Interlayer Separation in Graphene Paper Comprising Electrochemically Exfoliated Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Graphene Papers Using EEG Flakes
2.2. Characterization of EEG Paper
2.3. Interlayer Separation in EEG Paper
3. Results and Discussion
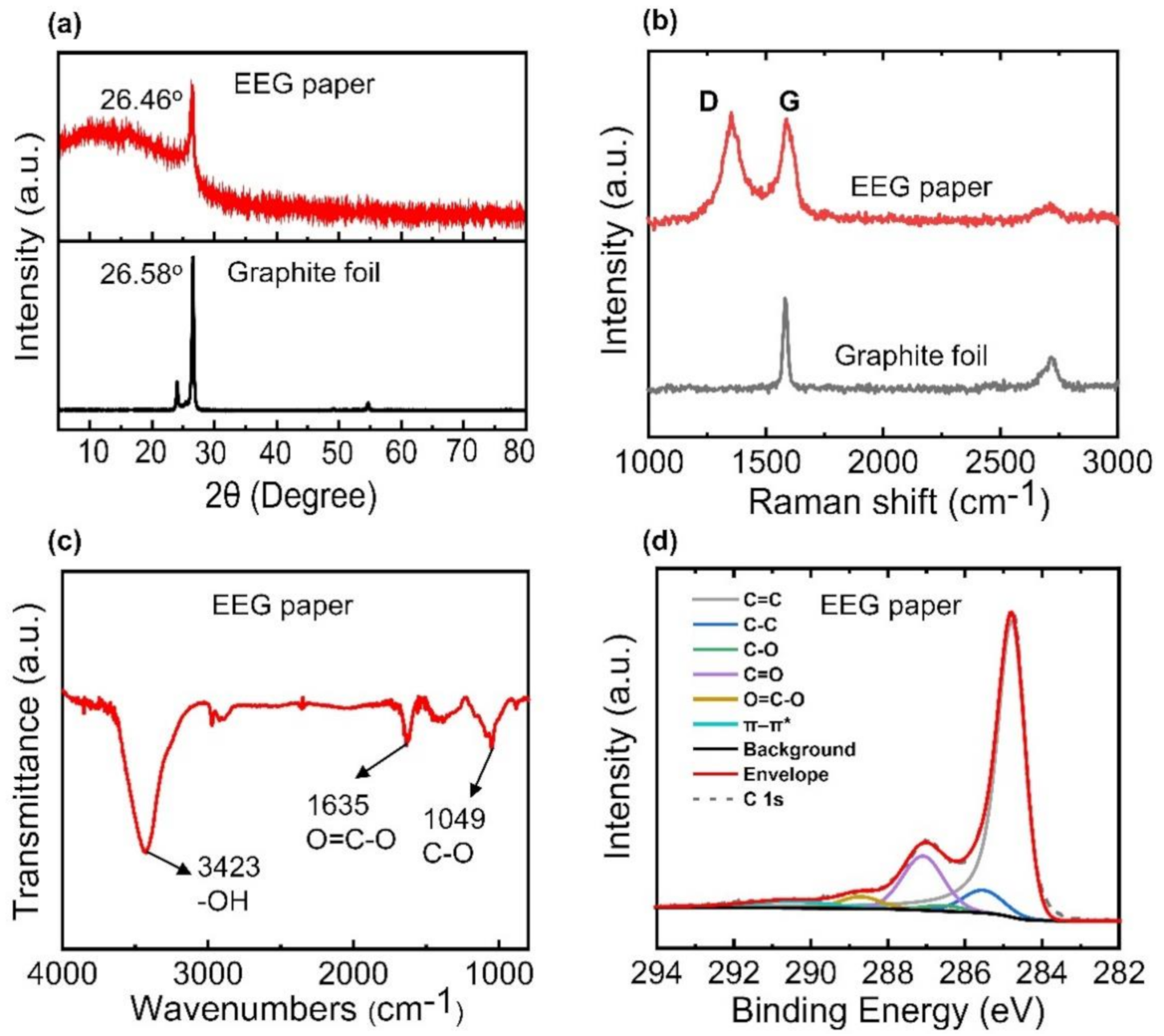
3.1. Characterization of EEG Papers
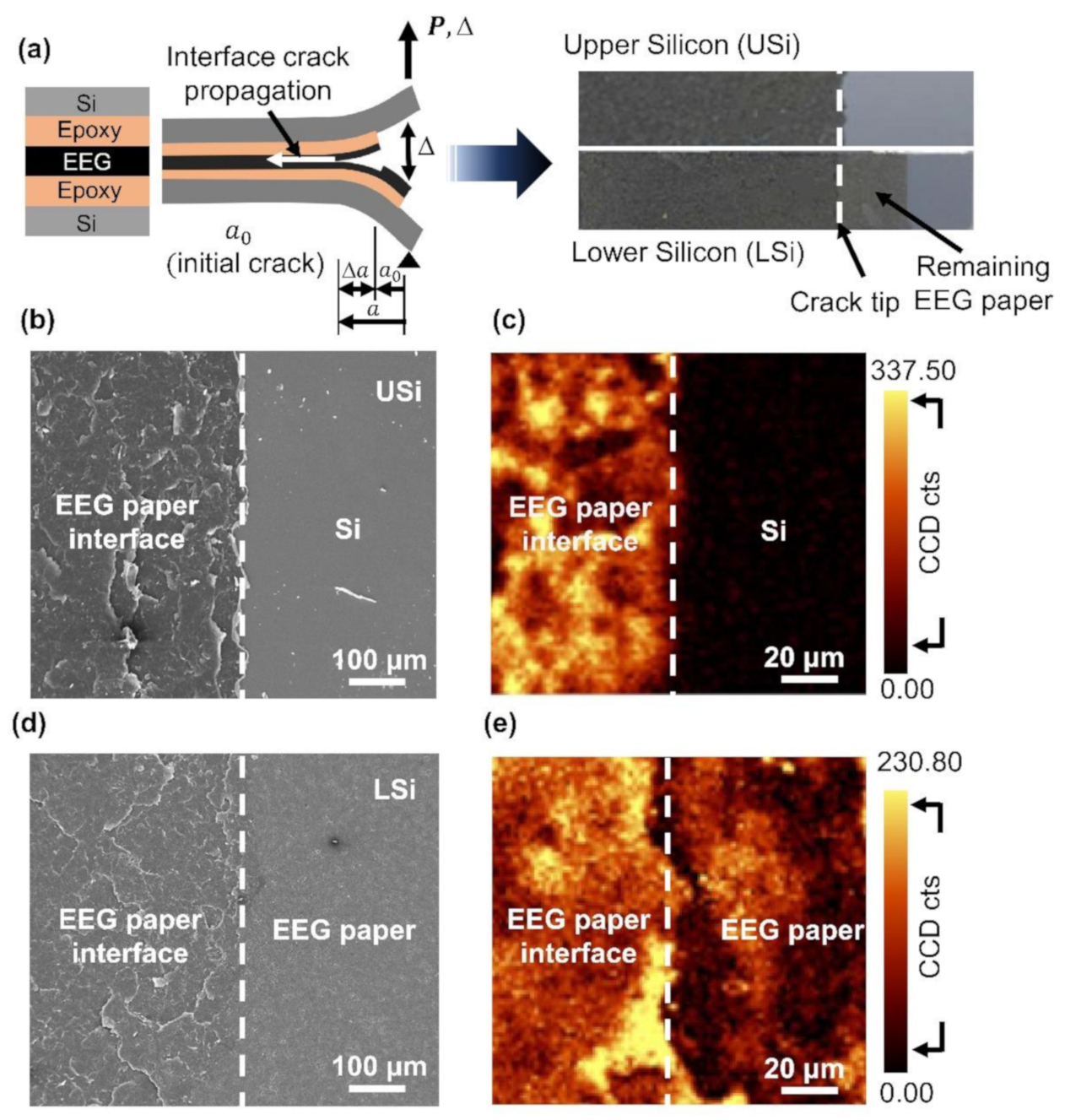
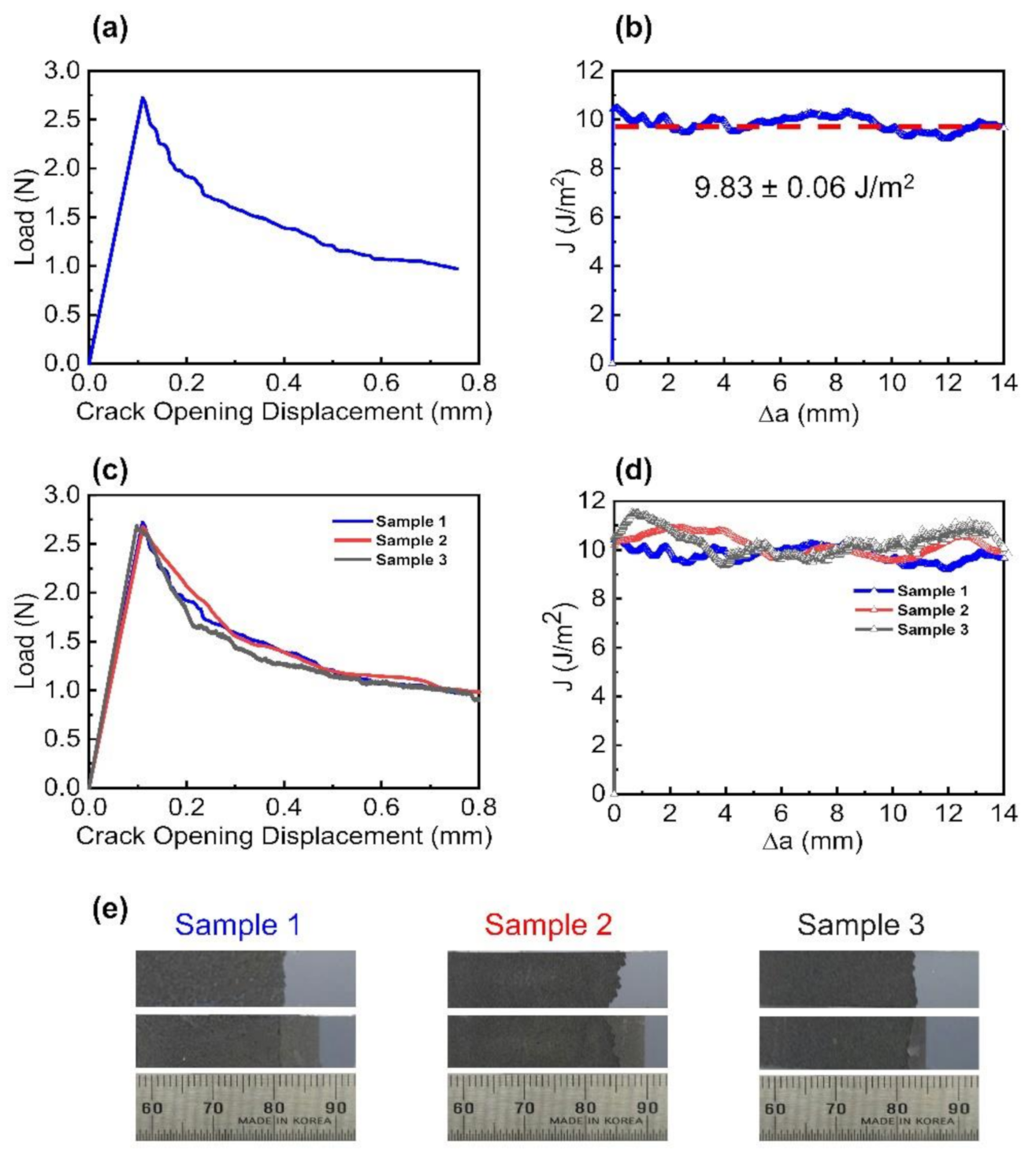
3.2. Interlayer Separation in the EEG Paper Using Mode I Fracture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lee, S.; An, B.-S.; Kim, T.-H.; Yang, C.-W.; Suk, J.W.; Baik, S. Dependence of the In-Plane Thermal Conductivity of Graphene on Grain Misorientation. Chem. Mater. 2017, 29, 10409–10417. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Avouris, P. Graphene: Electronic and Photonic Properties and Devices. Nano Lett. 2010, 10, 4285–4294. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, J.-H. Graphene for flexible and wearable device applications. Carbon 2017, 120, 244–257. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, C.; Li, Z.; Su, J.; Yi, Z.; Yao, W.; Wu, P.; Liu, Z.; Cheng, S.; Pan, M. Multi-band and high-sensitivity perfect absorber based on monolayer graphene metamaterial. Diam. Relat. Mater. 2021, 111, 108227. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Y.; Liu, C.; Zhang, T.; Zhang, B.; Wang, L.; Deng, X.; Bai, Y.; Wang, X. A tunable terahertz metamaterial absorber composed of elliptical ring graphene arrays with refractive index sensing application. Results Phys. 2020, 16, 103012. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z. Gated graphene island-enabled tunable charge transfer plasmon terahertz metamodulator. Nanoscale 2019, 11, 8091–8095. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic Confinement and Coherence in Patterned Epitaxial Graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Suk, J.W.; Ruoff, R.S. Graphene-Based Actuators. Small 2010, 6, 210–212. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Vo, T.T.; Lee, H.-J.; Kim, S.-Y.; Suk, J.W. Synergistic Effect of Graphene/Silver Nanowire Hybrid Fillers on Highly Stretchable Strain Sensors Based on Spandex Composites. Nanomaterials 2020, 10, 2063. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Lim, T.; Ho, B.T.; Suk, J.W. High-performance and thermostable wire supercapacitors using mesoporous activated graphene deposited on continuous multilayer graphene. J. Mater. Chem. A 2021, 9, 4800–4809. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Piao, C.; Jang, H.; Lim, T.; Kim, H.; Choi, H.R.; Hao, Y.; Suk, J.W. Enhanced dynamic performance of twisted and coiled soft actuators using graphene coating. Compos. Part B Eng. 2019, 178, 107499. [Google Scholar] [CrossRef]
- Eigler, S.; Hirsch, A. Chemistry with graphene and graphene oxide—challenges for synthetic chemists. Angew. Chem. Int. Ed. 2014, 53, 7720–7738. [Google Scholar] [CrossRef] [Green Version]
- Parvez, K.; Li, R.; Puniredd, S.R.; Hernandez, Y.; Hinkel, F.; Wang, S.; Feng, X.; Müllen, K. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano 2013, 7, 3598–3606. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Luo, F.; Wu, H.; Liu, Y.; Zhang, C.; Chen, J. One-step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphite. Adv. Funct. Mater. 2008, 18, 1518–1525. [Google Scholar] [CrossRef]
- Yu, P.; Lowe, S.E.; Simon, G.P.; Zhong, Y.L. Electrochemical exfoliation of graphite and production of functional graphene. Curr. Opin. Colloid Interface Sci. 2015, 20, 329–338. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, D.W.; Makotchenko, V.G.; Nazarov, A.S.; Fedorov, V.E.; Kim, Y.H.; Choi, J.Y.; Kim, J.M.; Yoo, J.B. One-step exfoliation synthesis of easily soluble graphite and transparent conducting graphene sheets. Adv. Mater. 2009, 21, 4383–4387. [Google Scholar] [CrossRef]
- Su, C.-Y.; Lu, A.-Y.; Xu, Y.; Chen, F.-R.; Khlobystov, A.N.; Li, L.-J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef]
- Shinde, D.B.; Debgupta, J.; Kushwaha, A.; Aslam, M.; Pillai, V.K. Electrochemical unzipping of multi-walled carbon nanotubes for facile synthesis of high-quality graphene nanoribbons. J. Am. Chem. Soc. 2011, 133, 4168–4171. [Google Scholar] [CrossRef]
- Munuera, J.; Paredes, J.; Villar-Rodil, S.; Ayán-Varela, M.; Martínez-Alonso, A.; Tascón, J. Electrolytic exfoliation of graphite in water with multifunctional electrolytes: En route towards high quality, oxide-free graphene flakes. Nanoscale 2016, 8, 2982–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvez, K.; Wu, Z.-S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Mullen, K. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wu, Z.S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible in-plane micro-supercapacitors by direct printing of solution-processable electrochemically exfoliated graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, C.; Halder, A.; Wang, H.; Chi, Q. Graphene papers: Smart architecture and specific functionalization for biomimetics, electrocatalytic sensing and energy storage. Mater. Chem. Front. 2017, 1, 37–60. [Google Scholar] [CrossRef]
- Karthick, R.; Chen, F. Free-standing graphene paper for energy application: Progress and future scenarios. Carbon 2019, 150, 292–310. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Liu, X.; Cheng, X.; Li, Y.; Sang, L.; Ma, J.; Wang, J.; He, J.; Wang, M.; Zhang, Y. Unveiling the thickness-dependent mechanical properties of graphene papers by in situ SEM tension. RSC Adv. 2019, 9, 4609–4615. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Brüller, S.; Wu, Z.-S.; Liu, Z.; Parvez, K.; Dong, R.; Richard, F.; Samorì, P.; Feng, X.; Müllen, K. Organic radical-assisted electrochemical exfoliation for the scalable production of high-quality graphene. J. Am. Chem. Soc. 2015, 137, 13927–13932. [Google Scholar] [CrossRef] [Green Version]
- Suk, J.W.; Murali, S.; An, J.; Ruoff, R.S. Mechanical measurements of ultra-thin amorphous carbon membranes using scanning atomic force microscopy. Carbon 2012, 50, 2220–2225. [Google Scholar] [CrossRef]
- Na, S.R.; Suk, J.W.; Tao, L.; Akinwande, D.; Ruoff, R.S.; Huang, R.; Liechti, K.M. Selective mechanical transfer of graphene from seed copper foil using rate effects. ACS Nano 2015, 9, 1325–1335. [Google Scholar] [CrossRef]
- Xu, C.; Yang, T.; Kang, Y.; Li, Q.; Xue, T.; Liechti, K.M.; Huang, R.; Qiu, W. Rate-Dependent Decohesion Modes in Graphene-Sandwiched Interfaces. Adv. Mater. Interfaces 2019, 6, 1901217. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- López, V.; Sundaram, R.S.; Gómez-Navarro, C.; Olea, D.; Burghard, M.; Gómez-Herrero, J.; Zamora, F.; Kern, K. Chemical Vapor Deposition Repair of Graphene Oxide: A Route to Highly-Conductive Graphene Monolayers. Adv. Mater. 2009, 21, 4683–4686. [Google Scholar] [CrossRef]
- Mao, X.; Zhu, L.; Liu, H.; Chen, H.; Ju, P.; Li, W. Synthesis of graphene via electrochemical exfoliation in different electrolytes for direct electrodeposition of a Cu/graphene composite coating. RSC Adv. 2019, 9, 35524–35531. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.; Zhang, L.; Li, R.; Wu, J.; Hedhili, M.N.; Wang, P. Are vacuum-filtrated reduced graphene oxide membranes symmetric? Nanoscale 2016, 8, 1108–1116. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.; Ham, M.-H.; Park, H.B.; Kim, C.-M.; Song, J.-h.; Kim, I.S. Tunable semi-permeability of graphene-based membranes by adjusting reduction degree of laminar graphene oxide layer. J. Membr. Sci. 2018, 547, 73–79. [Google Scholar] [CrossRef]
- Ambrosi, A.; Pumera, M. Electrochemically exfoliated graphene and graphene oxide for energy storage and electrochemistry applications. Chem. A Eur. J. 2016, 22, 153–159. [Google Scholar] [CrossRef]
- Cao, J.; He, P.; Mohammed, M.A.; Zhao, X.; Young, R.J.; Derby, B.; Kinloch, I.A.; Dryfe, R.A. Two-step electrochemical intercalation and oxidation of graphite for the mass production of graphene oxide. J. Am. Chem. Soc. 2017, 139, 17446–17456. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; He, C.; Dai, L.; Liu, J.; Wang, L. Rationally designed surfactants for few-layered graphene exfoliation: Ionic groups attached to electron-deficient π-conjugated unit through alkyl spacers. ACS Nano 2014, 8, 6663–6670. [Google Scholar] [CrossRef]
- Wang, H.; Wei, C.; Zhu, K.; Zhang, Y.; Gong, C.; Guo, J.; Zhang, J.; Yu, L.; Zhang, J. Preparation of graphene sheets by electrochemical exfoliation of graphite in confined space and their application in transparent conductive films. ACS Appl. Mater. Interfaces 2017, 9, 34456–34466. [Google Scholar] [CrossRef] [PubMed]
- Eigler, S.; Enzelberger-Heim, M.; Grimm, S.; Hofmann, P.; Kroener, W.; Geworski, A.; Dotzer, C.; Röckert, M.; Xiao, J.; Papp, C. Wet chemical synthesis of graphene. Adv. Mater. 2013, 25, 3583–3587. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Lahaye, R.J.; Park, M.-H.; An, K.H.; Kim, I.J.; Yang, C.-W.; Park, C.Y.; Ruoff, R.S.; Lee, Y.H. Evidence of graphitic AB stacking order of graphite oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef]
- Achee, T.C.; Sun, W.; Hope, J.T.; Quitzau, S.G.; Sweeney, C.B.; Shah, S.A.; Habib, T.; Green, M.J. High-yield scalable graphene nanosheet production from compressed graphite using electrochemical exfoliation. Sci. Rep. 2018, 8, 14525. [Google Scholar] [CrossRef]
- Lin, X.; Shen, X.; Zheng, Q.; Yousefi, N.; Ye, L.; Mai, Y.-W.; Kim, J.-K. Fabrication of Highly-Aligned, Conductive, and Strong Graphene Papers Using Ultralarge Graphene Oxide Sheets. ACS Nano 2012, 6, 10708–10719. [Google Scholar] [CrossRef]
- Koenig, S.P.; Boddeti, N.G.; Dunn, M.L.; Bunch, J.S. Ultrastrong adhesion of graphene membranes. Nat. Nanotechnol. 2011, 6, 543–546. [Google Scholar] [CrossRef]
- Sanchez, D.A.; Dai, Z.; Wang, P.; Cantu-Chavez, A.; Brennan, C.J.; Huang, R.; Lu, N. Mechanics of spontaneously formed nanoblisters trapped by transferred 2D crystals. Proc. Natl. Acad. Sci. USA 2018, 115, 7884–7889. [Google Scholar] [CrossRef]
- Suk, J.W.; Na, S.R.; Stromberg, R.J.; Stauffer, D.; Lee, J.; Ruoff, R.S.; Liechti, K.M. Probing the adhesion interactions of graphene on silicon oxide by nanoindentation. Carbon 2016, 103, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Semoto, T.; Tsuji, Y.; Tanaka, H.; Yoshizawa, K. Role of Edge Oxygen Atoms on the Adhesive Interaction between Carbon Fiber and Epoxy Resin. J. Phys. Chem. C 2013, 117, 24830–24835. [Google Scholar] [CrossRef]
- Megra, Y.T.; Suk, J.W. Adhesion properties of 2D materials. J. Phys. D Appl. Phys. 2019, 52, 364002. [Google Scholar] [CrossRef]
- Na, S.R.; Kim, Y.; Lee, C.; Liechti, K.M.; Suk, J.W. Adhesion and Self-Healing between Monolayer Molybdenum Disulfide and Silicon Oxide. Sci. Rep. 2017, 7, 14740. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.D.; Lim, T.; Lim, S.; Suk, J.W. Interlayer Separation in Graphene Paper Comprising Electrochemically Exfoliated Graphene. Nanomaterials 2021, 11, 865. https://doi.org/10.3390/nano11040865
Nguyen DD, Lim T, Lim S, Suk JW. Interlayer Separation in Graphene Paper Comprising Electrochemically Exfoliated Graphene. Nanomaterials. 2021; 11(4):865. https://doi.org/10.3390/nano11040865
Chicago/Turabian StyleNguyen, Dang Du, TaeGyeong Lim, Soomook Lim, and Ji Won Suk. 2021. "Interlayer Separation in Graphene Paper Comprising Electrochemically Exfoliated Graphene" Nanomaterials 11, no. 4: 865. https://doi.org/10.3390/nano11040865
APA StyleNguyen, D. D., Lim, T., Lim, S., & Suk, J. W. (2021). Interlayer Separation in Graphene Paper Comprising Electrochemically Exfoliated Graphene. Nanomaterials, 11(4), 865. https://doi.org/10.3390/nano11040865






