Chem. C 2011, 115, 17768–17773. [Google Scholar] [CrossRef]
- Banhart, F. Irradiation effects in carbon nanostructures. Rep. Prog. Phys. 1999, 62, 1181–1221. [Google Scholar] [CrossRef]
- Yu, K.; Xu, T.; Wu, X.; Wang, W.; Zhang, H.; Zhang, Q.; Tang, L.; Sun, L. In Situ Observation of Crystalline Silicon Growth from SiO2 at Atomic Scale. Research 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, S.; Huth, M.; Jungwirth, F. Precursors for direct-write nanofabrication with electrons. J. Mater. Chem. C 2020, 8, 15884–15919. [Google Scholar] [CrossRef]
- Hari, S.; Trompenaars, P.H.F.; Mulders, J.J.L.; Kruit, P.; Hagen, C.W. Combined Focused Electron Beam-Induced Deposition and Etching for the Patterning of Dense Lines without Interconnecting Material. Micromachines 2020, 12, 8. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.C.; Minton, T.K. Morphological Changes at a Silver Surface Resulting from Exposure to Hyperthermal Atomic Oxygen. J. Phys. Chem. C 2007, 111, 6763–6771. [Google Scholar] [CrossRef]
- Schmid, M.; Leonardelli, G.; Tscheließnig, R.; Biedermann, A.; Varga, P. Oxygen adsorption on Al(111): Low transient mobility. Surf. Sci. 2001, 478, L355–L362. [Google Scholar] [CrossRef]
- Carlisle, C.; Fujimoto, T.; Sim, W.; King, D. Atomic imaging of the transition between oxygen chemisorption and oxide film growth on Ag{111}. Surf. Sci. 2000, 470, 15–31. [Google Scholar] [CrossRef]
- Pazzi, V.I.; Tantardini, G.F. Dynamics of oxygen adsorption on Ag(110): Surface motion effects. Surf. Sci. 1997, 377–379, 572–577. [Google Scholar] [CrossRef]
- Barth, J.V.; Zambelli, T.; Wintterlin, J.; Schuster, R.; Ertl, G. Direct observation of mobility and interactions of oxygen molecules chemisorbed on the Ag(110) surface. Phys. Rev. B 1997, 55, 12902–12905. [Google Scholar] [CrossRef]
- Czanderna, A.W. The Adsorption of Oxygen on Silver. J. Phys. Chem. 1964, 68, 2765–2771. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, T.; Zhu, Y.; Wang, W.; Zhang, H.; Yuan, D.; Sun, L. In Situ Atomic-Scale Observation of Silver Oxidation Triggered by Electron Beam Irradiation. Nanomaterials 2021, 11, 1021. https://doi.org/10.3390/nano11041021
Zhang H, Xu T, Zhu Y, Wang W, Zhang H, Yuan D, Sun L. In Situ Atomic-Scale Observation of Silver Oxidation Triggered by Electron Beam Irradiation. Nanomaterials. 2021; 11(4):1021. https://doi.org/10.3390/nano11041021
Chicago/Turabian StyleZhang, Hui, Tao Xu, Yatong Zhu, Wen Wang, Hao Zhang, Dundong Yuan, and Litao Sun. 2021. "In Situ Atomic-Scale Observation of Silver Oxidation Triggered by Electron Beam Irradiation" Nanomaterials 11, no. 4: 1021. https://doi.org/10.3390/nano11041021





