Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges
Abstract
1. Introduction
2. Photoconductivity of Nanocrystalline Metal Oxides
3. Activation of Gas Sensitivity of Semiconductor Metal Oxides under UV Light
4. Activation of Gas Sensitivity of Semiconductor Metal Oxides under Visible Light
4.1. The Role of Impurity Absorption
4.2. Doping of Wide-Gap Oxides
4.3. Spectral Sensitization
4.3.1. Dyes Sensitization
4.3.2. Sensitization by Particles of Narrow-Gap Semiconductors
4.3.3. Sensitization with Semiconductor Quantum Dots
4.4. Using the Plasmon Resonance Effect
5. Mechanisms of Light Activated Gas Sensing
5.1. Light-Activated Sensor Response to Oxidizing Gases
5.2. Light-Activated Sensor Response to Reducing Gases
6. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurlo, A. Insights into the Mechanism of Gas Sensor Operation. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, M.A., Mathur, S., Kolmakov, A., Eds.; Integrated Analytical Systems; Springer: New York, NY, USA, 2013; pp. 3–34. [Google Scholar]
- Gurlo, A.; Bârsan, N.; Weimar, U. Gas Sensors Based on Semiconducting Metal Oxides. In Metal oxides: Chemistry and Application; Fierro, J.L.G., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 683–738. [Google Scholar]
- Yamazoe, N.; Shimanoe, K. Theory of power laws for semiconductor gas sensors. Sens. Actuators B 2008, 128, 566–573. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Mizsei, J. How can sensitive and selective semiconductor gas sensor be made? Sens. Actuators B 1995, 23, 173–176. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Wang, J.; Shen, H.; Xia, Y.; Komarneni, S. Light-activated room-temperature gas sensors based on metal oxide nanostructures: A review on recent advances. Ceram. Int. 2021, 47, 7353–7368. [Google Scholar] [CrossRef]
- Suh, J.M.; Eom, T.H.; Cho, S.H.; Kim, T.; Jang, H.W. Light-activated gas sensing: A perspective of integration with micro-LEDs and plasmonic nanoparticles. Mater. Adv. 2021, 2, 827–844. [Google Scholar] [CrossRef]
- Xu, F.; HO, H.P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Liu, X.; Zhang, J. Room-Temperature Gas Sensors under Photoactivation: From Metal Oxides to 2D Materials. Nano-Micro Lett. 2020, 12, 164. [Google Scholar] [CrossRef]
- Galazka, Z. Growth, characterization, and properties of bulk SnO2 single crystals. Phys. Status Solidi (A) 2014, 211, 66–73. [Google Scholar] [CrossRef]
- Özgür, Ü.; Morkoç, H. Optical Properties of ZnO and Related Alloys. In Zinc Oxide Bulk, Thin Films and Nanostructures; Jagadish, C., Pearton, S., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006; pp. 175–239. [Google Scholar]
- Dar, M.I.; Chandiran, A.K.; Graetzel, M.; Nazeeruddin, M.K.; Shivashankar, S.A. Controlled synthesis of TiO2 nanoparticles and nanospheres using a microwave assisted approach for their application in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 1662–1667. [Google Scholar] [CrossRef]
- Weiher, R.L.; Ley, R.P. Optical Properties of Indium Oxide. J. Appl. Phys. 1966, 37, 299–302. [Google Scholar] [CrossRef]
- Kleperis, J.; Zubkans, J.; Lusis, A.R. Nature of fundamental absorption edge of WO3. In Optical Organic and Semiconductor Inorganic Materials; Silinsh, E.A., Medvids, A., Lusis, A.R., Ozols, A.O., Eds.; SPIE: Riga, Latvia, 1997; pp. 186–191. [Google Scholar]
- Collins, R.J.; Thomas, D.G. Photoconduction and Surface Effects with Zinc Oxide Crystals. Phys. Rev. 1958, 112, 388–395. [Google Scholar] [CrossRef]
- Cunningham, R.D.; Marton, J.P.; Schlesinger, M. Photoconductivity in SnO2 Crystals. J. Appl. Phys. 1969, 40, 4664–4665. [Google Scholar] [CrossRef]
- Medved, D.B. Photoconductivity and chemisorption kinetics in sintered zinc oxide semiconductor. J. Phys. Chem. Solids 1961, 20, 255–267. [Google Scholar] [CrossRef]
- Bojorge, C.D. Nanocrystalline ZnO Photoconductivity Measurements. Procedia Mater. Sci. 2012, 1, 614–619. [Google Scholar] [CrossRef]
- Gurwitz, R.; Cohen, R.; Shalish, I. Interaction of light with the ZnO surface: Photon induced oxygen breathing, oxygen vacancies, persistent photoconductivity, and persistent photovoltage. J. Appl. Phys. 2014, 115, 033701. [Google Scholar] [CrossRef]
- Messias, F.R.; Scalvi, L.V.A.; Li, M.S.; Santilli, C.V.; Pulcinelli, S.H. Oxygen related defects excitation and photoconductivity dependence of SnO2 Sol-Gel films with several light sources. Radiat. Eff. Defects Solids 1999, 150, 391–395. [Google Scholar] [CrossRef]
- Forsh, E.A.; Abakumov, A.M.; Zaytsev, V.B.; Konstantinova, E.A.; Forsh, P.A.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. Optical and photoelectrical properties of nanocrystalline indium oxide with small grains. Thin Solid Films 2015, 595, 25–31. [Google Scholar] [CrossRef]
- Madel, M.; Huber, F.; Mueller, R.; Amann, B.; Dickel, M.; Xie, Y.; Thonke, K. Persistent photoconductivity in ZnO nanowires: Influence of oxygen and argon ambient. J. Appl. Phys. 2017, 121, 124301. [Google Scholar] [CrossRef]
- Dixit, A.; Panguluri, R.P.; Sudakar, C.; Kharel, P.; Thapa, P.; Avrutsky, I.; Naik, R.; Lawes, G.; Nadgorny, B. Robust room temperature persistent photoconductivity in polycrystalline indium oxide films. Appl. Phys. Lett. 2009, 94, 252105. [Google Scholar] [CrossRef]
- Brinzari, V. Mechanism of band gap persistent photoconductivity (PPC) in SnO2 nanoscrystalline films: Nature of local states, simulation of PPC and comparison with experiment. Appl. Surf. Sci. 2017, 411, 437–448. [Google Scholar] [CrossRef]
- Forsh, E.A.; Martyshov, M.N.; Forsh, P.A.; Kashkarov, P.K. Transient Photoconductivity in Nanocrystalline Indium Oxide. J. Nanoelectron. Optoelectron. 2014, 9, 124–127. [Google Scholar] [CrossRef]
- Madelung, O. Localized States. In Introduction to Solid-State Theory: Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1978; Volume 2, pp. 377–434. [Google Scholar]
- Brinzari, V.; Ivanov, M.; Cho, B.K.; Kamei, M.; Korotcenkov, G. Photoconductivity in In2O3 nanoscale thin films: Interrelation with chemisorbed-type conductometric response towards oxygen. Sens. Actuators B 2010, 148, 427–438. [Google Scholar] [CrossRef]
- Micheletti, F.B.; Mark, P. Effects of chemisorbed oxygen on the electrical properties of chemically sprayed CdS thin films. Appl. Phys. Lett. 1967, 10, 136. [Google Scholar] [CrossRef]
- Van Hove, H.; Luyckx, A. Photoconductivity decay of ZnO crystals in oxygen. Solid State Commun. 1966, 4, 603–606. [Google Scholar] [CrossRef]
- Saura, J. Gas-sensing properties of SnO2 pyrolytic films subjected to ultraviolet radiation. Sens. Actuators B 1994, 17, 211–214. [Google Scholar] [CrossRef]
- Camagni, P.; Faglia, G.; Galinetto, P.; Perego, C.; Samoggia, G.; Sberveglieri, G. Photosensitivity activation of SnO2 thin film gas sensors at room temperature. Sens. Actuators B 1996, 31, 99–103. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G. UV light activation of tin oxide thin films for NO2 sensing at low temperatures. Sens. Actuators B 2001, 78, 73–77. [Google Scholar] [CrossRef]
- Li, T.; Zeng, W.; Shi, D.; Hussain, S. UV-enhanced hydrogen sensor based on nanocone-assembled 3D SnO2 at low temperature. Mater. Lett. 2015, 161, 648–651. [Google Scholar] [CrossRef]
- Jeng, C.C.; Chong, P.J.H.; Chiu, C.C.; Jiang, G.J.; Lin, H.J.; Wu, R.J.; Wu, C.H. A dynamic equilibrium method for the SnO2-based ozone sensors using UV-LED continuous irradiation. Sens. Actuators B 2014, 195, 702–706. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cimalla, V.; Kunzer, M.; Passow, T.; Pletschen, W.; Kappeler, O.; Wagner, J.; Ambacher, O. Near-UV LEDs for integrated InO-based ozone sensors. Phys. Status Solidi (C) 2010, 7, 2177–2179. [Google Scholar] [CrossRef]
- Wagner, T.; Kohl, C.D.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. UV light-enhanced NO2 sensing by mesoporous In2O3: Interpretation of results by a new sensing model. Sens. Actuators B 2013, 187, 488–494. [Google Scholar] [CrossRef]
- Trocino, S.; Frontera, P.; Donato, A.; Bucassa, C.; Scarpino, L.A.; Antonucci, P.; Neri, G. Gas sensing properties under UV radiation of In2O3 nanostructures processed by electrospinning. Mater. Chem. Phys. 2014, 147, 35–41. [Google Scholar] [CrossRef]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV effect on NO2 sensing properties of nanocrystalline In2O3. Sens. Actuators B 2016, 231, 491–496. [Google Scholar] [CrossRef]
- Gonzalez, O.; Roso, S.; Vilanova, X.; Llobet, E. Enhanced detection of nitrogen dioxide via combined heating and pulsed UV operation of indium oxide nano-octahedra. Beilstein J. Nanotechnol. 2016, 7, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, L.; Yuan, X.; Li, Y.; Li, C.; Yin, M.; Fan, X. Room temperature photoelectric NO2 gas sensor based on direct growth of walnut-like In2O3 nanostructures. J. Alloys Compd. 2019, 782, 1121–1126. [Google Scholar] [CrossRef]
- Shen, Y.; Zhong, X.; Zhang, J.; Li, T.; Zhao, S.; Cui, B.; Wei, D.; Zhang, Y.; Wei, K. In-situ growth of mesoporous In2O3 nanorod arrays on a porous ceramic substrate for ppb-level NO2 detection at room temperature. Appl. Surf. Sci. 2019, 498, 143873. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Li, X.; Wang, J.; Tang, Z. UV activated hollow ZnO microspheres for selective ethanol sensors at low temperatures. Sens. Actuators B 2016, 232, 158–164. [Google Scholar] [CrossRef]
- Cui, J.; Shi, L.; Xie, T.; Wang, D.; Lin, Y. UV-light illumination room temperature HCHO gas-sensing mechanism of ZnO with different nanostructures. Sens. Actuators B 2016, 227, 220–226. [Google Scholar] [CrossRef]
- Fabbri, B.; Gaiardo, A.; Giberti, A.; Guidi, V.; Malagù, C.; Martucci, A.; Sturaro, M.; Zonta, G.; Gherardi, S.; Bernardoni, P. Chemoresistive properties of photo-activated thin and thick ZnO films. Sens. Actuators B 2016, 222, 1251–1256. [Google Scholar] [CrossRef]
- Vuong, N.M.; Chinh, N.D.; Hien, T.T.; Quang, N.D.; Kim, D.; Kim, H.; Yoon, S.G.; Kim, D. Gas-Sensing Properties of ZnO Nanorods at Room Temperature Under Continuous UV Illumination in Humid Air. J. Nanosci. Nanotechnol. 2016, 16, 10346–10350. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Chai, X.; Hu, Z.; Deng, Y. UV-Light-Activated ZnO Fibers for Organic Gas Sensing at Room Temperature. J. Phys. Chem. C 2010, 114, 1293–1298. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, H.; Sun, Y.; Li, M.; Liu, J. UV-activated room temperature single-sheet ZnO gas sensor. Micro Nano Lett. 2017, 12, 813–817. [Google Scholar] [CrossRef]
- Kumar, R.R.; Murugesan, T.; Chang, T.-W.; Lin, H.-N. Defect controlled adsorption/desorption kinetics of ZnO nanorods for UV-activated NO2 gas sensing at room temperature. Mater. Lett. 2021, 287, 129257. [Google Scholar] [CrossRef]
- Giberti, A.; Malagù, C.; Guidi, V. WO3 sensing properties enhanced by UV illumination: An evidence of surface effect. Sens. Actuators B 2012, 165, 59–61. [Google Scholar] [CrossRef]
- Trawka, M.P.; Smulko, J.M.; Hasse, L.Z.; Granqvist, C.G.; Ionescu, R.; Llobet, E.; Annanouch, F.E.; Kish, L.B. UV-Light-Induced Fluctuation Enhanced Sensing by WO3-Based Gas Sensors. IEEE Sens. J. 2016, 16, 5152–5159. [Google Scholar] [CrossRef]
- Trawka, M.; Smulko, J.; Hasse, L.; Granqvist, C.G.; Annanouch, F.E.; Ionescu, R. Fluctuation enhanced gas sensing with WO3-based nanoparticle gas sensors modulated by UV light at selected wavelengths. Sens. Actuators B 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Gonzalez, O.; Welearegay, T.; Llobet, E.; Vilanova, X. Pulsed UV Light Activated Gas Sensing in Tungsten Oxide Nanowires. Procedia Eng. 2016, 168, 351–354. [Google Scholar] [CrossRef]
- Imran, M.; Abdul Haroon Rashid, S.S.A.; Sabri, Y.; Motta, N.; Tesfamichael, T.; Sonar, P.; Shafiei, M. Template based sintering of WO3 nanoparticles into porous tungsten oxide nanofibers for acetone sensing applications. J. Mater. Chem. C 2019, 7, 2961–2970. [Google Scholar] [CrossRef]
- Almaev, A.V.; Yakovlev, N.N.; Chernikov, E.V.; Tolbanov, O.P. Selective Sensors of Nitrogen Dioxide Based on Thin Tungsten Oxide Films under Optical Irradiation. Tech. Phys. Lett. 2019, 45, 1016–1019. [Google Scholar] [CrossRef]
- Gonzalez, O.; Welearegay, T.; Vilanova, X.; Llobet, E. Using the Transient Response of WO3 Nanoneedles under Pulsed UV Light in the Detection of NH3 and NO2. Sensors 2018, 18, 1346. [Google Scholar] [CrossRef] [PubMed]
- Tomić, M.; Fohlerova, Z.; Gràcia, I.; Figueras, E.; Cané, C.; Vallejos, S. UV-light activated APTES modified WO3-x nanowires sensitive to ethanol and nitrogen dioxide. Sens. Actuators B 2021, 328, 129046. [Google Scholar] [CrossRef]
- Manera, M.G.; Taurino, A.; Catalano, M.; Rella, R.; Caricato, A.P.; Buonsanti, R.; Cozzoli, P.D.; Martino, M. Enhancement of the optically activated NO2 gas sensing response of brookite TiO2 nanorods/nanoparticles thin films deposited by matrix-assisted pulsed-laser evaporation. Sens. Actuators B 2012, 161, 869–879. [Google Scholar] [CrossRef]
- Zhang, S.; Lei, T.; Li, D.; Zhang, G.; Xie, C. UV light activation of TiO2 for sensing formaldehyde: How to be sensitive, recovering fast, and humidity less sensitive. Sens. Actuators B 2014, 202, 964–970. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Peng, X.; Geng, Q.; Chen, X.; Dai, W.; Fu, X.; Wang, X. Comparative study of ultraviolet light and visible light on the photo-assisted conductivity and gas sensing property of TiO2. Sens. Actuators B 2017, 248, 724–732. [Google Scholar] [CrossRef]
- Xie, T.; Sullivan, N.; Steffens, K.; Wei, B.; Liu, G.; Debnath, R.; Davydov, A.; Gomez, R.; Motayed, A. UV-assisted room-temperature chemiresistive NO2 sensor based on TiO2 thin film. J. Alloys Compd. 2015, 653, 255–259. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B 2015, 211, 146–156. [Google Scholar] [CrossRef]
- Mun, Y.; Park, S.; An, S.; Lee, C.; Kim, H.W. NO2 gas sensing properties of Au-functionalized porous ZnO nanosheets enhanced by UV irradiation. Ceram. Int. 2013, 39, 8615–8622. [Google Scholar] [CrossRef]
- Wongrat, E.; Chalnek, N.; Chueaiarrom, C.; Samransuksamer, B.; Hongsith, N.; Choopun, S. Low temperature ethanol response enhancement of ZnO nanostructures sensor decorated with gold nanoparticles exposed to UV illumination. Sens. Actuators A 2016, 251, 188–197. [Google Scholar] [CrossRef]
- Kumar, G.; Li, X.; Du, Y.; Geng, Y.; Hong, X. UV-light enhanced high sensitive hydrogen (H2) sensor based on spherical Au nanoparticles on ZnO nano-structured thin films. J. Alloys Compd. 2019, 798, 467–477. [Google Scholar] [CrossRef]
- Joshi, M.; da Silva, L.F.; Shimizu, F.M.; Mastelaro, V.R.; M’Peko, J.C.; Lin, L.; Oliveira Jr, O.N. UV-assisted chemiresistors made with gold-modified ZnO nanorods to detect ozone gas at room temperature. Microchim. Acta 2019, 186, 418. [Google Scholar] [CrossRef]
- Li, Y.; Jin, H.; Sun, G.; Zhang, B.; Luo, N.; Lin, L.; Bala, H.; Cao, J.; Zhang, Z.; Wang, Y. Synthesis of novel porous ZnO octahedrons and their improved UV-light activated formaldehyde-sensing performance by Au decoration. Phys. E Low Dimens. Syst. Nanostruct. 2019, 106, 40–44. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Facile Synthesis and UV-Activated Gas Sensing Performance of Ag:ZnO Nano-Ellipsoids. ECS J. Solid State Sci. Technol. 2018, 7, Q3089–Q3093. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, T.; Wang, C.; Liu, D. UV-light-assisted ethanol sensing characteristics of g-C3N4/ZnO composites at room temperature. Appl. Surf. Sci. 2018, 441, 317–323. [Google Scholar] [CrossRef]
- Haridas, D.; Chowdhuri, A.; Sreenivas, K.; Gupta, V. Enhanced room temperature response of SnO2 thin film sensor loaded with Pt catalyst clusters under UV radiation for LPG. Sens. Actuators B 2011, 153, 152–157. [Google Scholar] [CrossRef]
- Deng, Q.; Gao, S.; Lei, T.; Ling, Y.; Zhang, S.; Xie, C. Temperature and light modulation to enhance the selectivity of Pt-modified zinc oxide gas sensor. Sens. Actuators B 2017, 247, 903–915. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Geng, X.; Luo, Y.; Zheng, B.; Zhang, C. Photon assisted room-temperature hydrogen sensors using PdO loaded WO3 nanohybrids. Int. J. Hydrog. Energy 2017, 42, 6425–6434. [Google Scholar] [CrossRef]
- Bouchikhi, B.; Chludziński, T.; Saidi, T.; Smulko, J.; Bari, N.E.; Wen, H.; Ionescu, R. Formaldehyde detection with chemical gas sensors based on WO3 nanowires decorated with metal nanoparticles under dark conditions and UV light irradiation. Sens. Actuators B 2020, 320, 128331. [Google Scholar] [CrossRef]
- Geng, X.; Lu, P.; Zhang, C.; Lahem, D.; Olivier, M.-G.; Debliquy, M. Room-temperature NO2 gas sensors based on rGO-ZnO1-x composites: Experiments and molecular dynamics simulation. Sens. Actuators B 2019, 282, 690–702. [Google Scholar] [CrossRef]
- Li, W.; Guo, C.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.L.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; Dai, M.; Liu, K.; Liu, Y.; Zhou, L.; Liu, F.; Liu, F.; Gao, Y.; Yan, X.; et al. Visible light activated excellent NO2 sensing based on 2D/2D ZnO/g-C3N4 heterojunction composites. Sens. Actuators B 2020, 304, 127287. [Google Scholar] [CrossRef]
- Shao, S.; Chen, Y.; Huang, S.; Jiang, F.; Wang, Y.; Koehn, R. A tunable volatile organic compound sensor by using PtOx/GQDs/TiO2 nanocomposite thin films at room temperature under visible-light activation. RSC Adv. 2017, 7, 39859–39868. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Development of highly sensitive ZnO/In2O3 composite gas sensor activated by UV-LED. Sens. Actuators B 2017, 241, 828–839. [Google Scholar] [CrossRef]
- Wei, N.; Cui, H.; Wang, X.; Xie, X.; Wang, M.; Zhang, L.; Tian, J. Hierarchical assembly of In2O3 nanoparticles on ZnO hollow nanotubes using carbon fibers as templates: Enhanced photocatalytic and gas-sensing properties. J. Colloid Interface Sci. 2017, 498, 263–270. [Google Scholar] [CrossRef]
- Sun, J.; Liu, F.; Zhong, T.; Xu, J.; Zhang, Y.; Lu, G. Effects of UV Light Illumination on the Gas Sensing Properties of ZnO-SnO2 Thick Film Sensor. Sens. Lett. 2011, 9, 824–827. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Da Silva, L.F.; M’Peko, J.C.; Catto, A.C.; Bernatdini, S.; Mastelaro, V.R.; Aguir, K.; Ribeiro, C.; Longo, E. UV-enhanced ozone gas sensing response of ZnO-SnO2 heterojunctions at room temperature. Sens. Actuators B 2017, 240, 573–579. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Li, X.; Li, X.; Lin, S.; Li, T.; Rumyantseva, M.N.; Gaskov, A.M. Room Temperature Formaldehyde Sensing of Hollow SnO2/ZnO Heterojunctions Under UV-LED Activation. IEEE Sens. J. 2019, 19, 7207–7214. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Tian, M.; Miao, J.; Zhang, H.; Sun, J. UV excitation NO2 gas sensor sensitized by ZnO quantum dots at room temperature. Sens. Actuators B 2018, 259, 526–531. [Google Scholar] [CrossRef]
- Li, X.; Gu, D.; Yang, Y.; Du, H.; Li, X. UV Light Activated SnO2/ZnO Nanofibers for Gas Sensing at Room Temperature. Front. Mater. 2019, 6, 158. [Google Scholar] [CrossRef]
- Nasriddinov, A.; Rumyantseva, M.; Marikutsa, A.; Gaskov, A.; Lee, J.H.; Kim, J.H.; Kim, J.Y.; Kim, S.S.; Kim, H.W. Sub-ppm Formaldehyde Detection by n-n TiO2@SnO2 Nanocomposites. Sensors 2019, 19, 3182. [Google Scholar] [CrossRef]
- Park, S.; Ko, H.; Lee, S.; Kim, H. & Lee, C. Light-activated gas sensing of Bi2O3-core/ZnO-shell nanobelt gas sensors. Thin Solid Films 2014, 570, 298–302. [Google Scholar]
- Bajpai, R.; Motayed, A.; Davydov, A.V.; Oleshko, V.P.; Aluri, G.S.; Bertness, K.A.; Rao, M.V.; Zaghloul, M.E. UV-assisted alcohol sensing using SnO2 functionalized GaN nanowire devices. Sens. Actuators B 2012, 171–172, 499–507. [Google Scholar] [CrossRef]
- Kneissl, M. A Brief Review of III-Nitride UV Emitter Technologies and Their Applications. In III-Nitride Ultraviolet Emitters: Technology and Applications; Springer Series in Materials Science; Springer: Cham, Switzerland, 2016; Volume 227, pp. 1–25. [Google Scholar]
- Pollack, I.B.; Lerner, B.M.; Ryerson, T.B. Evaluation of ultraviolet light-emitting diodes for detection of atmospheric NO2 by photolysis-chemiluminescence. J. Atmos. Chem. 2010, 65, 111–125. [Google Scholar] [CrossRef]
- Auf der Maur, M.; Pecchia, A.; Penazzi, G.; Rodrigues, W.; Di Carlo, A. Efficiency Drop in Green InGaN/GaN Light Emitting Diodes: The Role of Random Alloy Fluctuations. Phys. Rev. Lett. 2016, 116, 027401. [Google Scholar] [CrossRef]
- Gulyaev, A.M.; Van, L.V.; Sarach, O.B.; Mukhina, O.B. Increased sensitivity and selective capacity of gas sensors based on SnO2-x films exposed to light-emitting diodes. Meas. Tech. 2008, 51, 694–698. [Google Scholar] [CrossRef]
- Gulyaev, A.M.; Sarach, O.B.; Kotov, V.A.; Vanin, A.A.; Anufriev, Y.V.; Konovalov, A.V. Light-enhanced sensitivity of SnO2-x gas sensors. Semiconductors 2008, 42, 726–730. [Google Scholar] [CrossRef]
- Varechkina, E.N.; Rumyanrseva, M.N.; Vasiliev, R.B.; Konstantinova, E.A.; Gaskov, A.M. UV-VIS Photoconductivity of Nanocrystalline Tin Oxide. J. Nanoelectron. Optoelectron. 2012, 7, 623–628. [Google Scholar] [CrossRef]
- Zhang, C.; Boudiba, A.; De Marco, P.; Snyders, R.; Olivier, M.G.; Debliquy, M. Room temperature responses of visible-light illuminated WO3 sensors to NO2 in sub-ppm range. Sens. Actuators B 2013, 181, 395–401. [Google Scholar] [CrossRef]
- Giancaterini, L.; Emamjomeh, S.M.; De Marcellis, A.; Palange, E.; Resmini, A.; Anselmi-Tamburini, U.; Cantalini, C. The influence of thermal and visible light activation modes on the NO2 response of WO3 nanofibers prepared by electrospinning. Sens. Actuators B 2016, 229, 387–395. [Google Scholar] [CrossRef]
- Deng, L.; Ding, X.; Zeng, D.; Tian, S.; Li, H.; Xie, C. Visible-light activate mesoporous WO3 sensors with enhanced formaldehyde-sensing property at room temperature. Sens. Actuators B 2012, 163, 260–266. [Google Scholar] [CrossRef]
- Geng, Q.; He, Z.; Chen, X.; Dai, W.; Wang, X. Gas sensing property of ZnO under visible light irradiation at room temperature. Sens. Actuators B 2013, 188, 293–297. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Cui, J.; Chen, L.; Jiang, T.; Lin, Y. Study on formaldehyde gas-sensing of In2O3-sensitized ZnO nanoflowers under visible light irradiation at room temperature. J. Mater. Chem. 2012, 22, 12915–12920. [Google Scholar] [CrossRef]
- Zou, Z.; Qiu, Y.; Xu, J.; Guo, P.; Luo, Y.; Wang, C. Enhanced formaldehyde photoelectric response on ZnO film illuminated with visible light. J. Alloys Compd. 2017, 695, 2117–2123. [Google Scholar] [CrossRef]
- Klaus, D.; Klawinski, D.; Amrehn, S.; Tiemann, M.; Wagner, T. Light-activated resistive ozone sensing at room temperature utilizing nanoporous In2O3 particles: Influence of particle size. Sens. Actuators B 2015, 217, 181–185. [Google Scholar] [CrossRef]
- Xie, Y.; Cheong, F.C.; Varghese, B.; Zhu, Y.W.; Mahendiran, R.; Sow, C.H. Wavelength sensitive photo-sensing from discrete crystalline tungsten oxide nanowires. Sens. Actuators B 2011, 151, 320–326. [Google Scholar] [CrossRef]
- Lin, Z.; Liao, F.; Zhu, L.; Lu, S.; Sheng, M.; Gao, S.; Shao, M. Visible light enhanced gas sensing of CdSe nanoribbons of ethanol. CrystEngComm 2014, 16, 4231–4235. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, F.; Zhu, Y.; Sun, J.; Shao, M. Visible-light-enhanced gas sensing of CdSxSe1-x nanoribbons for acetic acid at room temperature. Sens. Actuators B 2015, 215, 497–503. [Google Scholar] [CrossRef]
- Li, H.Y.; Yoon, J.W.; Lee, C.S.; Lim, K.; Yoon, J.W.; Lee, J.H. Visible light assisted NO2 sensing at room temperature by CdS nanoflake array. Sens. Actuators B 2018, 255, 2963–2970. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Gupta, V.; Tomar, M. Synthesis of CdS nanoparticle by sol-gel method as low temperature NO2 sensor. Mater. Chem. Phys. 2020, 239, 121975. [Google Scholar] [CrossRef]
- Liu, X.H.; Yin, P.F.; Kulinich, S.A.; Zhou, Y.Z.; Mao, J.; Ling, T.; Du, X.W. Arrays of Ultrathin CdS Nanoflakes with High-Energy Surface for Efficient Gas Detection. ACS Appl. Mater. Interfaces 2017, 9, 602–609. [Google Scholar] [CrossRef]
- Kang, Y.; Pyo, S.; Jo, E.; Kim, J. Light-assisted recovery of reacted MoS2 for reversible NO2 sensing at room temperature. Nanotechnology 2019, 30, 355504. [Google Scholar] [CrossRef]
- Gu, D.; Li, X.; Wang, H.; Li, M.; Xi, Y.; Chen, Y.; Wang, J.; Rumyantseva, M.N.; Gaskov, A.M. Light enhanced VOCs sensing of WS2 microflakes based chemiresistive sensors powered by triboelectronic nanogenerators. Sens. Actuators B 2018, 256, 992–1000. [Google Scholar] [CrossRef]
- Gu, D.; Wang, X.; Liu, W.; Li, X.; Lin, S.; Wang, J.; Rumyantseva, M.N.; Gaskov, A.M. Visible-light activated room temperature NO2 sensing of SnS2 nanosheets based chemiresistive sensors. Sens. Actuators B 2020, 305, 127455. [Google Scholar] [CrossRef]
- Hung, P.T.; Hien, V.X.; Hoat, P.D.; Lee, S.; Lee, J.H.; Kim, J.J.; Heo, Y.W. Photo induced NO2 sensing properties of bismuth triiodide (BiI3) nanoplates at room temperature. Scripta Mater. 2019, 172, 17–22. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Hsieh, J.-C.; Chen, C.-A.; Chen, W.-L.; Meng, H.-F.; Lu, C.-J.; Horng, S.-F.; Zan, H.-W. Ultrasensitive detection of hydrogen sulfide gas based on perovskite vertical channel chemo-sensor. Sens. Actuators B 2021, 326, 128988. [Google Scholar] [CrossRef]
- Zhu, M.-Y.; Zhang, L.-X.; Yin, J.; Chen, J.-J.; Bie, L.-J.; Fahlman, B.D. Physisorption induced p-xylene gas-sensing performance of (C4H9NH3)2PbI4 layered perovskite. Sens. Actuators B 2019, 282, 659–664. [Google Scholar]
- Abbas, T.A.H. Light-Enhanced Vanadium Pentoxide (V2O5) Thin Films for Gas Sensor Applications. J. Electron. Mater. 2018, 47, 7331–7342. [Google Scholar] [CrossRef]
- Sreedhar, A.; Reddy, I.N.; Hoai Ta, Q.T.; Phuong Doan, T.H.; Shim, J.; Noh, J.-S. Unveiling the impact of interfacially engineered selective V2O5 nanobelt bundles with flake-like ZnO and Co–ZnO thin films for multifunctional visible-light water splitting and toxic gas sensing. J. Power Sources 2020, 478, 229081. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Y.; Ma, M.; Ren, J.; Liu, C.; Tan, J. Visible light-assisted formaldehyde sensor based on HoFeO3 nanoparticles with sub-ppm detection limit. Ceram. Int. 2020, 46, 16337–16344. [Google Scholar] [CrossRef]
- Geng, X.; Lahem, D.; Zhang, C.; Li, C.J.; Olivier, M.G.; Debliquy, M. Visible light enhanced black NiO sensors for ppb-level NO2 detection at room temperature. Ceram. Int. 2019, 45, 4253–4261. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.J.; Lu, Y.; Jiang, T.; Liu, B.; Lin, Y. Visible-Light-Assisted HCHO Gas Sensing Based on Fe-Doped Flowerlike ZnO at Room Temperature. J. Phys. Chem. C 2011, 115, 22939–22944. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Q.; Song, H.; Qin, P.; Lei, M.; Tie, B.; Wang, T. Room-temperature gas sensing properties of cobalt-doped ZnO Nanobelts with visible light irradiation. Appl. Phys. A 2011, 105, 387–392. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.J.; Lu, Y.; Jiang, T.; Chen, L.; Xie, T.; Lin, Y. Influence of annealing temperature on the photoelectric gas sensing of Fe-doped ZnO under visible light irradiation. Sens. Actuators B 2013, 177, 34–40. [Google Scholar] [CrossRef]
- Shao, S.; Chang, Y.; Long, Y. High performance of nanostructured ZnO film gas sensor at room temperature. Sens. Actuators B 2014, 204, 666–672. [Google Scholar] [CrossRef]
- Pi, M.; Zheng, L.; Luo, H.; Duan, S.; Li, C.; Yang, J.; Zhang, D.; Chen, S. Improved acetone gas sensing performance based on optimization of a transition metal doped WO3 system at room temperature. J. Phys. D Appl. Phys. 2021, 54, 155107. [Google Scholar] [CrossRef]
- Vogel, H. Ueber die Lichtempfindlichkeit des Bromsilbers für die sogenannten chemisch unwirksamen Farben. Ann. Phys. 1874, 226, 453–459. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Kamat, P.V. Quantum Dot Solar Cells. Semiconductor Nanocrystals as Light Harvestersy. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- Chatterjee, D.; Dasgupta, S. Visible light induced photocatalytic degradation of organic pollutants. J. Photochem. Photobiol. C 2005, 6, 205. [Google Scholar] [CrossRef]
- Yu, S.H.; Lee, Y.; Jang, S.K.; Kang, J.; Jeon, J.; Lee, C.; Lee, J.Y.; Kim, H.; Hwang, E.; Lee, S.; et al. Dye-Sensitized MoS2 Photodetector with Enhanced Spectral Photoresponse. ACS Nano 2014, 8, 8285–8291. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kim, S.; Yu, J.S. Drop-cast and dye-sensitized ZnO nanorod-based visible-light photodetectors. Phys. Status Solidi Rapid Res. Lett. 2013, 7, 659–663. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Komarneni, S. Highly sensitive, fast and reversible NO2 sensors at room-temperature utilizing nonplasmonic electrons of ZnO/Pd hybrids. Ceram. Int. 2020, 46, 8462–8468. [Google Scholar]
- Zheng, K.; Žídek, K.; Abdellah, M.; Zhang, W.; Chábera, P.; Lenngren, N.; Yartsev, A.; Pullerits, T. Ultrafast Charge Transfer from CdSe Quantum Dots to p-Type NiO: Hole Injection vs Hole Trapping. J. Phys. Chem. C 2014, 118, 18462–18471. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and Electrochemical Electron-Transfer Theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced electron transfer from semiconductor quantum dots to metal oxide nanoparticles. PNAS 2011, 108, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, Y.; Martinelli, E.; Catini, A.; Magna, G.; Pomarico, G.; Basoli, F.; Paolesse, R.; Di Natale, C. Gas-Sensitive Photoconductivity of Porphyrin-Functionalized ZnO Nanorods. J. Phys. Chem. C 2012, 116, 9151–9157. [Google Scholar] [CrossRef]
- Sivalingam, Y.; Magna, G.; Pomarico, G.; Catini, A.; Martinelli, E.; Paolesse, R.; Di Natale, C. The light enhanced gas selectivity of one-pot grown porphyrins coated ZnO nanorods. Sens. Actuators B 2013, 188, 475–481. [Google Scholar] [CrossRef]
- Magna, G.; Sivalingam, Y.; Babbi, A.; Martinelli, E.; Paolesse, R.; Di Natale, C. Drift Correction in a Porphyrin-coated ZnO Nanorods Gas Sensor. Procedia Eng. 2014, 87, 608–611. [Google Scholar] [CrossRef][Green Version]
- Mosciano, R.; Magna, G.; Catini, A.; Pomarico, G.; Martinelli, E.; Paolesse, R.; Di Natale, C. Room Temperature CO Detection by Hybrid Porphyrin-ZnO Nanoparticles. Procedia Eng. 2015, 120, 71–74. [Google Scholar] [CrossRef]
- Yang, M.; Wang, D.; Peng, L.; Zhao, Q.; Lin, Y.; Wei, X. Surface photocurrent gas sensor with properties dependent on Ru(dcbpy)2(NCS)2-sensitized ZnO nanoparticles. Sens. Actuators B 2006, 117, 80–85. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Nasriddinov, A.; Vladimirova, S.; Tokarev, S.; Fedorova, A.; Krylov, I.; Drozdov, K.; Baranchikov, A.; Gaskov, A. Photosensitive Organic-Inorganic Hybrid Materials for Room Temperature Gas Sensor Applications. Nanomaterials 2018, 8, 671. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Olivier, M.G.; Debliquy, M. Room temperature nitrogen dioxide sensors based on N719-dye sensitized amorphous zinc oxide sensors performed under visible-light illumination. Sens. Actuators B 2015, 209, 69–77. [Google Scholar] [CrossRef]
- Lukovskaya, E.V.; Fedorova, O.A.; Glazova, Y.A.; Fedorov, Y.V.; Anisimov, A.V.; Podolko, E.V.; Rumyantseva, M.N.; Gaskov, A.M.; Fages, F. Effect of light irradiation on the gas sensor characteristics of the SnO2 and ZnO modified by tetrathiafulvalene derivative. Org. Photonics Photovolt. 2015, 3, 54–63. [Google Scholar] [CrossRef]
- Tian, X.; Yang, X.; Yang, F.; Qi, T. A visible-light activated gas sensor based on perylenediimide-sensitized SnO2 for NO2 detection at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123621. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, D.; Zhou, J.; Ye, J.; Sun, Y.; Li, X.; Geng, Y.; Wang, J.; Du, Y.; Qian, Z. Visible light-activated room temperature NH3 sensor base on CuPc-loaded ZnO nanorods. Sens. Actuators B 2021, 327, 128911. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Wang, D.; Lin, Y.; Xie, T.; Zhai, J. Photoelectric properties of ZnO/Ag2S heterostructure and its photoelectric ethanol sensing characteristics. Mater. Chem. Phys. 2012, 133, 834–838. [Google Scholar] [CrossRef]
- Miao, Y.; Pan, G.; Sun, C.; He, P.; Cao, G.; Luo, C.; Zhang, L.; Li, H. Enhanced photoelectric responses induced by visible light of acetone gas sensors based on CuO-ZnO nanocomposites at about room temperature. Sens. Rev. 2018, 38, 311–320. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, D.; Peng, L.; Lin, Y.; Li, X.; Xie, T. Visible-light-induced photoelectric gas sensing to formaldehyde based on CdS nanoparticles- ZnO heterostructures. Sens. Actuators B 2010, 147, 234–240. [Google Scholar] [CrossRef]
- Zhai, J.; Wang, L.; Wang, D.; Li, H.; Zhang, Y.; He, D.; Xie, T. Enhancement of Gas Sensing Properties of CdS Nanowire/ZnO Nanosphere Composite Materials at Room Temperature by Visible-Light Activation. ACS Appl. Mater. Interfaces 2011, 3, 2253–2258. [Google Scholar] [CrossRef]
- Zou, Z.; Xie, C.; Zhang, S.; Yang, C.; Zhang, G.; Yang, L. CdS/ZnO nanocomposite film and its enhanced photoelectric response to UV and visible lights at low bias. Sens. Actuators B 2013, 188, 1158–1166. [Google Scholar] [CrossRef]
- Zou, Z.; Qiu, Y.; Xie, C.; Xu, J.; Luo, Y.; Wang, C.; Yan, H. CdS-TiO2 nanocomposite film and its enhanced photoelectric responses to dry air and formaldehyde induced by visible light at room temperature. J. Alloys Compd. 2015, 645, 17–23. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Srinivasan, P.; Jeyaprakash, B.G. Fabrication of highly selective formaldehyde sensor through a novel spray deposited ZnO/CdS heterostructured interface: A surface charge enhancement approach. J. Alloys Compd. 2018, 768, 1016–1028. [Google Scholar] [CrossRef]
- Wu, B.; Lin, Z.; Sheng, M.; Hou, S.; Xu, J. Visible-light activated ZnO/CdSe heterostructure-based gas sensors with low operating temperature. Appl. Surf. Sci. 2016, 360, 652–657. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Zhang, Z. Visible light assisted room-temperature NO2 gas sensor based on hollow SnO2-SnS2 nanostructures. Sens. Actuators B 2020, 324, 128754. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Liu, Z.; Zeng, Z.; Zhao, H.; Wang, X.; Xu, J. Light enhanced room temperature resistive NO2 sensor based on a gold-loaded organic-inorganic hybrid perovskite incorporating tin dioxide. Microchim. Acta 2019, 186, 47. [Google Scholar]
- Yu, W.W.; Qu, L.; Guo, W.; Peng, X. Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem. Mater. 2003, 15, 2854–2860. [Google Scholar] [CrossRef]
- Chen, O.; Chen, X.; Yang, Y.; Lynch, J.; Wu, H.; Zhuang, J.; Cao, Y.C. Synthesis of Metal Selenide Nanocrystals Using Selenium Dioxide as the Selenium Precursor. Angew. Chem. Int. Ed. 2008, 47, 8638–8641. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, R.B.; Dirin, D.N.; Gaskov, A.M. Semiconductor nanoparticles with spatial separation of charge carriers: Synthesis and optical properties. Russ. Chem. Rev. 2011, 80, 1139–1158. [Google Scholar] [CrossRef]
- Chizhov, A.; Vasiliev, R.; Rumyantseva, M.; Krylov, I.; Drozdov, K.; Batuk, M.; Hadermann, J.; Abakumov, A.; Gaskov, A. Light-Activated Sub-ppm NO2 Detection by Hybrid ZnO/QD Nanomaterials vs. Charge Localization in Core-Shell QD. Front. Mater. 2019, 6, 231. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuators B 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Il’in, A.S.; Fantina, N.P.; Martyshov, M.N.; Forsh, P.A.; Chizhov, A.S.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. Effect of cadmium-selenide quantum dots on the conductivity and photoconductivity of nanocrystalline indium oxide. Semiconductors 2016, 50, 607–611. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Marchevsky, A.V.; Karakulina, O.M.; Abakumov, A.M.; Gaskov, A.M. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Mordvinova, N.E.; Rumyantseva, M.N.; Krylov, I.V.; Drozdov, K.A.; Li, X.; Gaskov, A.M. The Effect of CdSe and InP Quantum Dots on the Interaction of ZnO with NO2 under Visible Light Irradiation. Russ. J. Inorg. Chem. 2018, 63, 512–518. [Google Scholar] [CrossRef]
- Hou, D.; Dev, A.; Frank, K.; Rosenauer, A.; Voss, T. Oxygen-Controlled Photoconductivity in ZnO Nanowires Functionalized with Colloidal CdSe Quantum Dots. J. Phys. Chem. C 2012, 116, 19604–19610. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Xia, Y.; Xiang, L. Near infrared light enhanced room-temperature NO2 gas sensing by hierarchical ZnO nanorods functionalized with PbS quantum dots. Sens. Actuators B 2018, 255, 2538–2545. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Drozdov, K.; Krylov, I.; Batuk, M.; Hadermann, J.; Filatova, D.; Khmelevsky, N.; Kozlovsky, V.; Maltseva, L.; et al. Photoresistive gas sensor based on nanocrystalline ZnO sensitized with colloidal perovskite CsPbBr3 nanocrystals. Sens. Actuators B 2021, 329, 129035. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Q.; Wang, B.; Yao, J.D.; Wang, G.W. Light-controlled C2H2 gas sensing based on Au ZnO nanowires with plasmon-enhanced sensitivity at room temperature. J. Mater. Chem. C 2015, 3, 7067–7074. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Q.; Liu, W. Au@ZnO nanostructures on porous silicon for photocatalysis and gas-sensing: The effect of plasmonic hot-electrons driven by visible-light. Mater. Res. Express 2016, 3, 085006. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, G.; Xu, M.; Su, Y.; Tai, H.; Du, H.; Jiang, Y. Visible light-assisted room temperature gas sensing with ZnO-Ag heterostructure nanoparticles. Sens. Actuators B 2018, 259, 269–281. [Google Scholar] [CrossRef]
- Xu, F.; Lv, H.F.; Wu, S.Y.; HO, H.P. Light-activated gas sensing activity of ZnO nanotetrapods enhanced by plasmonic resonant energy from Au nanoparticles. Sens. Actuators B 2018, 259, 709–716. [Google Scholar] [CrossRef]
- Cattabiani, N.; Baratto, C.; Zappa, D.; Comini, E.; Donarelli, M.; Ferroni, M.; Ponzoni, A.; Faglia, G. Tin Oxide Nanowires Decorated with Ag Nanoparticles for Visible Light-Enhanced Hydrogen Sensing at Room Temperature: Bridging Conductometric Gas Sensing and Plasmon-Driven Catalysis. J. Phys. Chem. C 2018, 122, 5026–5031. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Banik, M.; Gogurla, N.; Santra, S.; Ray, S.K.; Mukherjee, R. Light Trapping-Mediated Room-Temperature Gas Sensing by Ordered ZnO Nano Structures Decorated with Plasmonic Au Nanoparticles. ACS Omega 2019, 4, 12071–12080. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.; Xie, G.; Yao, M.; Pan, H.; Du, H.; Tai, H.; Du, X.; Su, Y. Enhancing visible light-activated NO2 sensing properties of Au NPs decorated ZnO nanorods by localized surface plasmon resonance and oxygen vacancies. Mater. Res. Express 2020, 7, 015924. [Google Scholar] [CrossRef]
- Wang, J.; Fan, S.; Xia, Y.; Yang, C.; Komarneni, S. Room-temperature gas sensors based on ZnO nanorod/Au hybrids: Visible-light-modulated dual selectivity to NO2 and NH3. J. Hazard. Mater. 2020, 381, 120919. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.A.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Barry, T.I.; Stone, F.S. The reactions of oxygen at dark and irradiated zinc oxide surfaces. Proc. R. Soc. Lond. A 1960, 255, 124–144. [Google Scholar]
- Klimovskii, A.O.; Lisachenko, A.A. Determination of the kinetic parameters for the photoadsorption and photodesorption of oxygen on zinc oxide. Kinet. Catal. 1991, 32, 373–377. [Google Scholar]
- Rodriguez, J.A.; Jirsak, T.; Dvorak, J.; Sambasivan, S.; Fischer, D. Reaction of NO2 with Zn and ZnO: Photoemission, XANES, and Density Functional Studies on the Formation of NO3. J. Phys. Chem. B 2000, 104, 319–328. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsumoto, K.; Ohgaki, T.; Sakaguchi, I.; Ohashi, N.; Hishita, S.; Haneda, H. Development of ZnO-based surface plasmon resonance gas sensor and analysis of UV irradiation effect on NO2 desorption from ZnO thin films. J. Ceram. Soc. Jpn. 2010, 118, 193–196. [Google Scholar] [CrossRef]
- Peng, L.; Xie, T.F.; Yang, M.; Wang, P.; Xu, D.; Pang, S.; Wang, D.J. Light induced enhancing gas sensitivity of copper-doped zinc oxide at room temperature. Sens. Actuators B 2008, 131, 660–664. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Q.; Wang, D.; Zhai, J.; Wang, P.; Pang, S.; Xie, T. Ultraviolet-assisted gas sensing: A potential formaldehyde detection approach at room temperature based on zinc oxide nanorods. Sens. Actuators B 2009, 136, 80–85. [Google Scholar] [CrossRef]
- Fan, S.-W.; Srivastava, A.K.; Dravid, V.P. UV-activated room-temperature gas sensing mechanism of polycrystalline ZnO. Appl. Phys. Lett. 2009, 95, 142106. [Google Scholar]
- Peng, X.; He, Z.; Yang, K.; Chen, X.; Wang, X.; Dai, W.; Fu, X. Correlation between donating or accepting electron behavior of the adsorbed CO or H2 and its oxidation over TiO2 under ultraviolet light irradiation. Appl. Surf. Sci. 2016, 360, 698–706. [Google Scholar]
- Wang, C.Y.; Kinzer, M.; Youn, S.K.; Ramgir, N.; Kunzer, M.; Kohler, K.; Zacharias, M.; Cimalla, V. Oxidation behaviour of carbon monoxide at the photostimulated surface of ZnO nanowires. J. Phys. D 2011, 44, 305302. [Google Scholar] [CrossRef]
- Sutka, A.; Eglitis, R.; Kuzma, A.; Smits, K.; Zukuls, A.; Prades, J.D.; Fabrega, C. Photodoping-Inspired Room-Temperature Gas Sensing by Anatase TiO2 Quantum Dots. ACS Appl. Nano Mater. 2021, 4, 2522–2527. [Google Scholar] [CrossRef]
- Gong, B.; Shi, T.; Zhu, W.; Liao, G.; Li, X.; Huang, J.; Zhou, T.; Tang, Z. UV irradiation-assisted ethanol detection operated by the gas sensor based on ZnO nanowires/optical fiber hybrid structure. Sens. Actuators B 2017, 245, 821–827. [Google Scholar]
- Casals, O.; Markiewicz, N.; Fabrega, C.; Gràcia, I.; Cane, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. A Parts Per Billion (ppb) Sensor for NO2 with Microwatt (μW) Power Requirements Based on Micro Light Plates. ACS Sens. 2019, 4, 822–826. [Google Scholar] [CrossRef]
- Markiewicz, N.; Casals, O.; Fabrega, C.; Gràcia, I.; Cané, C.; Wasisto, H.S.; Waag, A.; Prades, J.D. Micro light plates for low-power photoactivated (gas) sensors. Appl. Phys. Lett. 2019, 114, 053508. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.; Mo, H.; Kim, T.; Jeong, T.; Youn, C.J.; Hong, K.J. Sensing Mechanism and Behavior of Sputtered ZnCdO Ozone Sensors Enhanced by Photons for Room- Temperature Operation. J. Electron. Mater. 2013, 42, 720–725. [Google Scholar] [CrossRef]
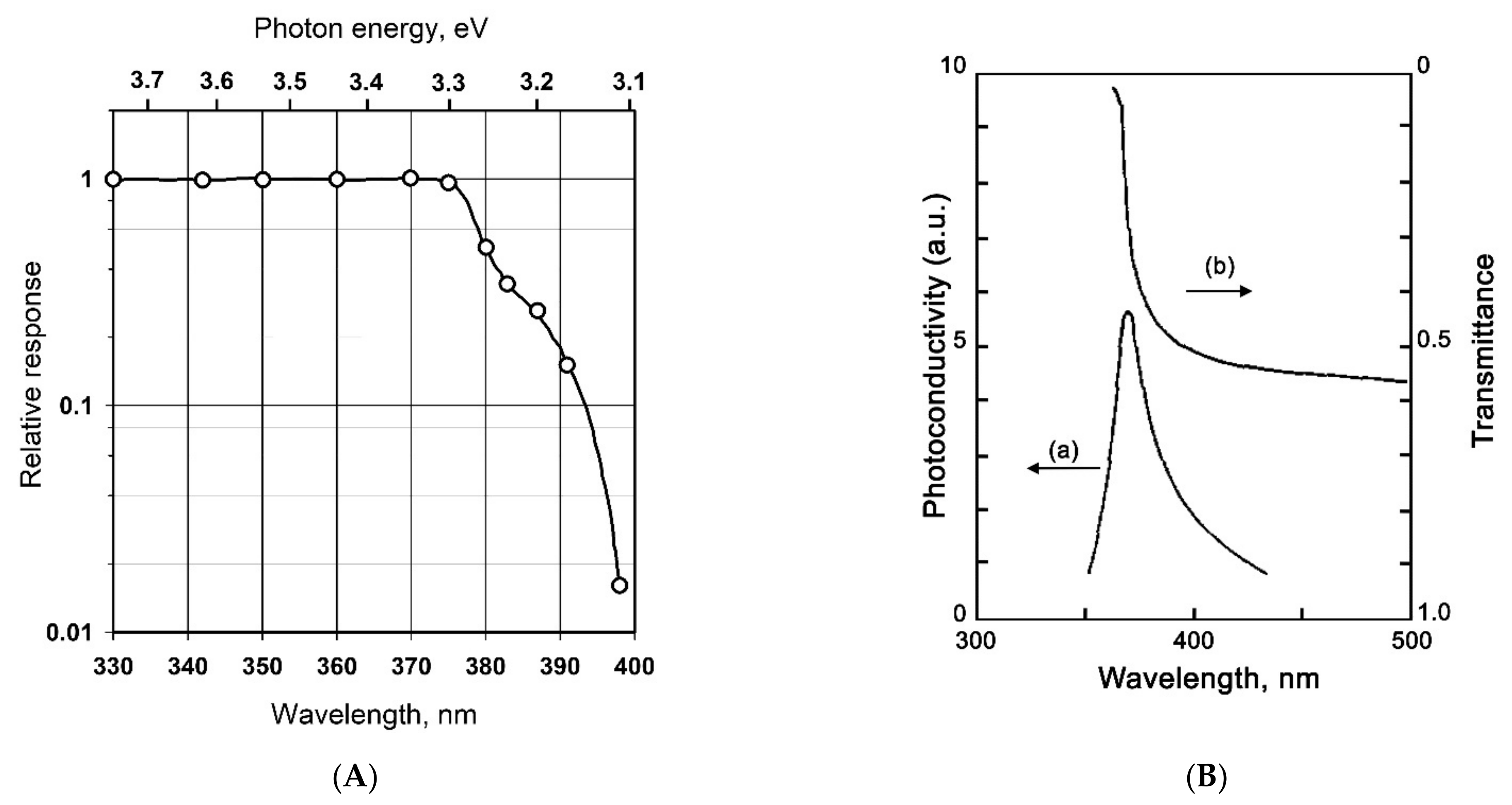
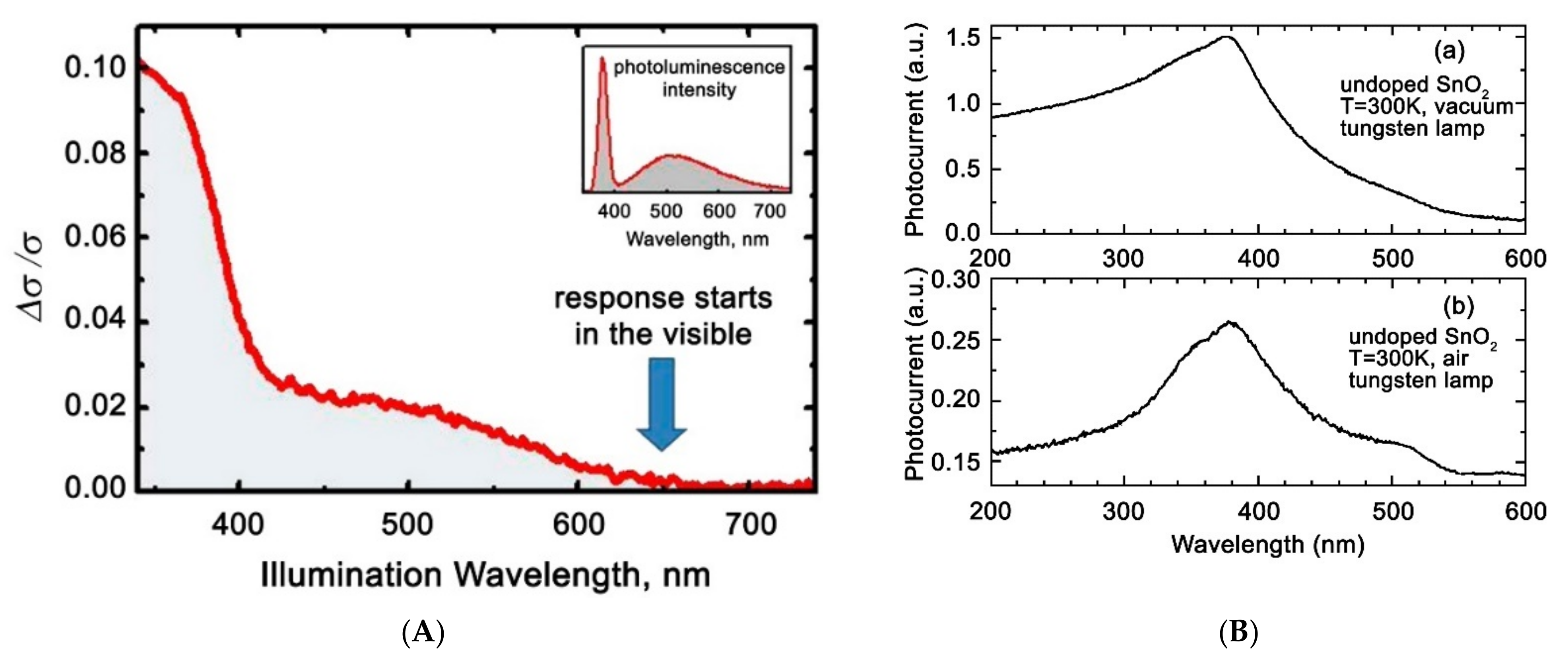
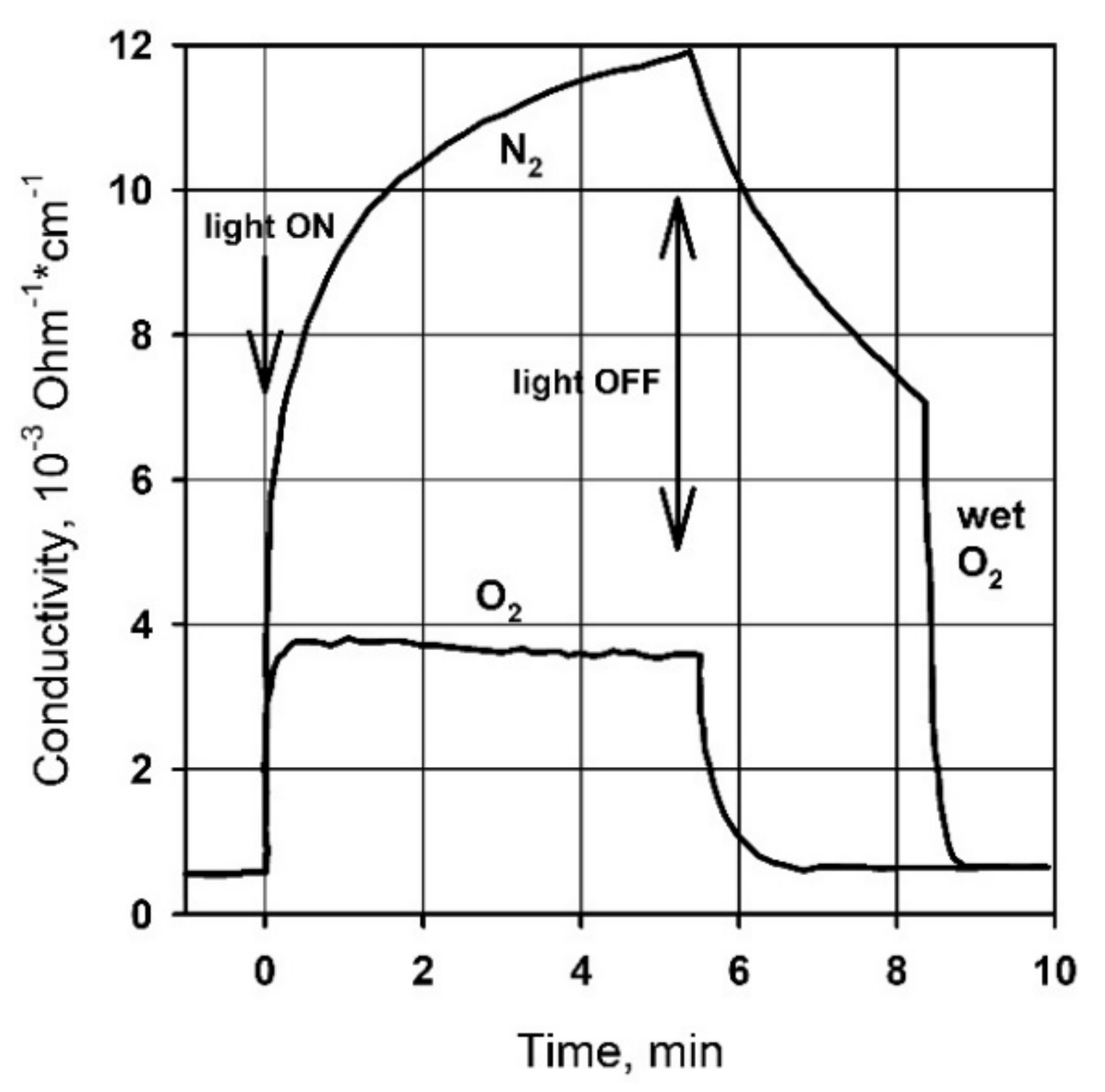
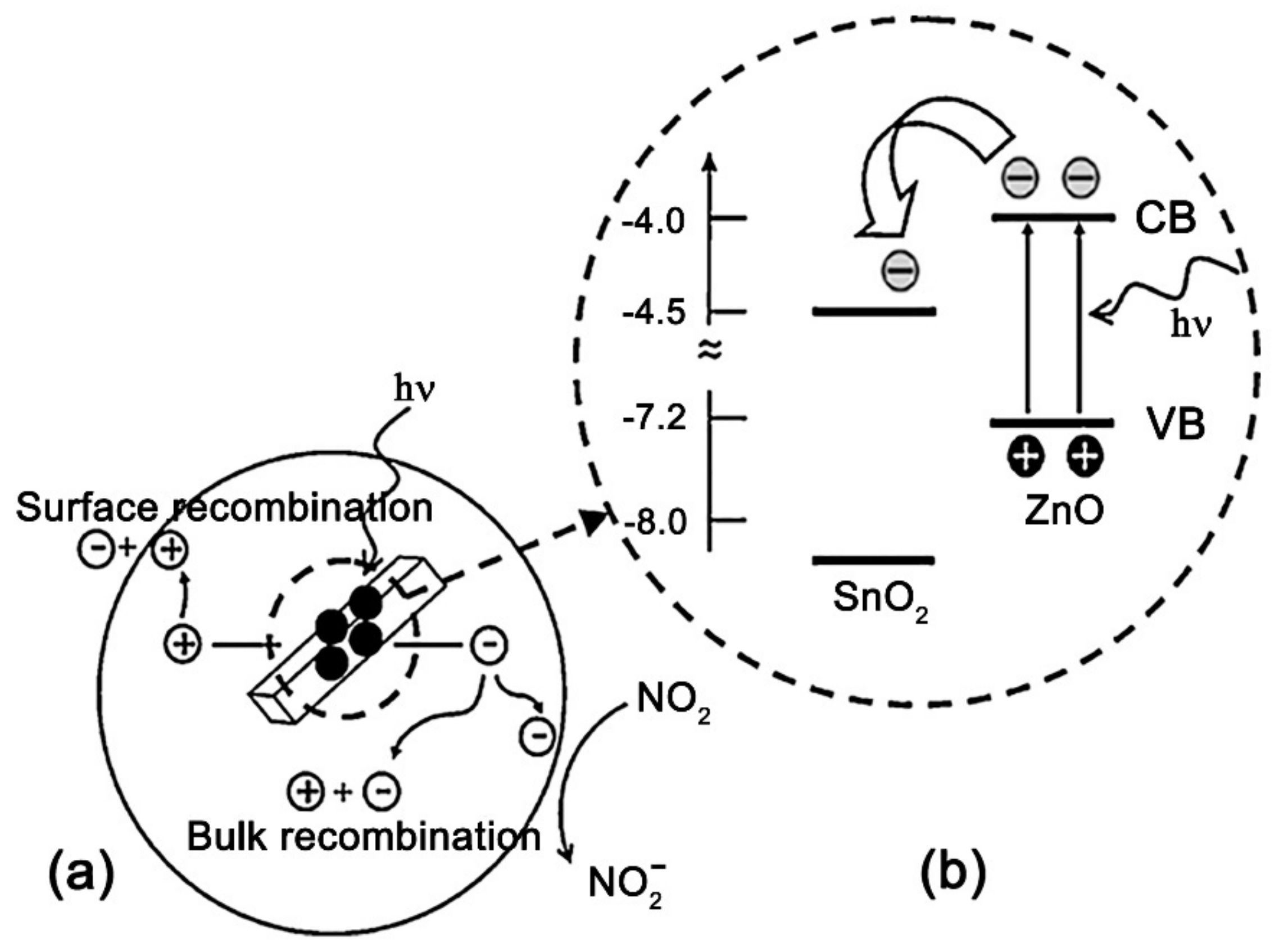


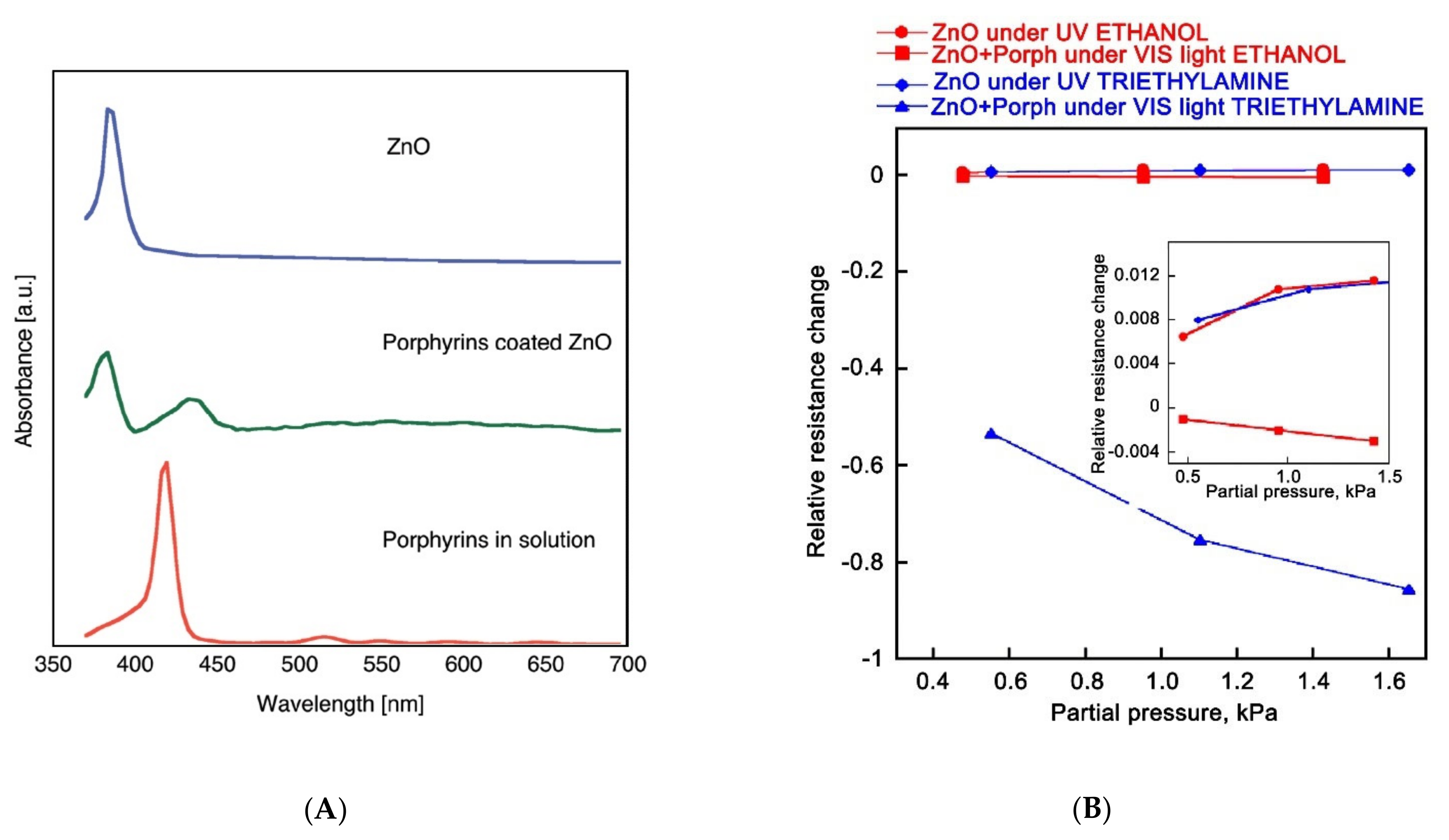
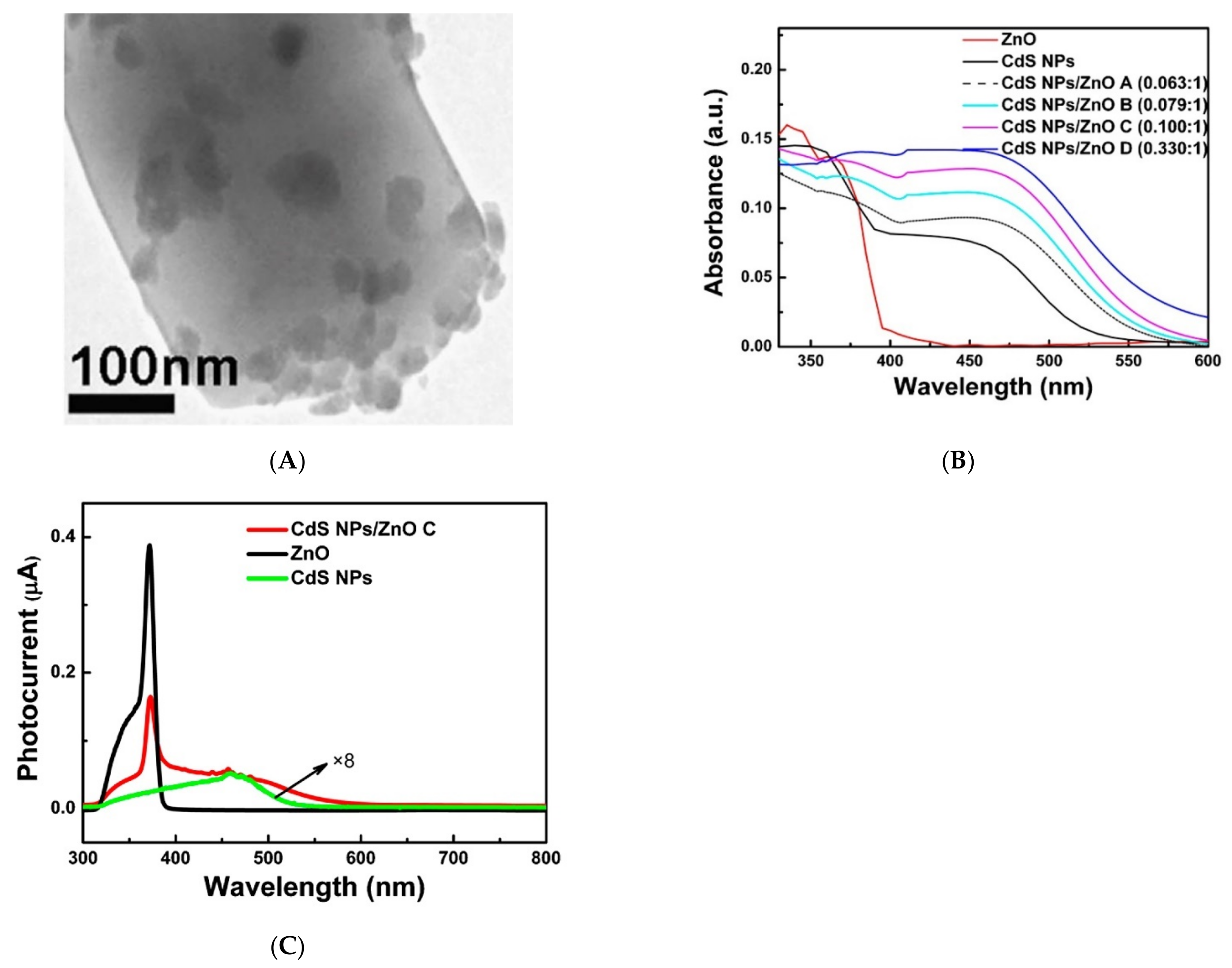


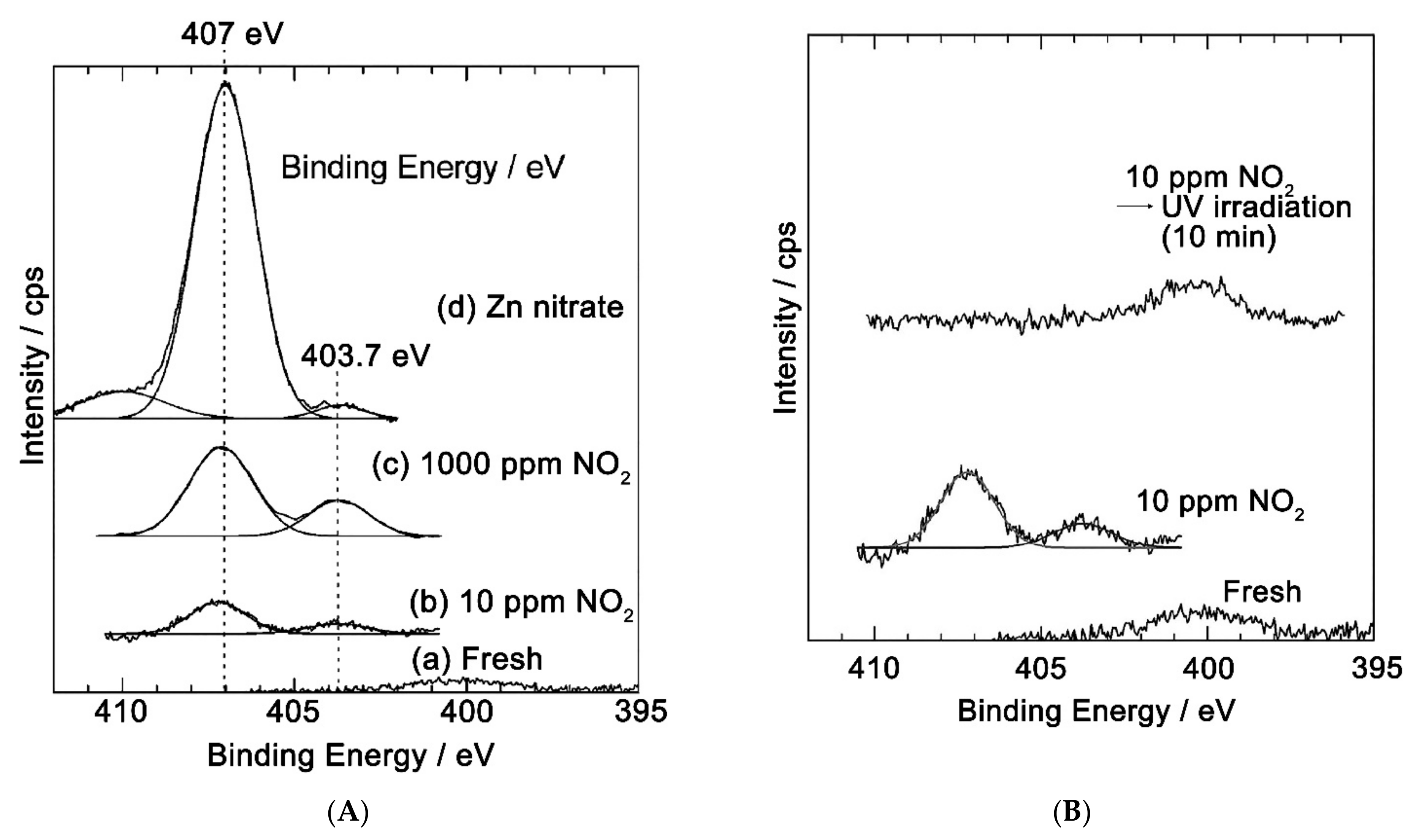
| Metal Oxide | SnO2 | ZnO | TiO2 | In2O3 | WO3 |
|---|---|---|---|---|---|
| Crystal structure | rutile | wurzite | rutile | bixbyite | monoclinic |
| Eg, eV | 3.5 d [11] | 3.4 d [12] | 3.2 i [13] | 3.75 d, 2.6 i,f [14] | 3.5 d, 2.6–2.8 d,i [15] |
| No | Sensing Material | Synthesis Method | Detected Gas | Concentration, Ppm | Temperature, °C | Irradiation Parameters | Sensor Signal 1 | Refs |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | SnO2 | spray pyrolysis | AC | 54 | RT | Mercury lamp, >200 nm, 20 mW/cm2 | 1.8 | [31] |
| 3 | nanocones SnO2 | hydrothermal | H2 | 100 | 50 | LED, 313 nm, 40W/m2 | 9.7 | [35] |
| 4 | SnO2 | RF sputtering | O3 | 5 | RT | LED, 370 nm | 1.3 | [36] |
| 5 | In2O3 | MOCVD | O3 | 0.5 | RT | LED, 400 nm | 1.6 | [37] |
| 6 | MP In2O3 | nanocasting | NO2 | 5 | 50 | LED, 400 nm | 7 | [38] |
| 7 | In2O3 | electrospinning | NO2 | 1 | RT | LED, 400 nm | 60 | [39] |
| 8 | In2O3 | sol-gel | NO2 | 8 | RT | LED, 380 nm, 5 mW/cm2 | 180 | [40] |
| 9 | In2O3 nanooctaedra | CVD | NO2 | 0.5 | RT | LED, 325 nm, 400 μW | 1.8 | [41] |
| 10 | walnut-like In2O3 | hydrolysis | NO2 | 2 | RT | LED 365 nm, 1.2 mW/cm2 | 3.6 | [42] |
| 11 | MP In2O3 NRs | hydrothermal | NO2 | 1 | RT | 365 nm, 6 W | 20.9 | [43] |
| 12 | ZnO hollow MSp | template synthesis | EtOH | 100 | 80 | LED 360 nm, 2 mW/cm2 | 11 | [45] |
| 13 | ZnO NFs | electrospinning | FA | 100 | RT | LED 365 nm | 12.61 | [46] |
| 14 | ZnO NRs | hydrothermal | H2S | 25 | RT | LED 354 nm, 1.22 μW/cm2 | 3.55 | [48] |
| 15 | ZnO NFs | electrospinning | EtOH | 60 | RT | Mercury lamp, 365 nm | 1.75 | [49] |
| 16 | WO3 NFs | electrospinning | AC | 12.5 | 350 | LED 365 nm, 2.024 mW/cm2 | 1.7 | [56] |
| 17 | WO3/Au | RF sputtering | NO2 | 10 | RT | LED 400 nm, 15 mW/cm2 | ~2.2 | [57] |
| 18 | TiO2 | Degussa P25 | FA | 100 | RT | LED 365 nm, 36 W/m2 | 9385.5 | [61] |
| 19 | TiO2 | RF sputtering | NO2 | 100 | RT | LED 365 nm | 2.3 | [63] |
| 20 | TiO2 NFs | electrospinning | H2 | 100 | 190 | UV lamp, 300-400 nm, 3.25 mW/cm2 | 45 | [64] |
| 21 | ZnO/Au NShs | sputtering | NO2 | 1 | RT | 365 nm, 1.2 mW/cm2 | 2.0525 | [65] |
| 22 | ZnO NWs /Au | sputtering | EtOH | 100 | RT | 254 mn, 4.1 mW/cm2 | 1.18 | [66] |
| 23 | ZnO/Au | RF sputtering | H2 | 1000 | 250 | 365 nm | 1.72 | [67] |
| 24 | ZnO/Au NRs | thermal evaporation | O3 | 0.03 | RT | LED 370 nm; 200 μW | ~108 | [68] |
| 25 | ZnO/Ag | CBD | NO2 | 5 | RT | LED 365 nm, 8 mW/cm2 | 1.98 | [70] |
| 23 | ZnO/g-C3N4 | in situ precipitation | EtOH | 104 | RT | 365 nm | 4.26 | [71] |
| 24 | SnO2/Pt clusters | RF sputtering | LPG | 200 | RT | UV lamp, 365 nm, 2 μW/cm2 | 4374.4 | [72] |
| 25 | SnO2/Pd | wet-impregnation | NO2 | 5 | 30 | LED 365 nm, 7 mW/cm2 | 1655 | [74] |
| 26 | SnO2/rGO hollow NFs | electrospinning | NO2 | 3 | RT | UV lamp, 365 nm, 97 mW/cm2 | ~2 | [78] |
| 27 | ZnO/In2O3 | coprecipitation | NO2 | 5 | RT | LED 365 nm, 25 mW/cm2 | 3.21 | [81] |
| 28 | ZnO/SnO2 | ball milling | EtOH | 10 | 250 | LED 380 nm, 60 mW | 10 | [83] |
| 29 | ZnO/SnO2 NRs | CBD | NO2 | 0.5 | 20 | LED, 380 nm | ~1065 | [84] |
| 30 | ZnO/SnO2 | hydrothermal | O3 | 0.02 | 26 | LED 325nm; 200 μW | 8 | [85] |
| 31 | ZnO/SnO2 hollow NSp | hydrothermal | FA | 100 | RT | LED 365 nm, 2 mW | ~8 | [86] |
| 32 | ZnO/SnO2 NFs | electrospinning | FA | 50 | RT | LED 365 nm | 2.3 | [88] |
| 33 | TiO2/SnO2 | ALD | FA | 0.6 | RT | LED 365 nm, 10 mW/cm2 | ~5 | [89] |
| 34 | SnO2/GaN NWs | MBE / RF sputtering | methanol | 500 | RT | Deut. lamp, 215-400 nm, 3.25 nW/cm2 | ~1.016 | [91] |
| No | Sensing Material | Synthesis Method | Detected Gas | Concentration, Ppm | Temperature, °C | Irradiation Parameters | Sensor Signal 1 | Refs |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 1 | SnO2-x | magnetron sputtering | EtOH | 4.5 | 155 | blue LED | 0.286 | [96] |
| 2 | WO3 | commercial powder | NO2 | 0.16 | RT | LED 580 nm, 340 mW/cm2 | 9.2 | [98] |
| 3 | WO3 NFs | electrospinning | NO2 | 0.4 | 75 | LED 430 nm, 770 μW/cm2 | 12.4 | [99] |
| 4 | MP WO3 | template synthesis | AC | 100 | 25 | 475 nm, 40W/m2 | 7.5 | [100] |
| 5 | WO3/PdO | hydrothermal/thermal | H2 | 40 | RT | LED 480 nm, 0.15 W/cm2 | 9.02 | [75] |
| 6 | ZnO | commercial powder | AC | 900 | 25 | visible LED | 1.2 | [101] |
| 7 | ZnO/In2O3 | hydrothermal | FA | 100 | RT | monochromator, 460 nm, 0.213 mW/cm2 | 4.19 | [102] |
| 8 | ZnO/In2O3 | solvothermal | EtOH | 100 | 260 | Xe lamp, >420 nm | 68.19 | [82] |
| 9 | ZnO | ball milling | FA | 100 | RT | white LED (400–800 nm, 35.5 mW/cm2) | 2.33 | [103] |
| 10 | MP In2O3 | nanocasting | O3 | 0.22 | RT | LED 460 nm, light int. approx. 10 cd | 120 | [104] |
| 11 | V2O5 thin film | spray pyrolysis | AC | sat. vapour | RT | green laser, 200 mW/m2 | 3.53 | [117] |
| 12 | Fe-doped ZnO (1%) | hydrothermal | FA | 100 | RT | laser 532 nm, 20 mW/cm2 | 2.87 | [121] |
| 13 | Co-doped ZnO (1%) | coprecipitation | EtOH | 18421 | RT | monochromator, 630 nm | 100 | [122] |
| 14 | ZnO/porph. complex | dip casting | triethylamine | 5500 | RT | white LED, | ~1.55 | [136] |
| 15 | ZnO/RuN3 | drop casting | CO | 27894 | RT | monochromator, 545 nm | 1.5 | [140] |
| 16 | In2O3/ Ru(II) complex | drop casting | NO2 | 2 | RT | LED, 630 nm | 100 | [141] |
| 17 | ZnO/N719-dye | dip casting | NO2 | 1.25 | RT | LED, 480 nm, 370 mW/cm2 | 1.43 | [142] |
| 18 | SnO2/PI | impregnation | NO2 | 0.5 | 30 | white LED, 400-700 nm, 3W | 131.6 | [144] |
| 19 | ZnO/Ag2S | cation exchange | EtOH | 500 | RT | laser 532 nm, 2 mW/cm2 | 45 | [146] |
| 20 | CuO(4.17%)/ZnO | sol-gel | AC | 500 | 30 | Xe lamp, 420-780 nm | 201.74 | [147] |
| 21 | ZnO/CdS | CBD | FA | 660 | RT | Xe lamp, >450 nm cut-off filter | 3.81 | [148] |
| 22 | CdS/TiO2 | SILAR | FA | 100 | RT | LED, 400–800 nm, 35.5 mW/cm2 | 2.54 | [151] |
| 23 | CdS/ZnO | liquid plasma spray | NO2 | 1 | RT | LED 510 nm, 50 mW/cm2 | 31.9 | [153] |
| 24 | ZnO/CdS | spray pyrolysis | FA | 10 | 29 | 400–800nm, 34.01mW/cm2 | 2.646 | [153] |
| 25 | ZnO/CdSe nanoribbons | therm. decomposition | EtOH | 25 | 160 | Xe lamp, 12.18 mW | 11 | [154] |
| 26 | ZnO/CdS@ZnTe QDs | drop cast | NO2 | 1 | RT | LED 535 nm, 20 mW/cm2 | 18 | [160] |
| 27 | ZnO/CdSe QDs | drop cast | NO2 | 0.85 | RT | LED 535 nm, 20 mW/cm2 | 20 | [161] |
| 28 | ZnO/InP QDs | drop cast | NO2 | 1 | RT | LED 535 nm, 20 mW/cm2 | 10.2 | [164] |
| 29 | ZnO /PbS QDs | CBD | NO2 | 1 | RT | LED 850 nm, 1 mW/cm2 | 1.24 | [166] |
| 30 | ZnO/Au NWs | CVD / sputtering | C2H2 | 100 | RT | laser 532 nm | ~1.2 | [169] |
| 31 | ZnO/Au NRs | sputtering | NH3 | 500 | RT | > 400 nm, 60 mW/cm2 | 1.68 | [170] |
| 32 | ZnO/Au | sputtering-annealing | EtOH | 500 | RT | mercury lamp, 600 mW/cm2 | 62 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chizhov, A.; Rumyantseva, M.; Gaskov, A. Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials 2021, 11, 892. https://doi.org/10.3390/nano11040892
Chizhov A, Rumyantseva M, Gaskov A. Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials. 2021; 11(4):892. https://doi.org/10.3390/nano11040892
Chicago/Turabian StyleChizhov, Artem, Marina Rumyantseva, and Alexander Gaskov. 2021. "Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges" Nanomaterials 11, no. 4: 892. https://doi.org/10.3390/nano11040892
APA StyleChizhov, A., Rumyantseva, M., & Gaskov, A. (2021). Light Activation of Nanocrystalline Metal Oxides for Gas Sensing: Principles, Achievements, Challenges. Nanomaterials, 11(4), 892. https://doi.org/10.3390/nano11040892








