Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with High Saturation Magnetization via the Surfactant Assisted Co-Precipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
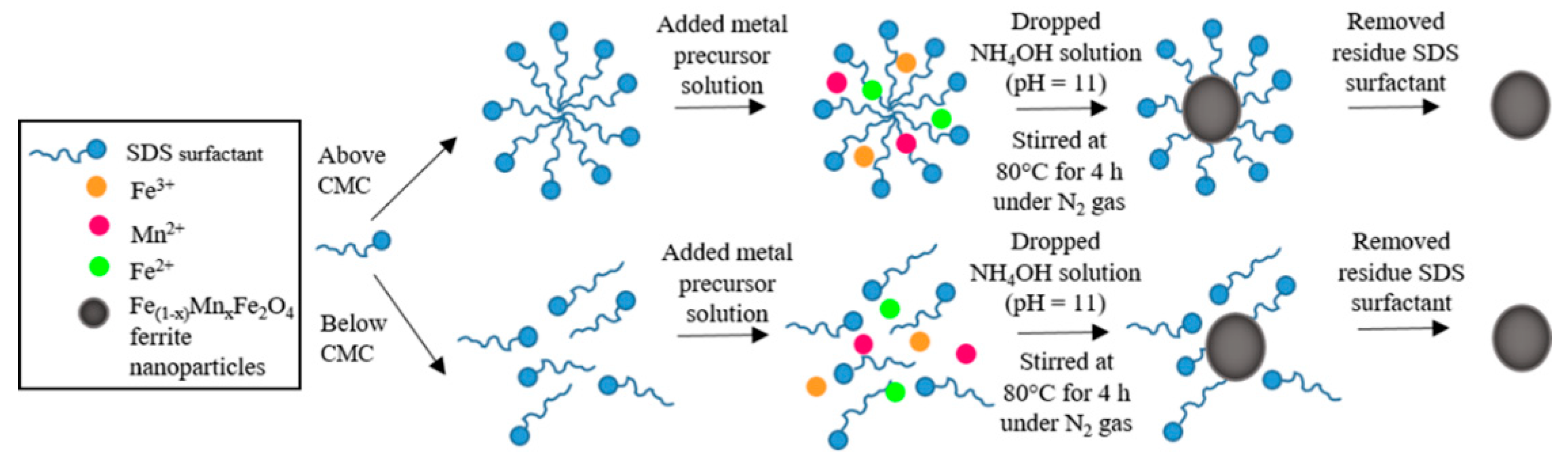
2.2. Synthesis of Ferrite Nanoparticles
2.2.1. Effect of Surfactant Types and Concentrations
2.2.2. Effect of Mn2+:Fe2+ Molar Ratios
2.3. Characterization of Ferrite Nanoparticles
3. Results
3.1. Characterization of Ferrite Nanoparticles
3.1.1. FT-IR Analysis
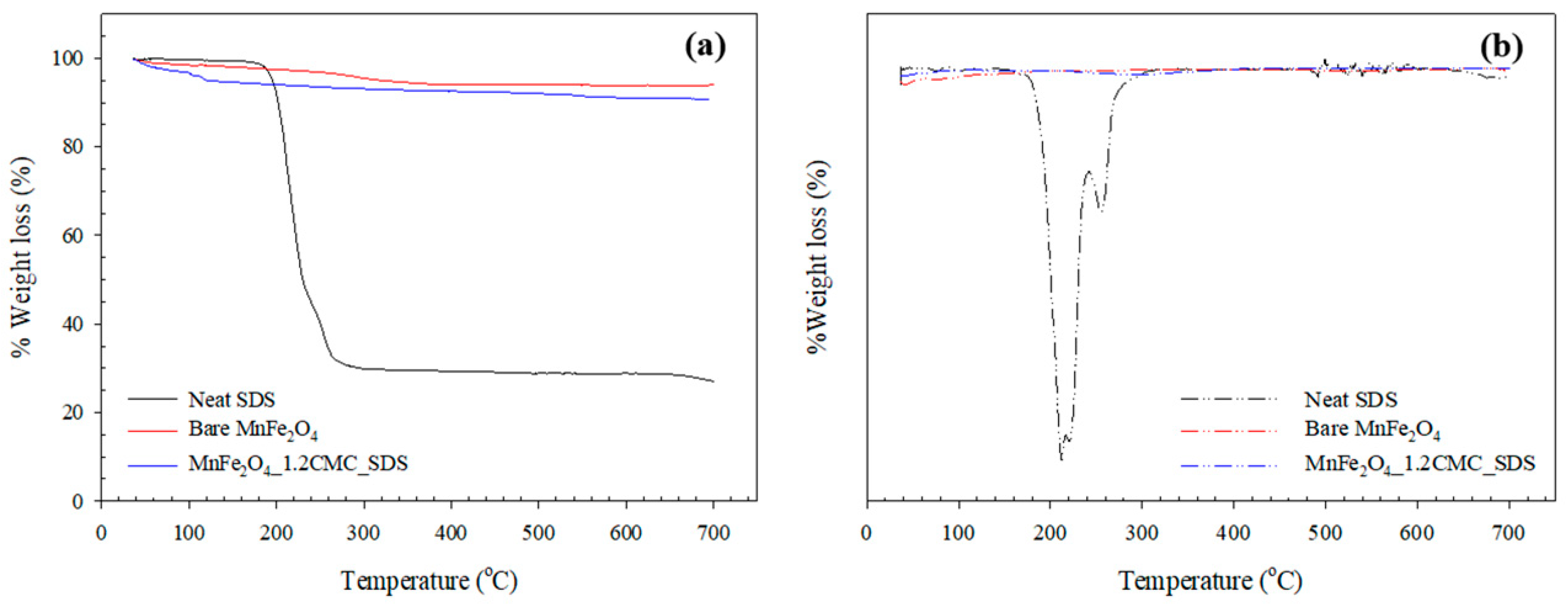
3.1.2. TGA Analysis
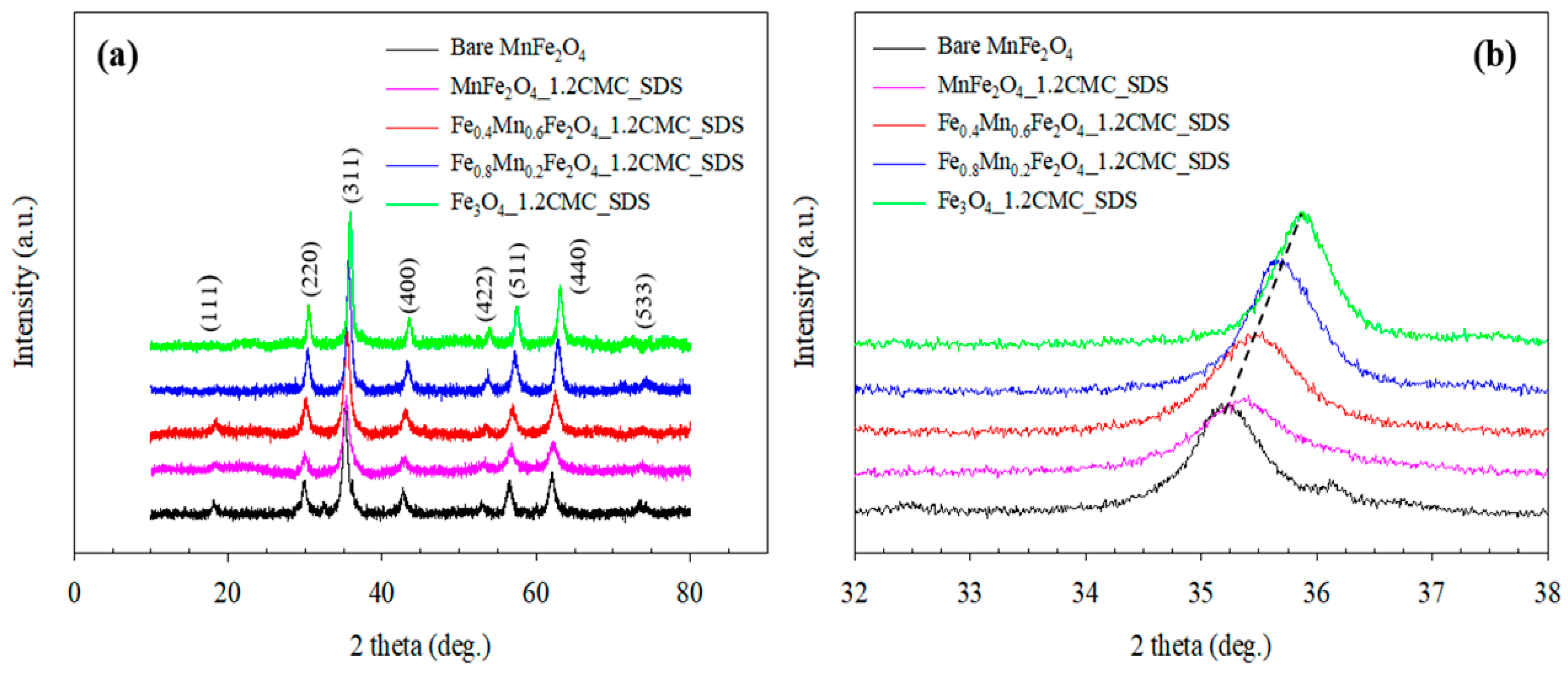
3.1.3. X-Ray Diffraction (XRD) Analysis
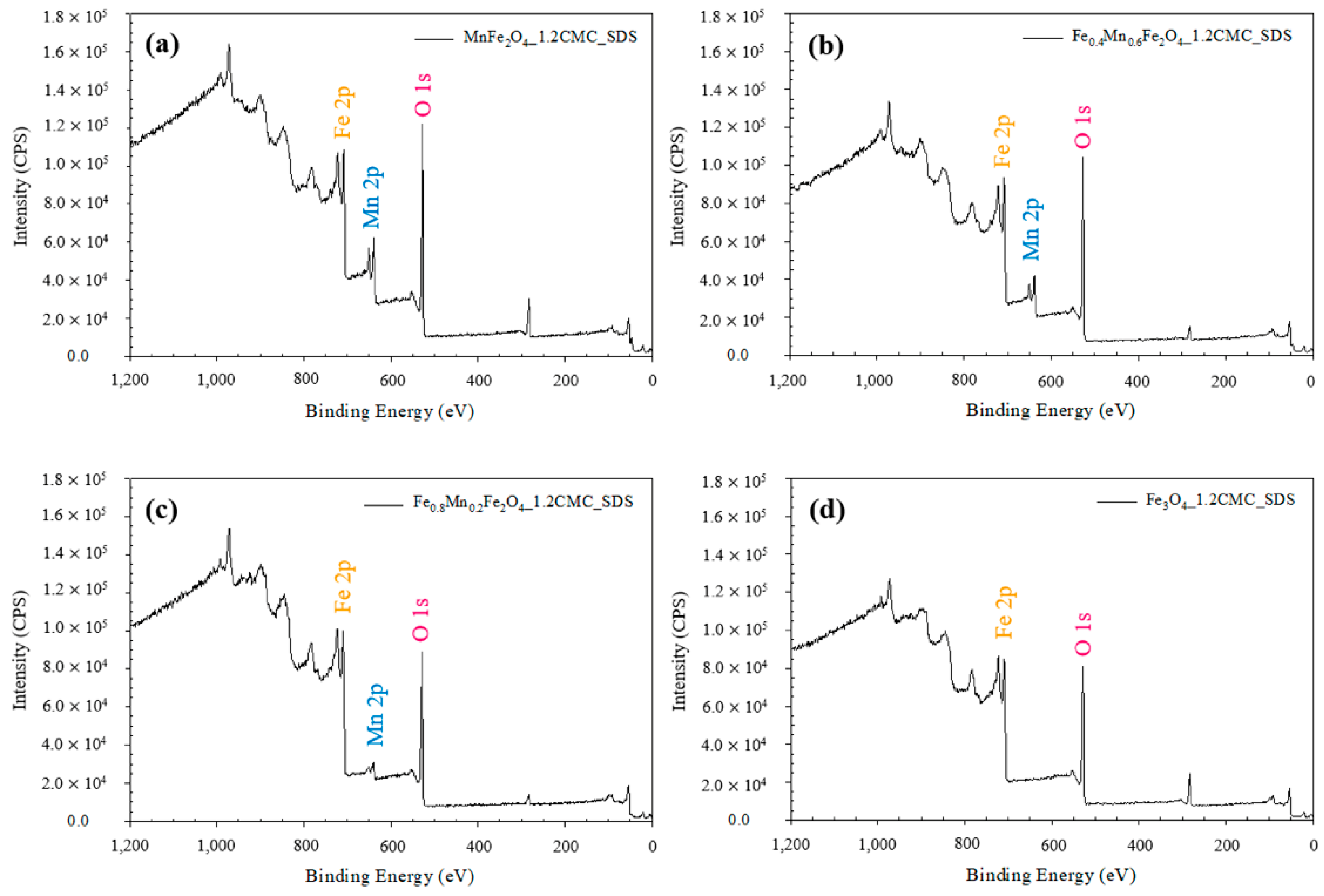
3.1.4. EDX and XPS Measurements
3.1.5. SEM and TEM Analysis
3.2. Electrical Conductivity Analysis
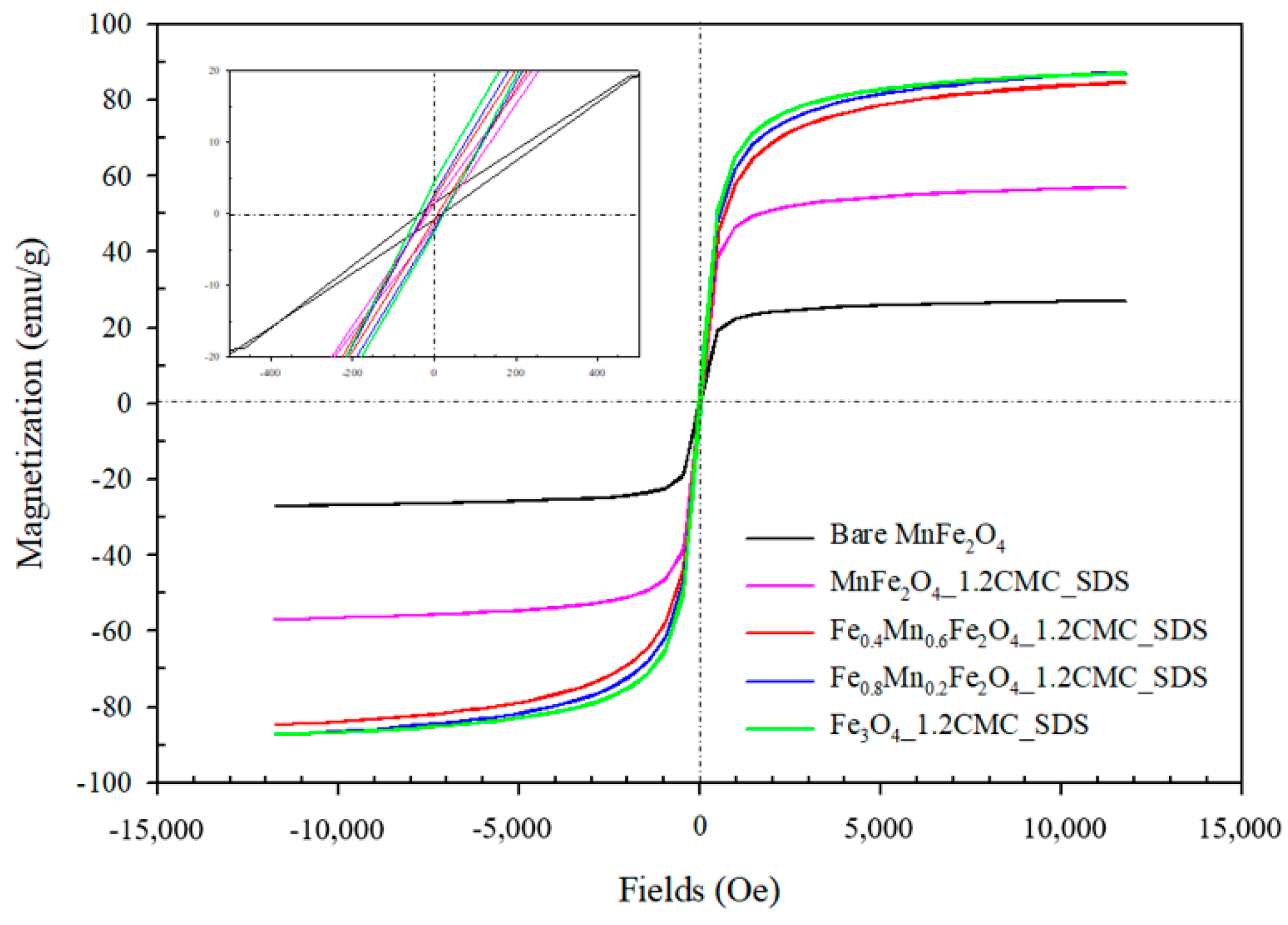
3.3. Vibrating Sample Magnetometer (VSM) Analysis
3.3.1. Effect of Surfactant Types
3.3.2. Effect of SDS Surfactant Concentrations
3.3.3. Effect of Mn2+:Fe2+ Molar Ratio Substitutions
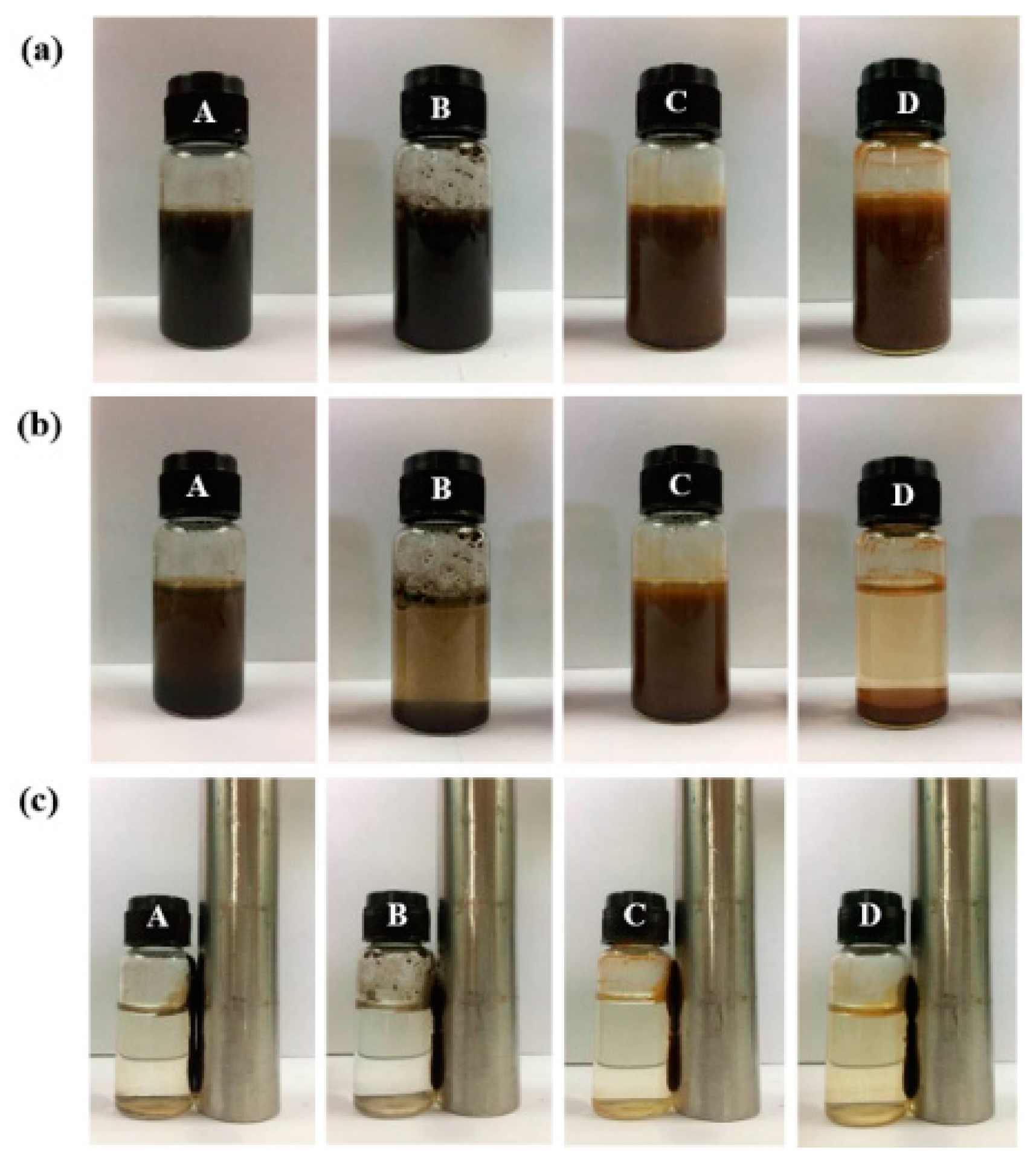
3.4. Dispersion Property Fe(1−x)MnxFe2O4 Ferrite Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, P.; Cui, B.; Bu, Y.; Yang, Z.; Wang, Y. Synthesis and characterization of mesoporous and hollow-mesoporous MxFe3−xO4 (M=Mg, Mn, Fe, Co, Ni, Cu, Zn) microspheres for microwave-triggered controllable drug delivery. J. Nanopart. Res. 2017, 19, 398. [Google Scholar] [CrossRef]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, R.R.; Pillai, P.B.; Gupta, S.N.; Iyengar, M.A.R.; Sood, A.K. Graphene Oxide−MnFe2O4 Magnetic Nanohybrids for Efficient Removal of Lead and Arsenic from Water. ACS Appl. Mater. Interfaces 2014, 6, 17426–17436. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M. Zinc ferrite nanoparticle as a magnetic catalyst: Synthesis and dye degradation. Mater. Res. Bill. 2013, 48, 4255–4260. [Google Scholar] [CrossRef]
- Mounkachi, O.; Lamouri, R.; Abraime, B.; Ez-Zahraouy, H.; El kenz, A.; Hamedoun, M.; Benyoussef, A. Exploring the magnetic and structural properties of Nd-doped Cobalt nanoferrite for permanent magnet applications. Ceram. Int. 2017, 43, 14401–14404. [Google Scholar] [CrossRef]
- Brito-Pereira, R.; Correia, D.M.; Ribeiro, C.; Francesko, A.; Etxebarria, I.; Perez-Alvarez, L.; Vilas, J.L.; Martins, P.; Lanceros-Mendez, S. Silk fibroin-magnetic hybrid composite electrospun fibers for tissue engineering applications. Compos. Part B 2018, 141, 70–75. [Google Scholar] [CrossRef]
- Cruz, M.M.; Ferreira, L.P.; Ramos, J.; Mendo, S.G.; Alves, A.F.; Godinho, M.; Carvalho, M.D. Enhance magnetic hyperthermia of CoFe2O4 and MnFe2O4 nanoparticles. J. Alloys Compd. 2017, 703, 370–380. [Google Scholar] [CrossRef]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron therapy, magnetic nanoparticles and hyperthermia: A promising combined tool for pancreatic cancer treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Almalki, F. Design and Synthesis of multi-functional superparamagnetic core-gold shell nanoparticles coated with chitosan and folate for targeted antitumor therapy. Nanomaterials 2021, 11, 32. [Google Scholar] [CrossRef]
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of breast cancer-bearing BALB/c mice with magnetic hyperthermia using dendrimer functionalized iron-oxide nanoparticles. Nanomaterials 2020, 10, 2310. [Google Scholar] [CrossRef]
- Qin, J.; Peng, H.; Ping, J.; Geng, Z. Facile synthesis of dual-functional nanoparticles co-loaded with ZnPc/Fe3O4 for PDT and magnetic resonance imaging. Mater. Res. Bill. 2019, 114, 90–94. [Google Scholar] [CrossRef]
- Vignesh, R.H.; Sankar, K.V.; Amaresh, S.; Lee, Y.S.; Selvan, R.K. Synthesis and characterization of MnFe2O4 nanoparticles for impedometric ammonia gas sensor. Sens. Actuators B 2015, 220, 50–58. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Magneto-electro-responsive material based on magnetite nanoparticles/polyurethane composites. Mater. Sci. Eng. C 2016, 61, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, M.; Moradmard, H.; Shayesteh, S.F. Cation distribution and magnetic properties of Cu-doped nanosized MnFe2O4 synthesized by the coprecipitation method. IEEE Trans. Magn. 2018, 54, 2500105. [Google Scholar] [CrossRef]
- Upadhyay, R.V.; Davies, K.J.; Wells, S.; Charles, S.W. Preparation and characterization of ultra-fine MnFe2O4 and MnxFe1-xFe2O4 spinel systems: I. particles. J. Magn. Magn. Mater. 1994, 132, 249–257. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterization and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Shanmugavel, T.; Raj, S.G.; Kumar, G.R.; Rajarajan, G. Synthesis and structural analysis of nanocrystalline MnFe2O4. Phys. Procedia 2014, 54, 159–163. [Google Scholar] [CrossRef]
- Zipare, K.; Dhumal, J.; Bandgar, S.; Mathe, V.; Shahane, G. Superparamagnetic manganese ferrite nanoparticles: Synthesis and magnetic properties. J. Nanosci. Nanoeng. 2015, 1, 178–182. [Google Scholar]
- Augustin, M.; Balu, T. Synthesis and characterization of metal (Mn, Zn) ferrite magnetic nanoparticles. Mater. Today 2015, 2, 923–927. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Hashim, M.; Shaari, A.H. Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2011, 323, 1745–1749. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Haq, I.U.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, H.; Li, G.; Liu, Y.; Leng, J. Cation distribution dependence of magnetic properties of sol-gel prepared MnFe2O4 spinel ferrite nanoparticles. J. Magn. Magn. Mater. 2010, 322, 3396–3400. [Google Scholar] [CrossRef]
- Gao, R.R.; Zhang, Y.; Yu, W.; Xiong, R.; Shi, J. Superparamagnetism and spin-glass like state for the MnFe2O4 nano-paricles synthesized by the thermal decomposition method. J. Magn. Magn. Mater. 2012, 324, 2534–2538. [Google Scholar] [CrossRef]
- Yanez-Vilar, S.; Sanchez-Andujar, M.; Gomez-Aguirre, C.; Mira, J.; Senaris-Rodriguez, M.A.; Castro-Garcia, S. A simple solvothermal synthesis of MFe2O4 (M = Mn, Co, and Ni) nanoparticles. J. Solid. State. Chem. 2009, 182, 2685–2690. [Google Scholar] [CrossRef]
- Yousuf, M.A.; Jabeen, S.; Shahi, M.N.; Khan, M.A.; Shakir, I.; Warsi, M.F. Magnetic and electrical properties of yttrium substituted manganese ferrite nanoparticles prepared via micro-emulsion route. Results Phys. 2020, 16, 102973. [Google Scholar] [CrossRef]
- Amighian, J.; Mozaffari, M.; Nasr, B. Preparation of nano-sized manganese ferrite (MnFe2O4) via coprecipitation method. Phys. Status. Solidi. C 2006, 3, 3188–3192. [Google Scholar] [CrossRef]
- Beeran, A.E.; Nazeer, S.S.; Fernandez, F.B.; Muvvala, K.S.; Wunderlich, W.; Anil, S.; Vellappally, S.; Rao, M.S.R.; John, A.; Jayasree, R.S.; et al. An aqueous method for the controlled manganese (Mn2+) substitution in superparamagnetic iron oxide nanoparticles for contrast enhancement in MRI. Phys. Chem. Chem. Phys. 2015, 17, 4609–4619. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, D.; Ma, Y.; Zhang, Z.; Che, H.; Mu, J.; Zhang, X.; Zhang, Z. Synthesis and characterization of polymer-coated manganese ferrite nanoparticles as controlled drug delivery. Appl. Surf. Sci. 2018, 428, 258–263. [Google Scholar] [CrossRef]
- Li, G.Y.; Jiang, Y.R.; Huang, K.L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Vadivel, M.; Babu, R.R.; Ramamurthi, K.; Arivanandhan, M. CTAB cationic surfactant assisted synthesis of CoFe2O4 magnetic nanoparticles. Ceram. Int. 2016, 42, 19320–19328. [Google Scholar] [CrossRef]
- Modaresi, N.; Afzalzadeh, R.; Aslibeiki, B.; Kameli, P. Competition between the impact of cation distribution and crystallite size on properties of MnxFe3-xO4 nanoparticles synthesized at room temperature. Ceram. Int. 2017, 43, 15382–15391. [Google Scholar] [CrossRef]
- Bateer, B.; Tian, C.; Qu, Y.; Du, S.; Yang, Y.; Ren, Z.; Pan, K.; Fu, H. Synthesis, size and magnetic properties of controllable MnFe2O4 nanoparticles with versatile surface functionalities. Dalton. Trans. 2014, 43, 9885–9891. [Google Scholar] [CrossRef]
- Goswami, P.P.; Choudhury, H.A.; Chakma, S.; Moholkar, V.S. Sonochemical synthesis and characterization of manganese ferrite nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 17848–17855. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Saeednia, S.; Mehran, M.; Dafeh, S.R. Modified structural and magnetic properties of nanocrystalline MnFe2O4 by pH in capping agent free co-precipitation method. J. Magn. Magn. Mater. 2017, 425, 31–36. [Google Scholar] [CrossRef]
- Ma, J.; Hou, Y.; Ji, T.; Zhao, J.; Zhang, S. Preparation of MnFe2O4 nanoparticles via a facile water-glycol solvothermal approach. Synth. React. Inorg. Metal. Org. Nano-Metal. Chem. 2016, 46, 1513–1518. [Google Scholar] [CrossRef]
- Lida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid. Interface Sci. 2007, 314, 274–280. [Google Scholar]
- Akbarzadeh, R.; Dehghani, H. Sodium-dodecyl-sulphate-assisted synthesis of Ni nanoparticles: Electrochemical properties. Bull. Mater. Sci. 2017, 40, 1361–1369. [Google Scholar] [CrossRef][Green Version]
- Lu, H.F.; Hong, R.Y.; Li, H.Z. Influence of surfactants on co-precipitation synthesis of strontium ferrite. J. Alloys Compd. 2011, 509, 10127–10131. [Google Scholar] [CrossRef]
- Doaga, A.; Cojocariu, A.M.; Amin, W.; Heib, F.; Bender, P.; Hempelmann, R.; Caltun, O.F. Synthesis and characterizations of manganese ferrites for hyperthermia applications. Mater. Chem. Phys. 2013, 143, 305–310. [Google Scholar] [CrossRef]
- Cheng, H.K.F.; Pan, Y.; Sahoo, N.G.; Chong, K.; Li, L.; Chan, S.H.; Zhao, J. Improvement in properties of multiwalled carbon nanotube/polypropylene nanocomposites through homogeneous dispersion with the aid of surfactants. J. Appl. Polym. Sci. 2011, 124, 1117–1127. [Google Scholar] [CrossRef]
- Guner, S.; Baykal, A.; Amir, M.D.; Gungunes, H.; Geleri, M.; Sozeri, H.; Shirsath, S.E.; Sertkol, M. Synthesis and characterization of oleylamine capped MnxFe1−xFe2O4 nanocomposite: Magneto-optical properties, cation distribution and hyperfine interaction. J. Alloys Compd. 2016, 688, 675–686. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wang, Z.L.; Chakoumakos, B.C.; Yin, J.S. Temperature dependence of cation distribution and oxidation state in magnetic Mn-Fe ferrite nanocrystal. J. Am. Chem. Soc. 1998, 120, 1800–1804. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B. 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Zhang, Y.; Nan, Z. Modified magnetic properties of MnFe2O4 by CTAB with coprecipitation method. Mater. Lett. 2015, 149, 22–24. [Google Scholar] [CrossRef]
- Iftikhar, A.; Islam, M.U.; Awan, M.S.; Ahmad, M.; Naseem, S.; Iqbal, M.A. Synthesis of super paramagnetic particles of Mn1-xMgxFe2O4 ferrites for hyperthermia applications. J. Alloys Compd. 2014, 601, 116–119. [Google Scholar] [CrossRef]
- Suresh, R.; Giribabu, K.; Manigandan, R. Electrochemical sensing property of Mn doped Fe3O4 nanoparticles. AIP Conf. Proc. 2013, 1512, 402–403. [Google Scholar]
- Bhattacharya, D.; Baksi, A.; Banerjee, I.; Ananthakrishnan, R.; Maiti, T.K.; Pramanik, P. Development of phosphonate modified Fe(1−x)MnxFe2O4 mixed ferrite nanoparticles: Novel peroxidase mimetics in enzyme linked immunosorbent assay. Talanta 2011, 86, 337–348. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A32 1976, 751–767. [Google Scholar] [CrossRef]
- Vadivel, M.; Babu, R.R.; Arivanandhan, M.; Ramamurthi, K.; Hayakawa, Y. Role of SDS surfactant concentrations on the structural, morphological, dielectric and magnetic properties of CoFe2O4 nanoparticles. RSC Adv. 2015, 5, 27060–27068. [Google Scholar] [CrossRef]
- Satalkar, M.; Kane, S.N. On the study of structural properties and cation distribution of Zn0.75−xNixMg0.15Cu0.1Fe2O4 nano ferrite: Effect of Ni addition. J. Phys. Conf. Ser. 2016, 755, 012050. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Yu, F.; Zhang, Y.; Ye, B.C.; Li, Y. Facile synthesis of hollow MnFe2O4 nanoboxes based on galvanic replacement reaction for fast and sensitive VOCs sensor. Sens. Actuators B 2018, 258, 589–596. [Google Scholar] [CrossRef]
- Maaz, K.; Karim, S.; Mashiatullah, A.; Liu, J.; Hou, M.D.; Sun, Y.M.; Duan, J.L.; Yao, H.J.; Mo, D.; Chen, Y.F. Structural analysis of nickel doped cobalt ferrite nanoparticles prepared by coprecipitation route. Physica B 2009, 404, 3947–3951. [Google Scholar] [CrossRef]
- Behera, C.; Choudhary, R.N.P.; Das, P.R. Size dependent electrical and magnetic properties of mechanically-activated MnFe2O4 nanoferrite. Ceram. Int. 2015, 41, 13042–13054. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Younas, M. Structural and transport properties of nanocrystalline MnFe2O4 synthesized by co-precipitation method. Solid Stat. Sci. 2012, 14, 1536–1542. [Google Scholar] [CrossRef]
- Lungu, A.; Malaescu, I.; Marin, C.N.; Vlazan, P.; Sfirloaga, P. The electrical properties of manganese ferrite powders prepared by two different methods. Physica B 2015, 462, 80–85. [Google Scholar] [CrossRef]
- Devi, E.C.; Soibam, I. Effect of Zn doping on the structural, electrical and magnetic properties of MnFe2O4 nanoparticles. Indian J. Phys. 2017, 91, 861–867. [Google Scholar] [CrossRef]
- Mohanty, S.; Panwar, V.; Khanduri, P. Development of PVA/Fe3O4 nanocomposite membrane. Mater. Today 2021, in press. [Google Scholar]
- Naseri, M.G.; Saion, E.B. Crystalization in spinel ferrite nanoparticles, Advances in Crystallization Processes. Intech 2012, 349–380. [Google Scholar] [CrossRef]
- Mercier, J.P.; Zambelli, G.; Kurz, W. Introduction to Materials Science; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Zhao, B.; Hua, B.; Wang, H.; Nan, Z. Modified magnetic properties of ZnLa0.02Fe1.98O4 clusters by anionic surfactant with solvothermal method. Mater. Lett. 2013, 92, 75–77. [Google Scholar] [CrossRef]
- Aslibeiki, B.; Kameli, P.; Ehsani, M.H. MnFe2O4 bulk, nanoparticles and film: A comparative study of structural and magnetic properties. Ceram. Int. 2016, 42, 12789–12795. [Google Scholar] [CrossRef]
- Ishikawa, M.; Tanaka, H.; Kawai, T. Preparation of highly conductive Mn-doped Fe3O4 thin films with spin polarization at room temperature using a pulsed-laser deposition technique. Appl. Phys. Lett. 2005, 86, 222504. [Google Scholar] [CrossRef]
- Amighian, J.; Karimzadeh, E.; Mozaffari, M. The effect of Mn2+ substitution on magnetic properties of MnxFe3-xO4 nanoparticles prepared by coprecipitation method. J. Magn. Magn. Mater. 2013, 332, 157–162. [Google Scholar] [CrossRef]
- Li, Y.H.; Kouh, T.; Shim, I.B.; Kim, C.S. Investigation of cation distribution in single crystalline Fe3-xMnxO4 microspheres based on Mossbauer spectroscopy. J. Appl. Phys. 2012, 111, 07B544. [Google Scholar] [CrossRef]
- Kumar, S.; Ravikumar, C.; Bandyopadhyaya, R. State of dispersion of magnetic nanoparticles in an aqueous medium: Experiments and Monte Carlo simulation. Langmuir 2010, 26, 18320–18330. [Google Scholar] [CrossRef] [PubMed]
- Asuha, S.; Wan, H.L.; Zhao, S.; Deligeer, W.; Wu, H.Y.; Song, L.; Tegus, O. Water-soluble, mesoporous Fe3O4: Synthesis, characterization, and properties. Ceram. Int. 2012, 38, 6579–6584. [Google Scholar] [CrossRef]
- Mei, Z.; Dhanale, A.; Gangaharan, A.; Sardar, D.K.; Tang, L. Water dispersion of magnetic nanoparticles with selective biofunctionality for enhanced plasmonic biosensing. Talanta 2016, 151, 23–29. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V.; Vazquez, A.; Pena, Y.; Gomez, I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-aqueous solvents: Recent trends. RSC Adv. 2014, 4, 45354. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Niziol, M.; Szymczyk, P.; Wisniewski, P.; Guiseppi-Elie, A. 3D printed stimuli-responsive magnetic nanoparticle embedded alginate-methylcellulose hydrogel actuators. Addit. Manuf. 2020, 34, 101275. [Google Scholar] [CrossRef]
- Reizabal, A.; Costa, C.M.; Pereira, N.; Perez-Alvarez, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Silk fibroin based magnetic nanocomposites for actuator applications. Adv. Eng. Mater. 2020, 22, 2000111. [Google Scholar] [CrossRef]
- Varga, Z.; Filipcsei, G.; Zrinyi, M. Magnetic field sensitive functional elastomers with tuneable elastic modulus. Polymer 2006, 47, 227–233. [Google Scholar] [CrossRef]
- Mitsumata, T.; Ohori, S. Magnetic polyurethane elastomers with wide range modulation of elasticity. Polym. Chem. 2011, 2, 1063. [Google Scholar] [CrossRef]
- Varga, Z.; Filipcsei, G.; Szilagyi, A.; Zrinyi, M. Electric and magnetic field-structured smart composites. Macromol. Symp. 2005, 227, 123–133. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H.; Park, H.; Lee, K.Y. Controlling the porous structure of alginate ferrogel for anticancer drug delivery under magnetic stimulation. Carbohydr. Polym. 2019, 223, 115045. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Roco, C.; Deleris, A.; Spoerri, P.; Cezar, C.; Weaver, J.; Vandenburgh, H.; Mooney, D. Improved magnetic regulation of delivery profiles from ferrogels. Biomaterials 2018, 161, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hu, S.H.; Liu, K.H.; Liu, D.M.; Chen, S.Y. Study on controlled drug permeation of magnetic-sensitive ferrogels: Effect of Fe3O4 and PVA. J. Control. Release 2008, 126, 228–236. [Google Scholar] [CrossRef]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Reddy, N.N.; Ravindra, S.; Reddy, N.M.; Rajinikanth, V.; Raju, K.M.; Vallabhapurapu, V.S. Temperature responsive hydrogel magnetic nanocomposites for hyperthermia and metal extraction applications. J. Magn. Magn. 2015, 394, 237–244. [Google Scholar] [CrossRef]
- Haring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Diaz, D.D. Magentic gel composites for hyperthermia cancer therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef]

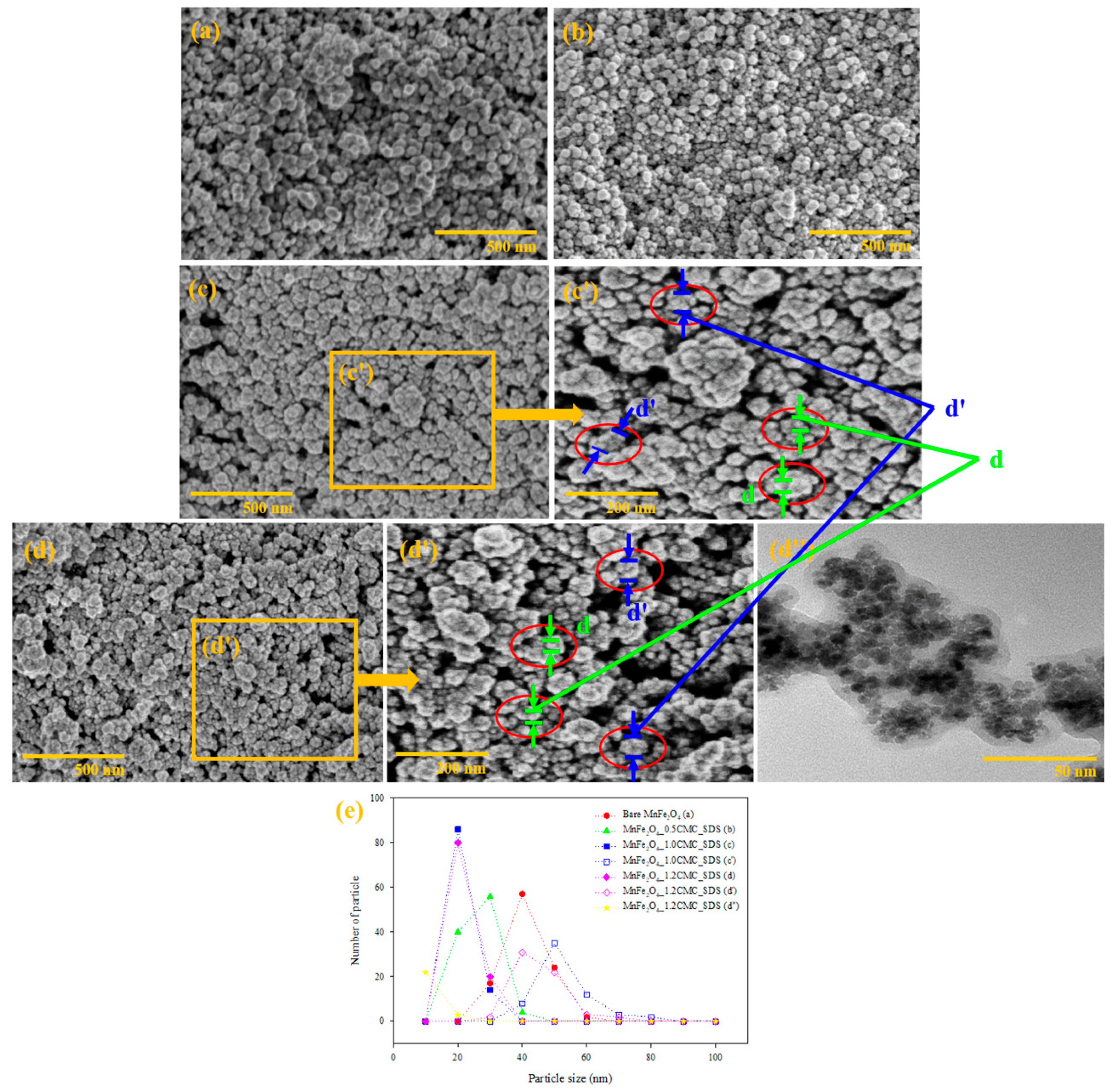
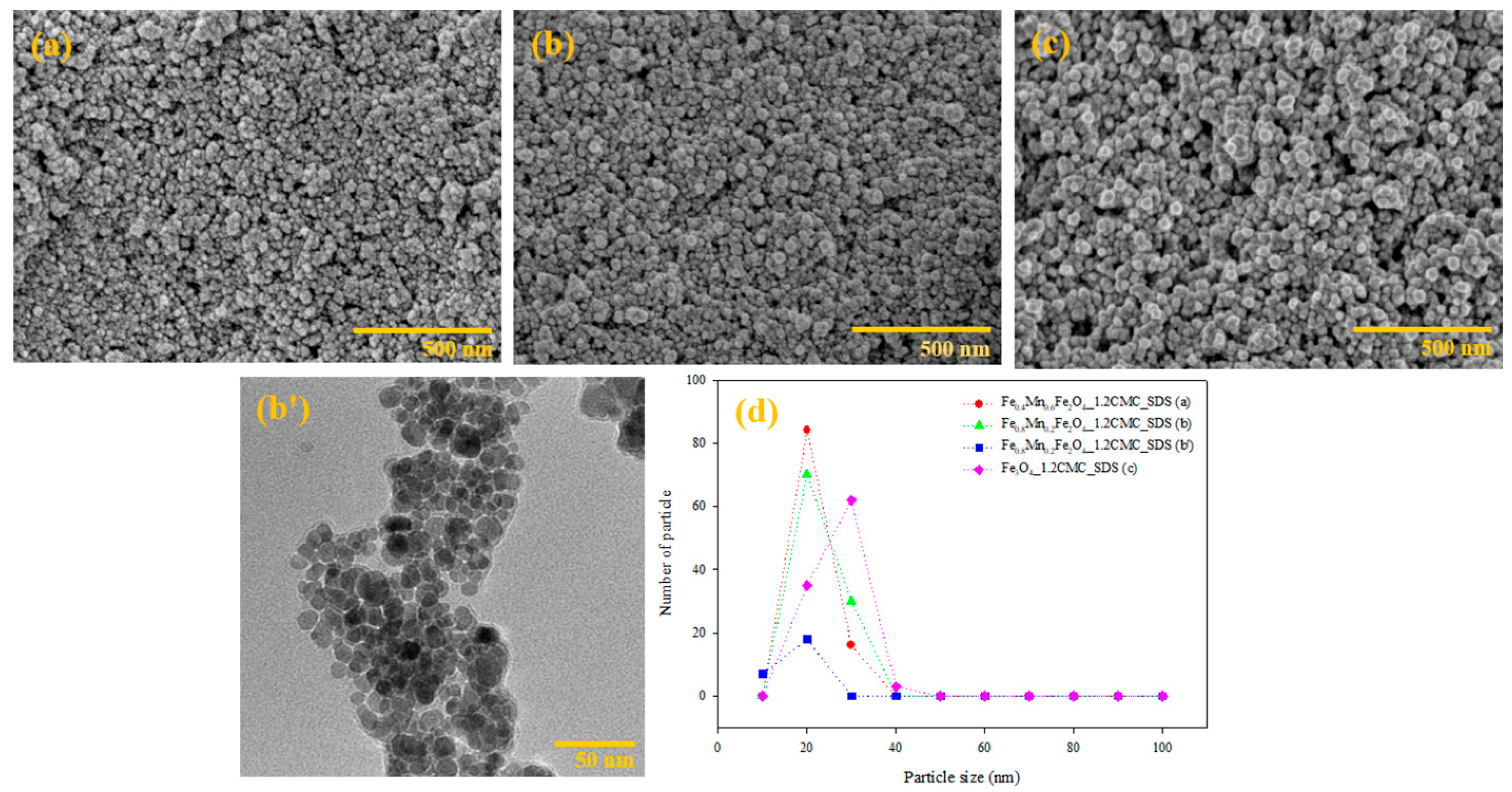

| Sample | D (nm) | a (Å) | Vcell (Å3) | LA (Å) | LB (Å) | (×103 Kg/m3) | S (m2/g) | d (nm) | d’ (nm) |
|---|---|---|---|---|---|---|---|---|---|
| Bare MnFe2O4 | 10.6 | 8.39 | 591 | 3.66 | 2.99 | 5.19 | 111 | 36 ± 6 | - |
| MnFe2O4_0.5CMC_SDS | 10.5 | 8.39 | 591 | 3.66 | 2.99 | 5.19 | 113 | 22 ± 5 | - |
| MnFe2O4_1.0CMC_SDS | 8.47 | 8.39 | 591 | 3.63 | 2.97 | 5.19 | 137 | 17 ± 3 | 49 ± 8 |
| MnFe2O4_1.2CMC_SDS | 7.80 | 8.46 | 604 | 3.66 | 2.99 | 5.07 | 152 | 17 ± 3 | 40 ± 8 |
| Fe0.4Mn0.6Fe2O4_1.2CMC_SDS | 7.82 | 8.39 | 590 | 3.63 | 2.97 | 5.20 | 148 | 18 ± 2 | - |
| Fe0.8Mn0.2Fe2O4_1.2CMC_SDS | 10.8 | 8.35 | 582 | 3.62 | 2.95 | 5.28 | 106 | 18 ± 3 | - |
| Fe3O4_1.2CMC_SDS | 13.3 | 8.30 | 572 | 3.60 | 2.94 | 5.38 | 83.9 | 22 ± 4 | - |
| Sample | Ms (emu/g) | Hc (Oe) | Mr (emu/g) | Magnetic Moment in the Units of Bohr Magneton |
|---|---|---|---|---|
| Bare MnFe2O4 | 27 | 27 | 1.6 | 1.1 |
| MnFe2O4_0.5CMC_SDS | 55-6 | 19 | 2.4 | 2.3 |
| MnFe2O4_1.0CMC_SDS | 73 | 20 | 2.8 | 3.1 |
| MnFe2O4_1.2CMC_SDS | 57 | 17 | 1.5 | 2.4 |
| Fe0.4Mn0.6Fe2O4_1.2CMC_SDS | 85 | 16 | 2.4 | 3.6 |
| Fe0.8Mn0.2Fe2O4_1.2CMC_SDS | 88 | 23 | 2.8 | 3.7 |
| Fe3O4_1.2CMC_SDS | 87 | 31 | 4.6 | 3.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotjanasuworapong, K.; Lerdwijitjarud, W.; Sirivat, A. Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with High Saturation Magnetization via the Surfactant Assisted Co-Precipitation. Nanomaterials 2021, 11, 876. https://doi.org/10.3390/nano11040876
Rotjanasuworapong K, Lerdwijitjarud W, Sirivat A. Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with High Saturation Magnetization via the Surfactant Assisted Co-Precipitation. Nanomaterials. 2021; 11(4):876. https://doi.org/10.3390/nano11040876
Chicago/Turabian StyleRotjanasuworapong, Kornkanok, Wanchai Lerdwijitjarud, and Anuvat Sirivat. 2021. "Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with High Saturation Magnetization via the Surfactant Assisted Co-Precipitation" Nanomaterials 11, no. 4: 876. https://doi.org/10.3390/nano11040876
APA StyleRotjanasuworapong, K., Lerdwijitjarud, W., & Sirivat, A. (2021). Synthesis and Characterization of Fe0.8Mn0.2Fe2O4 Ferrite Nanoparticle with High Saturation Magnetization via the Surfactant Assisted Co-Precipitation. Nanomaterials, 11(4), 876. https://doi.org/10.3390/nano11040876





