Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review
Abstract
:1. Introduction
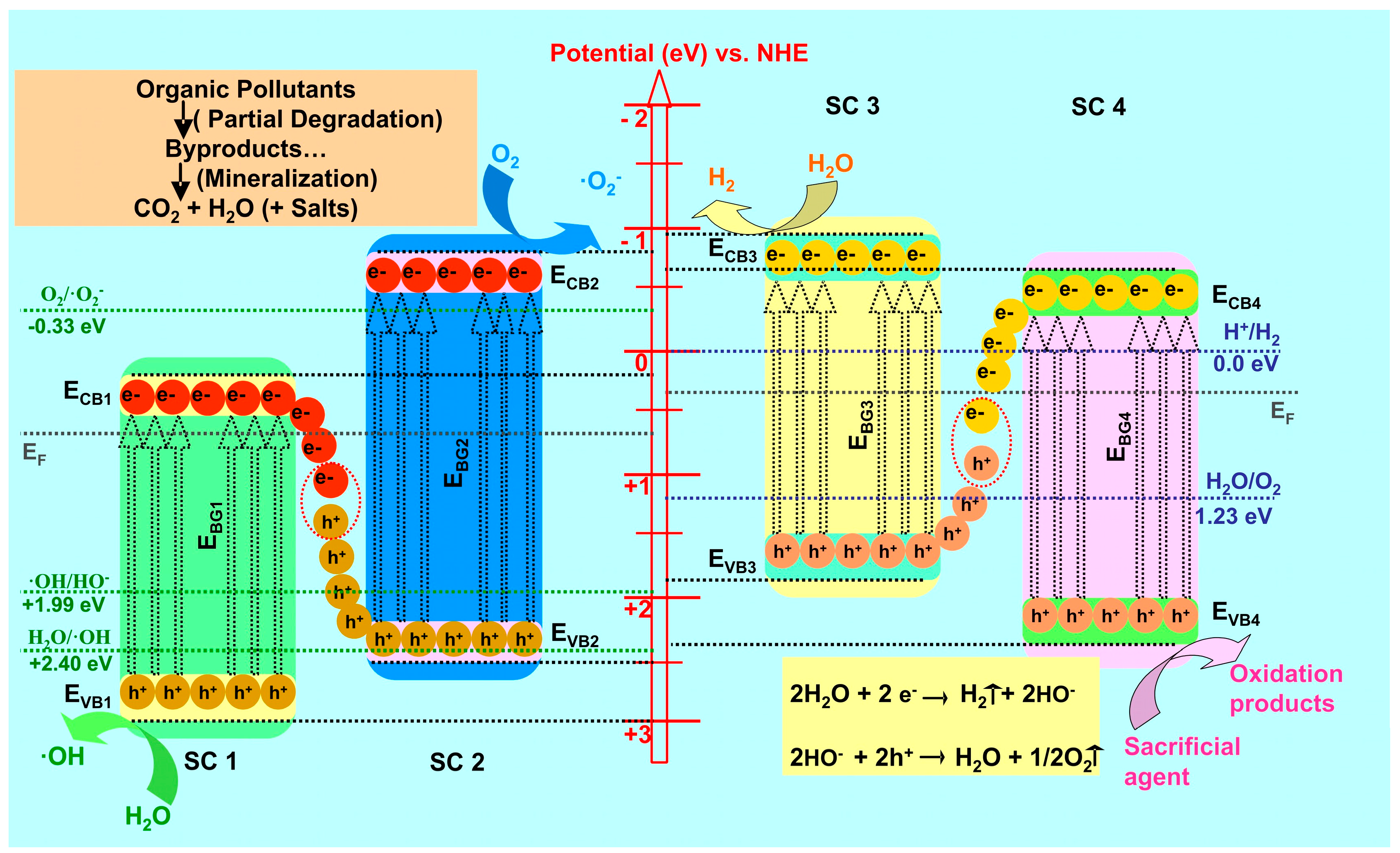
2. S-Scheme Heterojunction Mechanism
3. S-Scheme Heterostructure Photocatalytic Applications
3.1. Photocatalytic Removal of Organic Pollutant
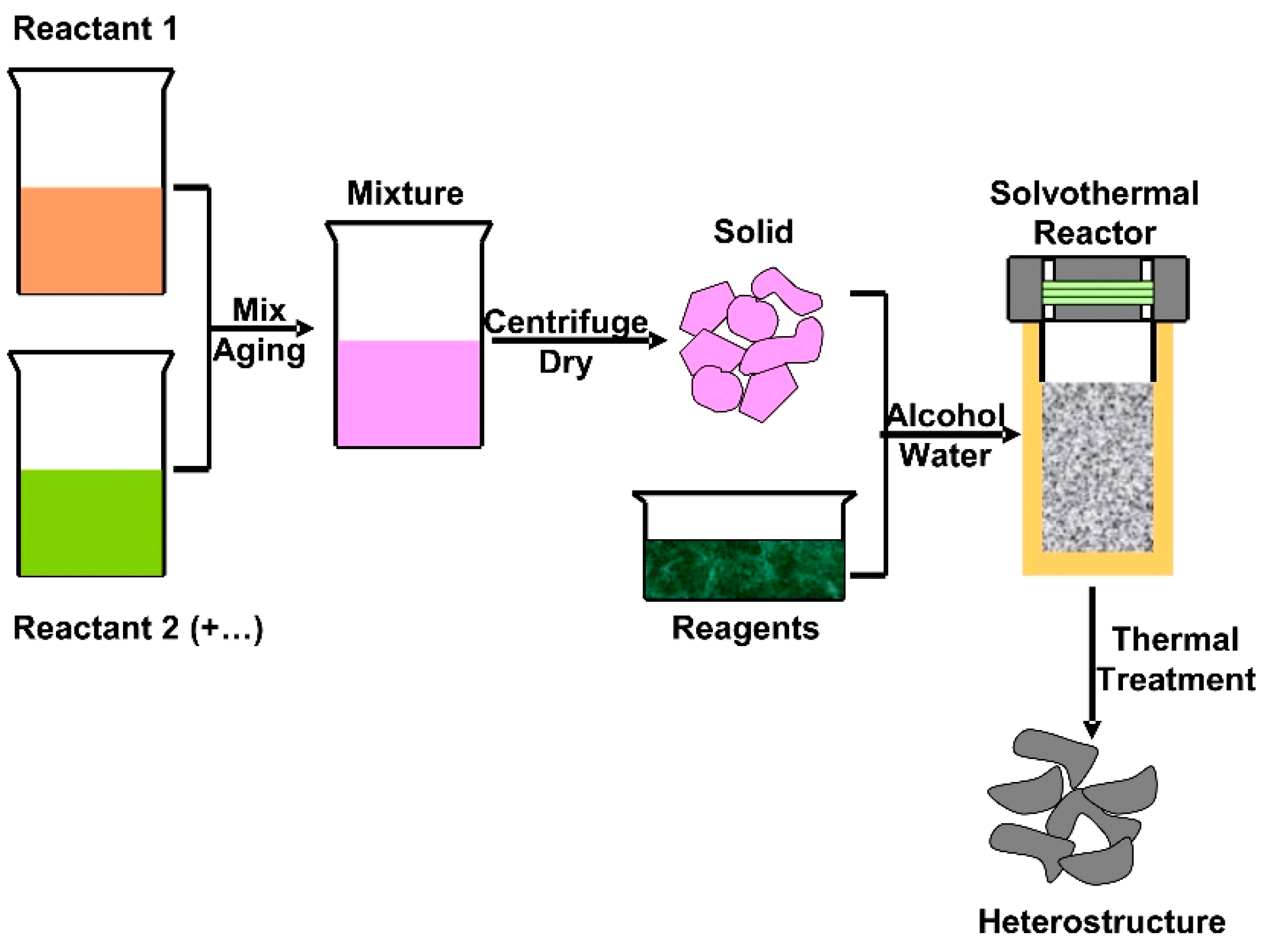
3.1.1. Heterostructures Obtained by Solvothermal Method
3.1.2. Heterostructures Obtained by Hydrothermal Method
3.1.3. Heterostructures Obtained by One-Pot and Precipitation Methods
3.1.4. Heterostructures Obtained by Other Methods
3.2. Photocatalytic Water Splitting for Hydrogen Production
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Liu, R.; Kuikka, S. Quantifying and predicting ecological and human health risks for binary heavy metal pollution accidents at the watershed scale using Bayesian Networks. Environ. Pollut. 2021, 269, 116125. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Van Kollenburg, G.H.; van Manen, H.J.; Jansen, J.J. Low-cost handheld NIR spectroscopy for identification of organic solvents and low-level quantification of water contamination. Talanta 2021, 223, 121865. [Google Scholar] [CrossRef] [PubMed]
- Shadrin, D.; Nikitin, A.; Tregubova, P.; Terekhova, V.; Jana, R.; Matveev, S.; Pukalchik, M. An Automated Approach to Groundwater Quality Monitoring—Geospatial Mapping Based on Combined Application of Gaussian Process Regression and Bayesian Information Criterion. Water 2021, 13, 400. [Google Scholar] [CrossRef]
- Bagdadee, A.M.; Zhang, L. Electrical power crisis solution by the developing renewable energy based power generation expansion. Energ. Rep. 2020, 6, 480–490. [Google Scholar] [CrossRef]
- Stoupos, N.; Kiohos, A. Energy commodities and advanced stock markets: A post-crisis approach. Resour. Policy 2020, 70, 101887. [Google Scholar] [CrossRef]
- Baneto, M.; Enesca, A.; Mihoreanu, C.; Lare, Y.; Jondo, K.; Napo, K.; Duta, A. Effects of the growth temperature on the properties of spray deposited CuInS2 thin films for photovoltaic applications. Ceram. Int. 2015, 41, 4742–4749. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Mehr, M.Y.; van Driel, W.D.; Zhang, K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials 2021, 14, 763. [Google Scholar] [CrossRef]
- Gonzalez, Z.; Yus, J.; Bravo, Y.; Sanchez-Herencia, A.J.; Ferrari, B. Exploitation of Lignocellulose Fiber-Based Biotemplates to Improve the Performance of an Immobilized TiO2 Photocatalyst. Catalysts 2021, 11, 156. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L.; Duta, A. Charge carriers injection in tandem semiconductors for dyes mineralization. Appl. Catal. B 2015, 162, 352–363. [Google Scholar] [CrossRef]
- Guo, Z.; Huo, W.; Zhang, Y. Heterojunction interface of zinc oxide and zinc sulfide promoting reactive molecules activation and carrier separation toward efficient photocatalysis. J. Colloid Interfac. Sci. 2021, 588, 826–837. [Google Scholar] [CrossRef]
- Xiao, W.; Su, Y.; Zhang, P. Flower-like hierarchical architecture of BiOI/ZnO p-n junction composites with high-efficient visible-light photodegradation activities. Solid State Sci. 2020, 108, 106432. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liao, H.H.; Sung, P.J. Well-behaved Ge n+/p shallow junction achieved by plasma immersion ion implantation. Vacuum 2020, 180, 109528. [Google Scholar] [CrossRef]
- Zhu, X.T.; Xu, Y.; Ao, Z. Investigation of the electronic structure of two-dimensional GaN/Zr2CO2 hetero-junction: Type-II band alignment with tunable bandgap. Appl. Surf. Sci. 2021, 542, 148505. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Wang, Y. Reasonable design of Cu2MoS4 heterophase junction for highly efficient photocatalysis. J. Alloy. Compound. 2020, 826, 154076. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, X.Q.; Fan, Z.Q. First-principles study on the electronic transport properties of M/SiC Schottky junctions (M = Ag, Au and Pd). Phys. Lett. A 2020, 384, 126732. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, J.; Meng, M. Rationally constructing of a novel 2D/2D WO3/Pt/g-C3N4 Schottky-Ohmic junction towards efficient visible-light-driven photocatalytic hydrogen evolution and mechanism insight. J. Colloid Interfac. Sci. 2021, 586, 176–587. [Google Scholar] [CrossRef]
- Sharma, S.; Ibhadon, A.O.; Francesconi, M.G.; Mehta, S.K.; Elumalai, S.; Kansal, S.K.; Umar, A.; Baskoutas, S. Bi2WO6/C-Dots/TiO2: A Novel Z-Scheme Photocatalyst for the Degradation of Fluoroquinolone Levofloxacin from Aqueous Medium. Nanomaterials 2020, 10, 910. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P. Recent advances in silver bromide-based Z-scheme photocatalytic systems for environmental and energy applications: A review. J. Environ. Chem. Eng. 2021, 9, 105157. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J. S-Scheme Heterojunction Photocatalyst. Chem 2020, 6, 1543–1559. [Google Scholar] [CrossRef]
- Fominski, V.; Romanov, R.; Fominski, D.; Soloviev, A.; Rubinkovskaya, O.; Demin, M.; Maksimova, K.; Shvets, P.; Goikhman, A. Performance and Mechanism of Photoelectrocatalytic Activity of MoSx/WO3 Heterostructures Obtained by Reactive Pulsed Laser Deposition for Water Splitting. Nanomaterials 2020, 10, 871. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Acar, G.; Iqbal, M.J.; Chaudhry, M.U. Large Area Emission in p-Type Polymer-Based Light-Emitting Field-Effect Transistors by Incorporating Charge Injection Interlayers. Materials 2021, 14, 901. [Google Scholar] [CrossRef]
- Li, H.; Hou, J.; Jiang, D. Hexagonal borophene sandwiched between blue phosphorenes: A novel bonding heterostructure as an anchoring material for lithium-sulfur batteries. Appl. Surf. Sci. 2021, 545, 148770. [Google Scholar] [CrossRef]
- Li, W.; You, Y.; Choi, J.H. Vacancy defects in the vertical heterostructures of graphene and MoS2. Surf. Sci. 2021, 707, 121809. [Google Scholar] [CrossRef]
- Fu, J.W.; Xu, Q.L.; Low, J.X.; Jiang, C.J.; Yu, J.G. Ultrathin 2D/2D WO3/g-C3N4 stepscheme H2-production photocatalyst. Appl. Catal. B 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Ge, H.N.; Xu, F.Y.; Cheng, B.; Yu, J.G.; Ho, W.K. S-Scheme heterojunction TiO2/CdS nanocomposite nanofiber as H2-production photocatalyst. Chem. Cat. Chem. 2019, 11, 6301–6309. [Google Scholar]
- Yang, J.W.; Ahn, S.H.; Jang, H.W. Crucial role of heterostructures in highly advanced water splitting photoelectrodes. Curr. Opin. Green Sustain. Chem. 2021, 29, 100454. [Google Scholar] [CrossRef]
- Guo, C.; Tian, K.; Hu, Y. Approach of fermi level and electron-trap level in cadmium sulfide nanorods via molybdenum doping with enhanced carrier separation for boosted photocatalytic hydrogen production. J. Colloid Interfac. Sci. 2021, 583, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, A.; Salavati-Niasari, M. Transition metal selenides and diselenides: Hydrothermal fabrication, investigation of morphology, particle size and and their applications in photocatalyst. Adv. Colloid Interfac. Sci. 2021, 287, 102321. [Google Scholar] [CrossRef] [PubMed]
- Narenuch, T.; Senasu, T.; Nanan, S. Solvothermal synthesis of CTAB capped and SDS capped BiOCl photocatalysts for degradation of rhodamine B (RhB) dye and fluoroquinolone antibiotics. J. Solid State Chem. 2021, 294, 121824. [Google Scholar] [CrossRef]
- Zhao, Q.; Feng, G.; Jiang, W. Nonhydrolytic sol-gel in-situ synthesis of novel recoverable amorphous Fe2TiO5/C hollow spheres as visible-light driven photocatalysts. Mater. Des. 2020, 194, 108928. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Li, Z. Fabrication of black phosphorus nanosheets/BiOBr visible light photocatalysts via the co-precipitation method. Colloid. Surf. A. 2021, 612, 125967. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, Q.; Leung, D.Y.C. A novel Au/g-C3N4 nanosheets/CeO2 hollow nanospheres plasmonic heterojunction photocatalysts for the photocatalytic reduction of hexavalentchromium and oxidation of oxytetracycline hydrochloride. Chem. Eng. J. 2021, 409, 128185. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, X.; Liu, X. Magnetically separable and recyclable ternary photocatalyst MnxZn1−xFe2O4/BiVO4/MnO2 with excellent photocatalytic activity. Vacuum 2021, 187, 110133. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, W.; Xu, J. A novel AgCl-based visible-light photocatalyst through in-situ assembly of carbon dots for efficient dye degradation and hydrogen evolution. Sustain. Mater. Technol. 2021, 27, e00242. [Google Scholar]
- Wang, Y.; Wang, Q.; Gao, S. CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol. 2020, 242, 116775. [Google Scholar] [CrossRef]
- Chowdhury, E.H.; Rahman, M.H.; Islam, M.M. Investigation of the mechanical properties and fracture mechanisms of graphene/WSe2 vertical heterostructure: A molecular dynamics study. Comp. Mater. Sci. 2021, 188, 110231. [Google Scholar] [CrossRef]
- Tian, H.; Wu, H.; Huang, Y. Hydrothermal synthesis of m-BiVO4/t-BiVO4 heterostructure for organic pollutants degradation: Insight into the photocatalytic mechanism of exposed facets from crystalline phase controlling. J. Hazard. Mater. 2020, 399, 123159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Jian, W.; Bai, F.Q. Insights into the photocatalytic mechanism of the C4N/MoS2 heterostructure: A first-principle study. Chin. Chem. Lett. 2020, 31, 2319–2324. [Google Scholar] [CrossRef]
- Cao, W.; Jiang, C.; Wang, Y. A novel Z-scheme CdS/Bi4O5Br2 heterostructure with mechanism analysis: Enhanced photocatalytic performance. J. Alloys Compd. 2021, 861, 158554. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Lin, Z. ZIF-67-induced double-tubular 1D CeO2/Co3O4 heterostructures allowing electron transfer synergetic mechanism for enhanced photocatalytic performance. Mater. Lett. 2021, 289, 129391. [Google Scholar] [CrossRef]
- Dudita, M.; Bogatu, C.; Enesca, A.; Duta, A. The influence of the additives composition and concentration on the properties of SnOx thin films used in photocatalysis. Mater. Lett. 2011, 65, 2185–2189. [Google Scholar] [CrossRef]
- Kumar, G.J.; Sarathbavan, M.; Bharathi, K.K. Mechanism of analog bipolar resistive switching and work function in Au/Na0.5Bi0.5TiO3/Pt heterostructure thin films. Mater. Chem. Phys. 2021, 257, 123765. [Google Scholar] [CrossRef]
- Kovalskiy, V.A.; Eremenko, V.G.; Danilov, Y.A. On the mechanism of cross-hatch pattern formation in heterostructures with a small lattice mismatch. Appl. Surf. Sci. 2019, 479, 930–941. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, M.; Liu, B. Hierarchical Fe2O3 nanorods/TiO2 nanosheets heterostructure: Growth mechanism, enhanced visible-light photocatalytic and photoelectrochemical performances. Appl. Surf. Sci. 2019, 475, 380–388. [Google Scholar] [CrossRef]
- Tiwari, P.; Jaiswal, J.; Chandra, R. Hierarchal growth of MoS2@CNT heterostructure for all solid state symmetric supercapacitor: Insights into the surface science and storage mechanism. Electrochim. Acta 2019, 324, 134767. [Google Scholar] [CrossRef]
- Enesca, A.; Andronic, L. The Influence of Photoactive Heterostructures on the Photocatalytic Removal of Dyes and Pharmaceutical Active Compounds: A Mini-Review. Nanomaterials 2020, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Tryba, B.; Rychtowski, P.; Markowska-Szczupak, A.; Przepiórski, J. Photocatalytic Decomposition of Acetaldehyde on Different TiO2-Based Materials: A Review. Catalysts 2020, 10, 1464. [Google Scholar] [CrossRef]
- Zubair, U.; Bianco, S.; Bodoardo, S. Probing the interaction mechanism of heterostructured VOxNy nanoparticles supported in nitrogen-doped reduced graphene oxide aerogel as an efficient polysulfide electrocatalyst for stable sulfur cathodes. J. Power Sources 2020, 461, 228144. [Google Scholar] [CrossRef]
- Chen, L.; Ning, X.K.; Chen, M.J. Thickness-dependent of conduction mechanism and bias electric field modulation of transport properties for SrRuO3/PMN-PT heterostructures. Phys. Lett. A 2018, 382, 2989–2993. [Google Scholar] [CrossRef]
- Su, M.; Xu, R.; Chen, D. Heterostructured Bi2O2CO3/rGO/PDA photocatalysts with superior activity for organic pollutant degradation: Structural characterization, reaction mechanism and economic assessment. Ecotox. Environ. Saf. 2020, 204, 111112. [Google Scholar] [CrossRef]
- Ali, I.; Kim, J.-O. Continuous-Flow Photocatalytic Degradation of Organics Using Modified TiO2 Nanocomposites. Catalysts 2018, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Shtarev, D.S.; Shtareva, A.V.; Syuy, A.V. Synthesis, characterization, optoelectronic and photocatalytic properties of Sr2Bi2O5/SrCO3 and Sr3Bi2O6/SrCO3 heterostructures with varying SrCO3 content. Chemosphere 2021, 267, 129229. [Google Scholar] [CrossRef]
- Huang, L.; Yu, Y.; Xu, B. Molecule assembly of heterostructured TiO2@BiOCl via fenton-like reaction for enhanced solar energy conversion. Ceram. Int. 2020, 47, 10716–10723. [Google Scholar] [CrossRef]
- Lin, H.J.; Mo, Q.L.; Xiao, F.X. Unlocking photoredox selective organic transformation over metal-free 2D transition metal chalcogenides-MXene heterostructures. J. Catal. 2020, 391, 485–496. [Google Scholar] [CrossRef]
- Banumathi, S.; Uma, J.; Kumar, G.M. Rapid sun-light driven photocatalytic functions of 3D rGO/ZnO/Ag heterostructures via improved charge transfer kinetics. J. Mater. Res. Technol. 2021, 10, 1301–1309. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; He, Q. Recent progress on photocatalytic heterostructures with full solar spectral responses. Chem. Eng. J. 2020, 393, 124719. [Google Scholar] [CrossRef]
- Li, J.; Jin, B.; Jiao, Z. Rationally embedded zinc oxide nanospheres serving as electron transport channels in bismuth vanadate/zinc oxide heterostructures for improved photoelectrochemical efficiency. J. Colloid Interfac. Sci. 2021, 592, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Bilal, M.; Raj, A. Trends in predictive biodegradation for sustainable mitigation of environmental pollutants: Recent progress and future outlook. Sci. Total Environ. 2021, 770, 144561. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Ma, J.; Chen, Z.; Kang, B.; Liu, J.; Li, H.; Feng, Z.; Huang, J. Novel BP/BiOBr S-scheme nano-heterojunction for enhanced visible-light photocatalytic tetracycline removal and oxygen evolution activity. J. Hazard. Mater. 2020, 387, 121690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Deng, F.; Luo, X.; Dionysiou, D.D. Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions. Chem. Eng. J. 2020, 379, 122264. [Google Scholar] [CrossRef]
- Wang, R.; Shen, J.; Zhang, W.; Liu, Q.; Zhang, M.; Tang, H. Build-in electric field induced step-scheme TiO2/W18O49 heterojunction for enhanced photocatalytic activity under visible-light irradiation. Ceram. Int. 2020, 46, 23–30. [Google Scholar] [CrossRef]
- Hu, X.; Wang, G.; Wang, J.; Hu, Z.; Su, Y. Step-scheme NiO/BiOI heterojunction photocatalyst for rhodamine photodegradation. Appl. Surf. Sci. 2020, 511, 145499. [Google Scholar] [CrossRef]
- Xu, A.; Tu, W.; Shen, S.; Lin, Z.; Gao, N.; Zhong, W. BiVO4@MoS2 core-shell heterojunction with improved photocatalytic activity for discoloration of Rhodamine B. Appl. Surf. Sci. 2020, 528, 146949. [Google Scholar] [CrossRef]
- Dou, L.; Jin, X.; Chen, J.; Zhong, J.; Li, J.; Zeng, Y.; Duan, R. One-pot solvothermal fabrication of S-scheme OVs-Bi2O3/Bi2SiO5 microsphere heterojunctions with enhanced photocatalytic performance toward decontamination of organic pollutants. Appl. Surf. Sci. 2020, 527, 146775. [Google Scholar] [CrossRef]
- Pei, C.Y.; Chen, Y.G.; Wang, L.; Chen, W.; Huang, G.B. Step-scheme WO3/CdIn2S4 hybrid system with high visible light activity for tetracycline hydrochloride photodegradation. Appl. Surf. Sci. 2021, 535, 147682. [Google Scholar] [CrossRef]
- Wei, J.; Chen, Y.; Zhang, H.; Zhuang, Z.; Yu, Y. Hierarchically porous S-scheme CdS/UiO-66 photocatalyst for efficient 4-nitroaniline reduction. Chin. J. Catal. 2021, 42, 78–86. [Google Scholar] [CrossRef]
- Li, X.; Hu, T.; Zhang, J.; Dai, K.; Liang, C. Novel 2D SnNb2O6/Ag3VO4 S-scheme heterojunction with enhanced visible-light photocatalytic activity. Ceram. Int. 2021, 47, 7169–7176. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, W.; Zhai, Z.; Ren, K.; Wang, T.; Guan, H.; Shi, H. 2D/2D Bi2MoO6/g-C3N4 S-scheme heterojunction photocatalyst with enhanced visible-light activity by Au loading. J. Mater. Sci. Technol. 2020, 56, 216–226. [Google Scholar] [CrossRef]
- Lei, S.; Luo, R.; Li, H.; Chen, J.; Zhong, J.; Li, J. Ionic liquid assisted in-situ construction of S-scheme BiOI/Bi2WO6 heterojunctions with improved sunlight-driven photocatalytic performance. Inorg. Chem. Commun. 2020, 121, 108192. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Zheng, M.; Bi, H. One pot milling route to fabricate step-scheme AgI/I-BiOAc photocatalyst: Energy band structure optimized by the formation of solid solution. Appl. Surf. Sci. 2019, 489, 409–419. [Google Scholar] [CrossRef]
- Li, S.; Han, Q.; Ji, X.; Zahi, A.H.; Bi, H. Room-temperature one-step synthesis of tube-like S-scheme BiOBr/BiO(HCOO)Br-x heterojunction with excellent visible-light photocatalytic performance. Appl. Surf. Sci. 2020, 530, 147208. [Google Scholar] [CrossRef]
- Chen, J.; Liu, T.; Zhang, H.; Wang, B.; Zheng, W.; Wang, X.; Li, J.; Zhong, J. One-pot preparation of double S-scheme Bi2S3/MoO3/C3N4 heterojunctions with enhanced photocatalytic activity originated from the effective charge pairs partition and migration. Appl. Surf. Sci. 2020, 527, 146788. [Google Scholar] [CrossRef]
- Hu, T.; Dai, K.; Zhang, J.; Zhu, G.; Liang, C. One-pot synthesis of step-scheme Bi2S3/porous g-C3N4 heterostructure for enhanced photocatalytic performance. Mater. Lett. 2019, 257, 126740. [Google Scholar] [CrossRef]
- Pan, T.; Chen, D.; Xu, W.; Fang, J.; Wu, S.; Liu, Z.; Wu, K.; Fang, Z. Anionic polyacrylamide-assisted construction of thin 2D-2D WO3/g-C3N4 Step-scheme heterojunction for enhanced tetracycline degradation under visible light irradiation. J. Hazard. Mater. 2020, 393, 122366. [Google Scholar] [CrossRef]
- Jia, X.; Han, Q.; Liu, H.; Li, S.; Bi, H. A dual strategy to construct flowerlike S-scheme BiOBr/BiOAc1−xBrx heterojunction with enhanced visible-light photocatalytic activity. Chem. Eng. J. 2020, 399, 125701. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Xie, Q.; He, W.; Liu, S.; Li, C.; Zhang, J.; Wong, P.K. Bifunctional S-scheme g-C3N4/Bi/BiVO4 hybrid photocatalysts toward artificial carbon cycling. Chin. J. Catal. 2020, 41, 140–153. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yu, C.; Yang, K.; Li, X.; Dai, W.; Guan, J.; Shu, Q.; Huang, W. Construction of S-scheme g-C3N4/ZrO2 heterostructures for enhancing photocatalytic disposals of pollutants and electrocatalytic hydrogen evolution. Dyes Pigments 2020, 180, 108525. [Google Scholar] [CrossRef]
- Majhi, D.; Mishra, A.K.; Das, K.; Bariki, R.; Mishra, B.G. Plasmonic Ag nanoparticle decorated Bi2O3/CuBi2O4 photocatalyst for expeditious degradation of 17α-ethinylestradiol and Cr(VI) reduction: Insight into electron transfer mechanism and enhanced photocatalytic activity. Chem. Eng. J. 2020, 127506, in press. [Google Scholar]
- He, R.; Liu, H.; Liu, H.; Xu, D.; Zhang, L. S-scheme photocatalyst Bi2O3/TiO2 nanofiber with improved photocatalytic performance. J. Mater. Sci. Technol. 2020, 52, 145–151. [Google Scholar]
- Tao, J.; Yu, X.; Liu, Q.; Liu, G.; Tang, H. Internal electric field induced S–scheme heterojunction MoS2/CoAl LDH for enhanced photocatalytic hydrogen evolution. J. Colloid Interfac. Sci. 2021, 585, 470–479. [Google Scholar] [CrossRef]
- He, F.; Meng, A.; Cheng, B.; Ho, W.; Yu, J. Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal. 2020, 41, 9–20. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, D.; Yang, S.; Tian, Z.; Cheng, B.; Fan, J. Novel g-C3N4/g-C3N4 S-scheme isotype heterojunction for improved photocatalytic hydrogen generation. Appl. Surf. Sci. 2019, 495, 143555. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Ma, Q. CoAl LDH@Ni-MOF-74 S-Scheme Heterojunction for Efficient Hydrogen Evolution. Trans. Tianjin Univ. 2020, 27, 127–138. [Google Scholar] [CrossRef]
- Hu, T.; Dai, K.; Zhang, J.; Chen, S. Noble-metal-free Ni2P modified step-scheme SnNb2O6/CdSdiethylenetriamine for photocatalytic hydrogen production under broadband light irradiation. Appl. Catal. B 2020, 269, 118844. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Lv, H.; Ren, H.; Xing, X. Rational design of Au decorated Mn0.5Cd0.5S/WO3 step-scheme heterostructure with multichannel charge transfer and efficient H2 generation. Appl. Surf. Sci. 2020, 526, 146734. [Google Scholar] [CrossRef]
- Shi, J.; Zheng, B.; Mao, L.; Cheng, C.; Hu, Y.; Wang, H.; Li, G.; Jing, D.; Liang, X. MoO3/g-C3N4 Z-scheme (S-scheme) system derived from MoS2/melamine dual precursors for enhanced photocatalytic H2 evolution driven by visible light. Int. J. Hydrogen Energ. 2021, 46, 2927–2935. [Google Scholar] [CrossRef]
- Chen, Y.; Su, F.; Xie, H.; Wang, R.; Ding, C.; Huang, J.; Xu, Y.; Ye, L. One-step construction of S-scheme heterojunctions of N-doped MoS2 and Sdoped g-C3N4 for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 404, 126498. [Google Scholar] [CrossRef]
- Chen, X.; Hu, T.; Zhang, J.; Yang, C.; Dai, K.; Pan, C. Diethylenetriamine synergistic boosting photocatalytic performance with porous g-C3N4/CdS-diethylenetriamine 2D/2D S-scheme heterojunction. J. Alloys Compd. 2020, 863, 158068. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Yan, Y.; Liu, C.; Hu, X.; Liu, E.; Fan, J. A novel S-scheme 1D/2D Bi2S3/g-C3N4 heterojunctions with enhanced H2 evolution activity. Colloid. Surf. A 2021, 608, 125598. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Huo, Y.; Dai, K.; Li, S.; Chen, S. Two-dimensional sulfur- and chlorine-codoped g-C3N4/CdSe-amine heterostructures nanocomposite with effective interfacial charge transfer and mechanism insight. Appl. Catal. B 2021, 280, 119452. [Google Scholar] [CrossRef]
- Mu, J.; Teng, F.; Miao, H.; Wang, Y.; Hu, X. In-situ oxidation fabrication of 0D/2D SnO2/SnS2 novel Step-scheme heterojunctions with enhanced photoelectrochemical activity for water splitting. Appl. Surf. Sci. 2020, 501, 143974. [Google Scholar] [CrossRef]




| Tandem Composition and Band Gap (EG) | Synthesis Method | Morphology and SBET [m2/g] | Radiation Parameters | Photocatalytic Parameters | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light Spectra | Intensity [W] | Pollutant Concentration [mg/L] | Photocatalyst Dosage [mg/mL] | Time [min] | Efficiency [%] | Rate Constant [min−1] | ||||
| BP (black phosphorus) (1.68 eV)/BiOBr (2.73 eV) | Solvothermal | BP (black phosphorus) nanosheets/BiOBr nanosheets SBET = np * | Vis | 300 (Xe) | Tetracycline (TC) = 50 mg/L | 100 mg/100 mL | 90 | 85 | 0.021 | [62] |
| SnFe2O4 (1.88 eV)/ ZnFe2O4 (1.78 eV) | Solvothermal | SnFe2O4 nanoparticles/ ZnFe2O4 nanoparticles SBET = 68.76 | Vis | np | TC = 10 mg/L | 30 mg/100 mL | 120 | 93.2 | np | [63] |
| TiO2(3.0 eV)/W18O49 (2.78 eV) | Solvothermal | TiO2 nanosheets and W18O49 spindle-like SBET = np | Vis | np | Rhodamine B (RhB) = 10 mg/L | np | 75 | 82.1 | 0.0261 | [64] |
| TC = 30 mg/L | 80.3 | np | ||||||||
| NiO (3.23 eV)/BiOI (1.74 eV) | Solvothermal | NiO foam-like/BiOI flower-like microspheres SBET = 20.7 | Vis | 300 (Xe) | RhB = 4.8 mg/L | 0.04 mg/30 mL | 60 | 90 | 0.057 | [65] |
| BiVO4 (1.96 eV) @MoS2 (1.63 eV) | Solvothermal | BiVO4 nanorods @MoS2 sheets SBET = np | Vis | 500 (Xe) | RhB = 20 mg/L | np | 20 | 90 | 0.111 | [66] |
| Bi2O3 (2.40 eV)/Bi2SiO5 (3.64 eV) | Solvothermal | Bi2O3 microspheres/Bi2SiO5 flower-like microstructure SBET = 66.8 | Vis | 500 (Xe) | Methyl orange (MO) = 10 mg/L | 50 mg/50 mL | 420 | 67 | 0.0026 | [67] |
| Phenol = 10 mg/L | 360 | 30 | 0.0001 | |||||||
| WO3 (2.76 eV)/CdIn2S4 (1.94 eV) | Hydrothermal | WO3 nanorods/CdIn2S4 nanosheets SBET = 32 | Vis | 300 (Xe) | TC = 50 mg/L | 30 mg/30 mL | 50 | 95 | np | [68] |
| CdS (2.42 eV)/UiO-66 (2.75 eV) | Hydrothermal | CdS nanoparticles/UiO-66 nanoparticles SBET = np | Vis | 500 (Xe) | 4-nitroaniline = 20 mg/L | 40 mg/40 mL | 20 | 80 | 0.085 | [69] |
| SnNb2O6 (2.10 eV)/Ag3VO4 (2.16 eV) | Hydrothermal | SnNb2O6 flaky structure/Ag3VO4 nanoparticles SBET = 58.24 | Vis | 50 (LED) | Methylene blue (MB) = 20 mg/L | 30 mg/30 mL | 10 | 99 | 0.2256 | [70] |
| Bi2MoO6 (2.64 eV)/g-C3N4 (2.76 eV) | Photoreduction and hydrothermal | Bi2MoO6 sheet like/g-C3N4 nanosheets SBET = np | Vis | 300 (Xe) | RhB = 5 mg/L | 5 mg/100 mL | 40 | 97.6 | 0.0808 | [71] |
| BiOI (1.68 eV)/Bi2WO6 (2.60 eV) | Hydrothermal | BiOI flakes/Bi2WO6 nanosheets SBET = 23.31 | Vis | 500 (Xe) | RhB = 10 mg/L | 50 mg/50 mL | 150 | 90.1 | 0.0295 | [72] |
| MO = 10 mg/L | 72.1 | 0.00217 | ||||||||
| AgI (2.75 eV)/I-BiOAc (2.35 eV) | One-pot milling | AgI nanoparticles/I-BiOAc nanosheets SBET = 9.0 | Vis | 500 (Xe) | Methyl violet (MV) = 10 mg/L | 20 mg/50 mL | 300 | 94.4 | 0.047 | [73] |
| Bisphenol A (BPA) = 10 mg/L | 20 mg/50 mL | 300 | 71.1 | 0.035 | ||||||
| MO = 20 mg/L | 20 mg/50 mL | 120 | 83 | np | ||||||
| Malachite green (MG) = 20 mg/L | 20 mg/50 mL | 60 | 95 | np | ||||||
| BiOBr (2.62 eV)/BiO (HCOO)Br-x (2.96 eV) | Precipitation | BiOBr nanosheets/BiO (HCOO)Br-x tube SBET = 9.65 | Vis | 500 (Xe) | MG = 20 mg/L | 20 mg/50 mL | 60 | 100 | 0.064 | [74] |
| RhB = 20 mg/L | 120 | 98 | 0.024 | |||||||
| TC = 20 mg/L | 80 | np | ||||||||
| MO = 20 mg/L | 30 | |||||||||
| Bi2S3 (1.3 eV)/MoO3 (3.01 eV)/g-C3N4 (2.63 eV) | One-pot solid-state reaction | Bi2S3/MoO3/C3N4 lump-like structure SBET = 43.2 | Vis | 500 (Xe) | MO = 10 mg/L | 50 mg/50 mL | 120 | 78 | 0.0091 | [75] |
| Bi2S3 (2.50 eV)/ porous g-C3N4 (2.7 eV) | One-pot | Bi2S3 nanorods/ porous g-C3N4 nanosheets SBET = np | Vis | 50 (LED) | MB = 20 mg/L | 30 mg/30 mL | 90 | 90 | 0.0199 | [76] |
| WO3 (2.34 eV)/g-C3N4 (2.65 eV) | Template assisted polymer | WO3/g-C3N4 Nanosheets SBET = 43.03 | Vis | 300 (Xe) | TC = 20 mg/L | 50 mg/50 mL | 60 | 90.54 | 0.0378 | [77] |
| BiOBr (2.83 eV)/BiOAc1−xBrx (3.28 eV) | Co-precipitation | BiOBr nanosheets/BiOAc1−xBrx flower like SBET = 33.9 | Vis | 500 (Xe) | TC = 20 mg/L | 20 mg/50 mL | 120 | 99.2 | 0.023 | [78] |
| RhB = 20 mg/L | 99.4 | 0.033 | ||||||||
| LaNiO3 (2.42 eV)/TiO2 (3.2 eV) | Sol-gel | LaNiO3 nanoparticles/TiO2 nanoparticles SBET = np | UV-Vis | 300 (Hg) 300 (Xe) | MO = 10–20 mg/L | 100 mg/50 mL | 150 | 100 (10 mg/L) 92 (20 mg/L) | np | [79] |
| Ciprofloxacin (CIP) = 10 mg/L | 50 mg/50 mL | 210 | 54 | np | ||||||
| g-C3N4 (2.83 eV)/Bi/BiVO4 (2.4 eV) | In-situ reduction | g-C3N4 nanosheets/Bi nanoparticles/ BiVO4 nanoparticles SBET = 50 | Vis | 350 (Xe) | RhB = 10 mg/L | 50 mg/50 mL | 70 | 100 | 0.067 | [80] |
| g-C3N4 (2.7 eV)/ZrO2 (2.6 eV) | Calcination | g-C3N4 nanosheets/ZrO2 nanoparticles SBET = 116.4 | Vis | 300 (Xe) | RhB = 10 mg/L | 30 mg/50 mL | 150 | 82 | np | [81] |
| MO = 10 mg/L | 50 | |||||||||
| Acid orange II (AO II) = 10 mg/L | 98 | |||||||||
| Bi2O3 (2.8 eV)/CuBi2O4 (1.87 eV)/Ag | Photodeposition | Bi2O3 nanoplate/CuBi2O4 nanoparticles/Ag nanoparticles SBET = np | Vis | 250 (Xe) | 17-α Ethinylestradiol = 10 mg/L | 40 mg/100 mL | 120 | 94.6 | 0.0185 | [82] |
| Bi2O3 (2.77 eV)/TiO2 (3.0 eV) | In-situ photoreductive deposition | Bi2O3 rod-like/TiO2 nanofiber SBET = 51 | Vis | 300 (Xe) | Phenol = 100 mg/L | 50 mg/15 mL | 120 | 50 | np | [83] |
| Tandem Composition and Band Gap (EG) | Synthesis Method | Morphology and SBET [m2/g] | Radiation Parameters | Hydrogen Production | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Light Spectra | Intensity [W] | Sacrificial Agent | Photocatalyst Dosage [mg/L] | Time [min] | Evolution Rate [µmol/h] | ||||
| MoS2 (1.8 eV)/CoAl (2.1 eV) | Hydrothermal | MoS2 spherical/CoAl carnations SBET = np | Vis | 300 (Xe) | np * | 50 mg/80 mL | 300 | 17.1 | [84] |
| WO3 (3.2 eV)/TiO2 (2.6 eV)/rGO | Hydrothermal | WO3 nanoparticles/TiO2 nanoparticles/rGO nanosheets SBET = 165 | Vis | 350 (Xe) | np | 50 mg/80 mL | 180 | 12.29 | [85] |
| Melamine g-C3N4 (2.69 eV)/Urea g-C3N4 (2.81 eV) | Hydrothermal | Melamine g-C3N4 comb-like/Urea g-C3N4 laminar SBET = 46 | Vis | 300 (Xe) | Triethanolamine (TEOA) | 50 mg/100 mL | 180 | 29.9 | [86] |
| CoAl layered double hydroxides (LDH) (2.40 eV) @Ni- Metal–organic frameworks (MOF)-74 (2.37 eV) | Hydrothermal | CoAl LDH nanosheets @Ni-MOF-74 quadrilateral Structure SBET = np | Vis | 5 W (LED) | TEOA | 10 mg/30 mL | 350 | 213 | [87] |
| SnNb2O6 (2.25 eV)/CdS diethylenetriamine (2.51 eV) | Hydrothermal | SnNb2O6 nanosheets/CdS diethylenetriamine nanosheets SBET = 93.27 | Vis | 300 (Xe) | Na2S + Na2SO3 | 30 mg/50 mL | 240 | 234.24 | [88] |
| Mn0.5Cd0.5S (2.48 eV)/WO3 (2.7 eV) | Chemical deposition | Mn0.5Cd0.5S nanoparticles/WO3 nanorods SBET = np | Vis | 300 W (Xe) | Na2S + Na2SO3 | 50 mg/100 mL | 180 | 517.13 | [89] |
| MoO3/g-C3N4 (2.7 eV) | One-pot | MoO3 nanoparticles/g-C3N4 nanosheets SBET = np | Vis | 300 (Xe) | TEOA | 50 mg/200 mL | 480 | 25.62 | [90] |
| S-doped g-C3N4 (2.80 eV)/N-doped MoS2 (1.80 eV) | Thermal polycondensation | S-doped g-C3N4 nanosheets/N-doped MoS2 nanobelts SBET = 4.9 | Vis | 300 (Xe) | TEOA | 50 mg/100 mL | 240 | 32.92 | [91] |
| g-C3N4 (2.61 eV)/CdS-diethylenetriamine (2.68 eV) | Solvothermal | g-C3N4 nanosheets/CdS-diethylenetriamine nanosheets SBET = 36.6 | Vis | 300 (Xe) | Na2S+Na2SO3 | 50 mg/100 mL | 180 | 486.9 | [92] |
| Bi2S3 (1.60 eV)/g-C3N4 (2.78 eV) | Solvothermal | Bi2S3 nanorods/g-C3N4 nanosheets SBET = 58.1 | Vis | 300 (Xe) | Na2S + Na2SO3 | 30 mg/100 mL | 180 | 101.8 | [93] |
| g-C3N4 (2.85 eV)/CdSe-amine (1.86 eV) | Microwave solvothermal | g-C3N4 nanosheets/CdSe-amine flower like SBET = 73.1 | Vis | 300 (Xe) | Na2S + Na2SO3 | 20 mg/50 mL | 240 | 18.8 | [94] |
| SnO2 (3.7 eV)/SnS2 (2.2 eV) | Solvothermal | SnO2 nanoparticles/SnS2 nanosheets SBET = np | Vis | 300 (Xe) | Pure water | np | 180 | 5.5 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enesca, A.; Andronic, L. Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review. Nanomaterials 2021, 11, 871. https://doi.org/10.3390/nano11040871
Enesca A, Andronic L. Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review. Nanomaterials. 2021; 11(4):871. https://doi.org/10.3390/nano11040871
Chicago/Turabian StyleEnesca, Alexandru, and Luminita Andronic. 2021. "Photocatalytic Activity of S-Scheme Heterostructure for Hydrogen Production and Organic Pollutant Removal: A Mini-Review" Nanomaterials 11, no. 4: 871. https://doi.org/10.3390/nano11040871







