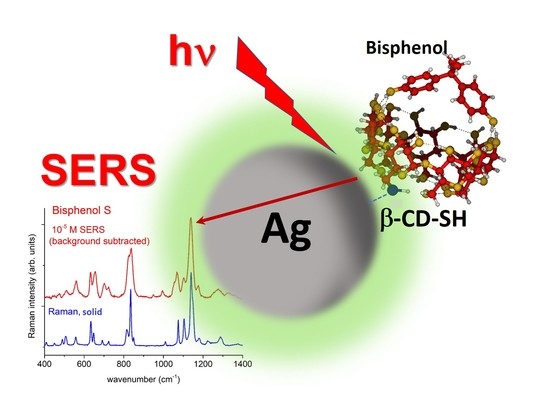
Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. AgNP Synthesis and Chemicals
2.2. Instrumentation
2.3. SERS Measurements
2.4. Multivariate Analysis of SERS Data
2.5. Theoretical Calculations
3. Results and Discussion
3.1. Raman and SERS Spectroscopy Results
3.2. DFT Calculations
3.3. Multivariate Analysis of SERS Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maffini, M.V.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Endocrine disruptors and reproductive health: The case of bisphenol-A. Mol. Cell. Endocrinol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Eladak, S.; Grisin, T.; Moison, D.; Guerquin, M.J.; N’Tumba-Byn, T.; Pozzi-Gaudin, S.; Benachi, A.; Livera, G.; Rouiller-Fabre, V.; Habert, R. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril. 2015, 103, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.D.; Kah, O.; Coumailleau, P. Estrogenic effects of several BPA analogs in the developing zebrafish brain. Front. Neurosci. 2016, 10, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EUR-Lex—32018R0213—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2018/213/oj (accessed on 9 March 2021).
- Pang, S.; Yang, T.; He, L. Review of surface enhanced Raman spectroscopic (SERS) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Tognaccini, L.; Ricci, M.; Gellini, C.; Feis, A.; Smulevich, G.; Becucci, M. Surface Enhanced Raman Spectroscopy for In-Field Detection of Pesticides: A Test on Dimethoate Residues in Water and on Olive Leaves. Molecules 2019, 24, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feis, A.; Gellini, C.; Ricci, M.; Tognaccini, L.; Becucci, M.; Smulevich, G. Surface-enhanced Raman scattering of glyphosate on dispersed silver nanoparticles: A reinterpretation based on model molecules. Vib. Spectrosc. 2020, 108, 103061. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2007; ISBN 9780471607311. [Google Scholar]
- Perumal, J.; Wang, Y.; Attia, A.B.E.; Dinish, U.S.; Olivo, M. Towards a point-of-care SERS sensor for biomedical and agri-food analysis applications: A review of recent advancements. Nanoscale 2021, 13, 553–580. [Google Scholar] [CrossRef]
- Cupil-Garcia, V.; Strobbia, P.; Crawford, B.M.; Wang, H.; Ngo, H.; Liu, Y.; Vo-Dinh, T. Plasmonic nanoplatforms: From surface-enhanced Raman scattering sensing to biomedical applications. J. Raman Spectrosc. 2021, 52, 541–553. [Google Scholar] [CrossRef]
- Koh, E.H.; Moon, J.Y.; Kim, S.Y.; Lee, W.C.; Park, S.G.; Kim, D.H.; Jung, H.S. A cyclodextrin-decorated plasmonic gold nanosatellite substrate for selective detection of bipyridylium pesticides. Analyst 2021, 146, 305–314. [Google Scholar] [CrossRef]
- Bakar, N.A.; Salleh, M.M.; Umar, A.A.; Shapter, J.G. Design and measurement technique of surface-enhanced Raman scattering for detection of bisphenol A. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 25008. [Google Scholar] [CrossRef]
- Lin, P.Y.; Hsieh, C.W.; Hsieh, S. Rapid and Sensitive SERS Detection of Bisphenol A Using Self-assembled Graphitic Substrates. Sci. Rep. 2017, 7, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Cheshari, E.C.; Qi, J.; Li, X. Silver microspheres coated with a molecularly imprinted polymer as a SERS substrate for sensitive detection of bisphenol A. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.Q.; Li, D.W.; Qu, L.L.; Long, Y.T. Surface-imprinted core-shell Au nanoparticles for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal. Chim. Acta 2013, 777, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, Q.; Zeng, X.; Liu, Q.; Hou, X.; Tian, Y.; Wu, L. Sensitive detection of bisphenol A by coupling solid phase microextraction based on monolayer graphene-coated Ag nanoparticles on Si fibers to surface enhanced Raman spectroscopy. Talanta 2018, 187, 13–18. [Google Scholar] [CrossRef]
- Feng, J.; Xu, L.; Cui, G.; Wu, X.; Ma, W.; Kuang, H.; Xu, C. Building SERS-active heteroassemblies for ultrasensitive Bisphenol A detection. Biosens. Bioelectron. 2016, 81, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.K.; Stanciu, L.A. Bisphenol A detection using gold nanostars in a SERS improved lateral flow immunochromatographic assay. Sens. Actuators B Chem. 2018, 276, 222–229. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Marques, A.; Veigas, B.; Araújo, A.; Pagará, B.; Baptista, P.V.; Águas, H.; Martins, R.; Fortunato, E. Paper-Based SERS Platform for One-Step Screening of Tetracycline in Milk. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F. Determination of bisphenol A in milk and dairy products by high-performance liquid chromatography with fluorescence detection. J. Food Prot. 2003, 66, 1439–1443. [Google Scholar] [CrossRef]
- Martens, H.; Karstang, T.; Næs, T. Improved selectivity in spectroscopy by multivariate calibration. J. Chemom. 1987, 1, 201–219. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01. 2016. Available online: https://gaussian.com/citation/ (accessed on 29 March 2021).
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitale. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Zheng, Y. Raman spectroscopy of “Bisphenol A”. J. Mol. Struct. 2016, 1108, 649–653. [Google Scholar] [CrossRef]
- Ullah, R.; Wang, X. Raman spectroscopy of Bisphenol ‘S’ and its analogy with Bisphenol ‘A’ uncovered with a dimensionality reduction technique. J. Mol. Struct. 2019, 1175, 927–934. [Google Scholar] [CrossRef]
- Barnett, S.M.; Vlckova, B.; Butler, I.S.; Kanigan, T.S. Surface-Enhanced Raman Scattering Spectroscopic Study of 17.alpha.-Ethinylestradiol on Silver Colloid and in Glass-Deposited Ag-17.alpha.-Ethinylestradiol Film. Anal. Chem. 1994, 66, 1762–1765. [Google Scholar] [CrossRef]
- Han, X.X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling Reaction-Based Ultrasensitive Detection of Phenolic Estrogens Using Surface-Enhanced Resonance Raman Scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef]
- De Bleye, C.; Dumont, E.; Hubert, C.; Sacré, P.Y.; Netchacovitch, L.; Chavez, P.F.; Hubert, P.; Ziemons, E. A simple approach for ultrasensitive detection of bisphenols by multiplexed surface-enhanced Raman scattering. Anal. Chim. Acta 2015, 888, 118–125. [Google Scholar] [CrossRef]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Functionalization of Ag nanoparticles with dithiocarbamate calix[4]arene as an effective supramolecular host for the surface-enhanced Raman scattering detection of polycyclic aromatic hydrocarbons. Langmuir 2006, 22, 10924–10926. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Garcia-Ramos, J.V.; Domingo, C.; Sanchez-Cortes, S. Self-assembly of a dithiocarbamate calix[4]arene on Ag nanoparticles and its application in the fabrication of surface-enhanced Raman scattering based nanosensors. Phys. Chem. Chem. Phys. 2009, 11, 1787–1793. [Google Scholar] [CrossRef]
- Lai, H.; Xu, F.; Zhang, Y.; Wang, L. Recent progress on graphene-based substrates for surface-enhanced Raman scattering applications. J. Mater. Chem. B 2018, 6, 4008–4028. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Han, X.; Xue, X.; Ji, W.; Qi, Z.; Liu, J.; Zhao, B.; Ozaki, Y. Sensing of polycyclic aromatic hydrocarbons with cyclodextrin inclusion complexes on silver nanoparticles by surface-enhanced Raman scattering. Analyst 2010, 135, 1389–1394. [Google Scholar] [CrossRef]
- Fang, C.; Bandaru, N.M.; Ellis, A.V.; Voelcker, N.H. Beta-cyclodextrin decorated nanostructured SERS substrates facilitate selective detection of endocrine disruptor chemicals. Biosens. Bioelectron. 2013, 42, 632–639. [Google Scholar] [CrossRef]
- Lofrumento, C.; Platania, E.; Ricci, M.; Becucci, M.; Castellucci, E.M. SERS Spectra of Alizarin Anion-Agn (n = 2, 4, 14) Systems: TDDFT Calculation and Comparison with Experiment. J. Phys. Chem. C 2016, 120, 12234–12241. [Google Scholar] [CrossRef]
- Ricci, M.; Lofrumento, C.; Becucci, M.; Castellucci, E.M. The Raman and SERS spectra of indigo and indigo-Ag2 complex: DFT calculation and comparison with experiment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 141–148. [Google Scholar] [CrossRef]
- López-Tocón, I.; Valdivia, S.; Soto, J.; Otero, J.C.; Muniz-Miranda, F.; Menziani, M.C.; Muniz-Miranda, M. A DFT approach to the surface-enhanced Raman scattering of 4-cyanopyridine adsorbed on silver nanoparticles. Nanomaterials 2019, 9, 1211. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, N.R.; Lucotti, A.; Tommasini, M.; Neri, F.; Trusso, S.; Ossi, P.M. SERS detection and DFT calculation of 2-naphthalene thiol adsorbed on Ag and Au probes. Sens. Actuators B Chem. 2016, 237, 545–555. [Google Scholar] [CrossRef]
- Varsanyi, G. Vibrational Spectra of Benzene Derivatives; Academic Press: Cambridge, MA, USA, 1969. [Google Scholar]
- Schaftenaar, G.; Noordik, J.H. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput. Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]






| Experimental | B3LYP/6-31G * | B3LYP/LanL2DZ | This Work | Refs. [32,33] | |||
|---|---|---|---|---|---|---|---|
| Raman | SERS | BPA | BPA | SH-CD-BPA b | CD-BPA b | Assignment a | Assignment c |
| 489 | 495 1 | 543 | 542 | 548 | 549 | δCCCskeletal | γ(CO) |
| 641 | 643 2 | 645 | 638 | 644 | 642 | 6b; δring + νCring-C-Cring | δ(CCC)r |
| 648 | 657 | 657 | 658 | 658 | 6a; δring | ν(CC) | |
| 735 | 735 | 744 | 752 | 756 | 754 | 4; τring | τ(ring), γ(CO) |
| 819 | 822 | 847 | 828 | 832 | 830 | 1; νring | γ(CH), τ(HCCC) |
| 834 | 836 | 857 | 839 | 842 | 841 | 1; νring + νCring-C-Cring | δ(CCC)r, γ(CH) |
| 920 | 920 | 950 | 937 | 938 | 927 | rCH3 + νCCH3 | ν(CC) |
| 938 | 938 | 958 | 955 | 954 | 951 | rCH3 + νCCH3 | ν(CC), ω(CCH)r |
| 1083 | 1110 | 1109 | 1112 | 1110 | δCHring + νCCring + rCH3 | ν(CC) | |
| 1113 | 1113 | 1133 | 1136 | 1139 | 1136 | δCHring + νCCring | ν(CC), ω(CCH)r |
| 1149 | 1178 | 1175 | 1186 | 1179 | δCHring + νCCH3 | ν(CC) | |
| 1182 | 1181 | 1210 | 1218 | 1215 | 1215 | δCOH + CHring | ν(CC), ω(CCH)r |
| 1234 | 1268 | 1258 | 1276 | 1279 | νCring-C-Cring + rCCH3 | ν(CO) | |
| 1257 | 1308 | 1274 | 1294 | 1310 | νCO + νCCring + δCHring | ν(CC) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.C.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials 2021, 11, 881. https://doi.org/10.3390/nano11040881
Roschi E, Gellini C, Ricci M, Sanchez-Cortes S, Focardi C, Neri B, Otero JC, López-Tocón I, Smulevich G, Becucci M. Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions. Nanomaterials. 2021; 11(4):881. https://doi.org/10.3390/nano11040881
Chicago/Turabian StyleRoschi, Eleonora, Cristina Gellini, Marilena Ricci, Santiago Sanchez-Cortes, Claudia Focardi, Bruno Neri, Juan Carlos Otero, Isabel López-Tocón, Giulietta Smulevich, and Maurizio Becucci. 2021. "Surface-Enhanced Raman Spectroscopy for Bisphenols Detection: Toward a Better Understanding of the Analyte–Nanosystem Interactions" Nanomaterials 11, no. 4: 881. https://doi.org/10.3390/nano11040881