Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio scaber: A Focus on Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis and Characterisation
2.2. Experimental Animals
2.3. Feeding Experiment
2.4. Injection Experiment
2.5. Haemolymph Collection
2.6. Immune Parameters
2.7. Statistical Analysis
3. Results
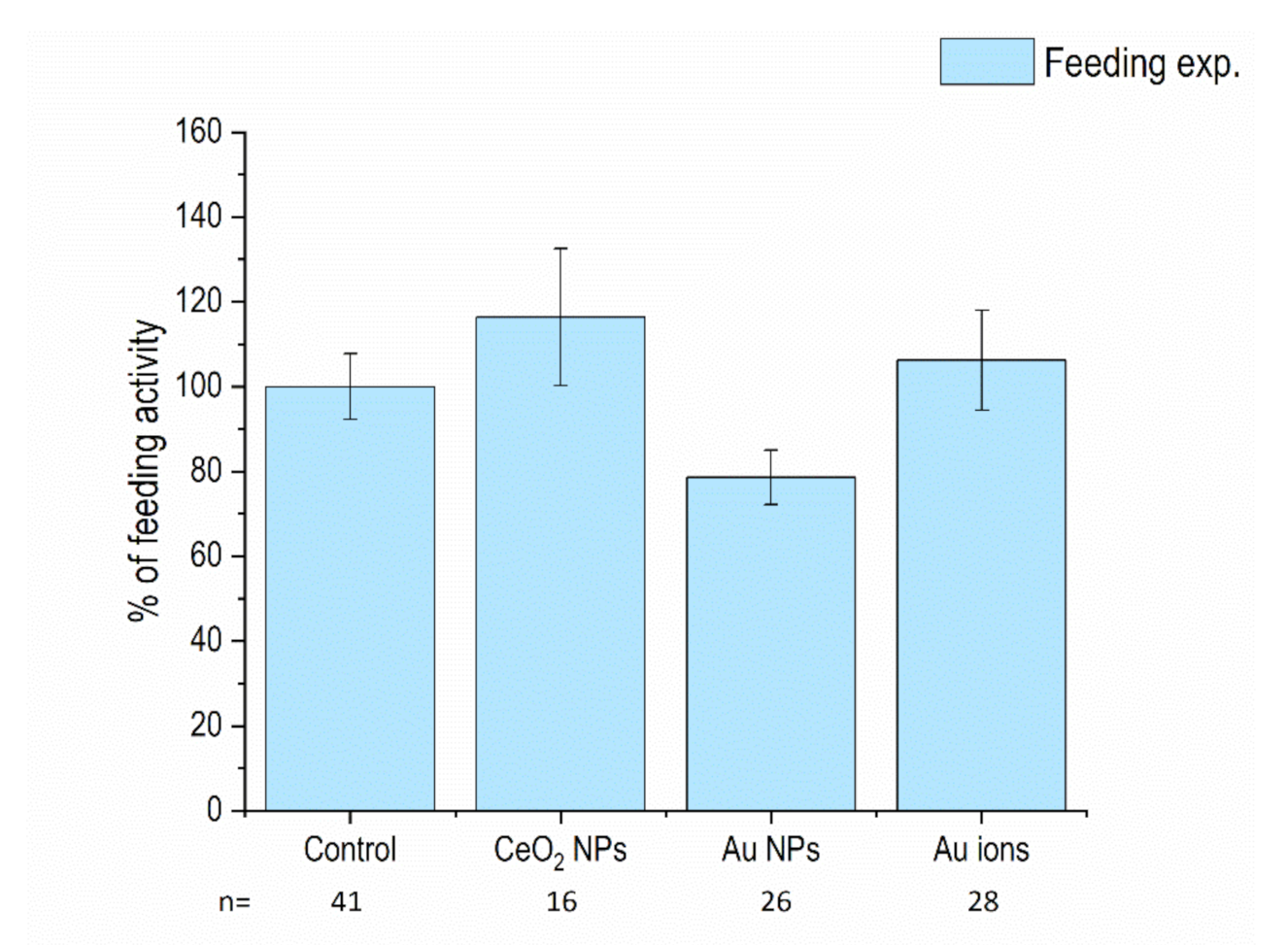
3.1. Feeding Activity of Isopods and Mortality
3.2. NO Levels and PO-Like Activity
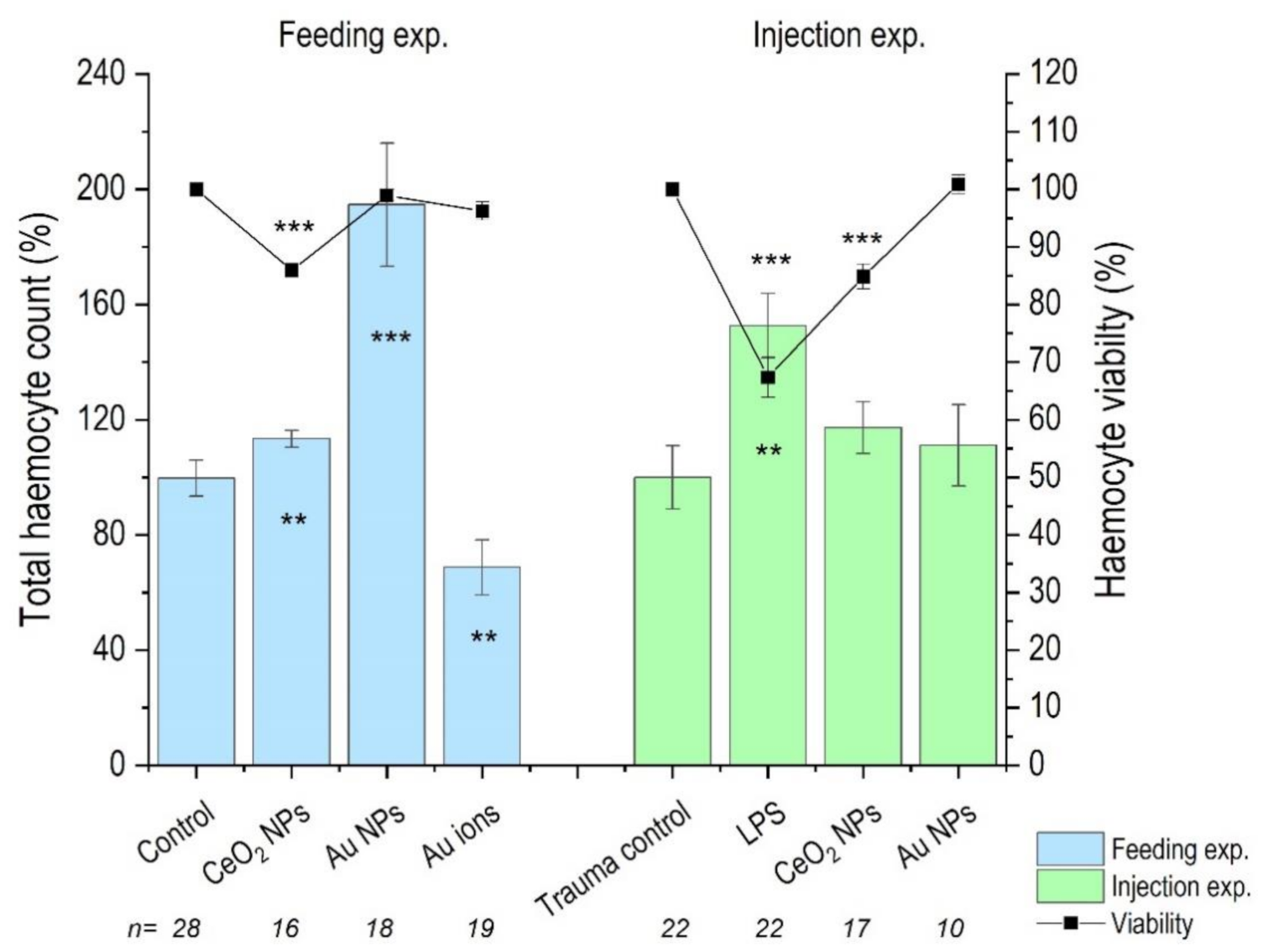
3.3. Haemocyte Count
4. Discussion
4.1. Feeding on Au NP-Dosed Diet
4.2. Feeding on CeO2 NP-Dosed Diet
4.3. Feeding on Au Ions-Dosed Diet
4.4. Injection of Stimuli into Dlood
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Biological Repeats (Number of Animals Used) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment (Feeding) | Number of Animals (Start of 2 Weeks) | Mortality | Removed (Gravid/Molting/Infected) | Feeding Activity | Haemocyte Count | Nitric Oxide * | Phenoloxidase-Like Activity ** | |
| Control | Total | 50 | 4 | 5 | 41 | 28 | 7 (32) | 13 (27) |
| 30 | 2 | 4 | 24 | 11 | ||||
| 20 | 2 | 1 | 17 | 17 | 1 (5) | 2 (4) | ||
| 6 (27) | ||||||||
| 11 (23) | ||||||||
| Cerium NPs | Total | 20 | 3 | 1 | 16 | 16 | 7 (32) | 14 (29) |
| 20 | 3 | 1 | 16 | 16 | 2 (9) | 2 (5) | ||
| 5 (23) | ||||||||
| 12 (24) | ||||||||
| Gold NPs | Total | 30 | 2 | 2 | 26 | 18 | 8 (34) | 12 (20) |
| 30 | 2 | 2 | 26 | 18 | ||||
| 8 (34) | ||||||||
| 12 (20) | ||||||||
| Gold Ions | Total | 30 | 2 | 0 | 28 | 19 | 8 (33) | 11 (15) |
| 30 | 2 | 0 | 28 | 19 | ||||
| 8 (33) | ||||||||
| 11 (15) | ||||||||
| Biological Repeats (Number of Animals Used) | ||||
|---|---|---|---|---|
| Treatment (Injected) | Haemocyte Count | Nitric Oxide * | Phenoloxidase-Like Activity ** | |
| Control | Total | 22 | 7 (31) | 23 (47) |
| 22 | 5 (8) | |||
| 6 (13) | ||||
| 7 (31) | 5 (13) | |||
| 4 (8) | ||||
| 3 (5) | ||||
| LPS | Total | 22 | 7 (35) | 23 (61) |
| 22 | ||||
| 6 (17) | ||||
| 5 (14) | ||||
| 3 (15) | 6 (14) | |||
| 6 (16) | ||||
| 4 (20) | ||||
| Cerium NPs | Total | 17 | 8 (38) | 12 (26) |
| 17 | 2 (9) | 9 (19) | ||
| 2 (10) | ||||
| 4 (19) | ||||
| 3 (7) | ||||
| Gold NPs | Total | 10 | 5 (24) | 12 (22) |
| 10 | 5 (9) | |||
| 1 (5) | ||||
| 4 (19) | 7 (13) | |||
References
- Usharauli, D.; Kamala, T. An identical mechanism governs self-nonself discrimination and effector class regulation. PeerJ Prepr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Schultz, K.T.; Grieder, F. Structure and Function of the Immune System. Toxicol. Pathol. 1987, 15, 262–264. [Google Scholar] [CrossRef] [Green Version]
- Boraschi, D.; Alijagic, A.; Auguste, M.; Barbero, F.; Ferrari, E.; Hernadi, S.; Mayall, C.; Michelini, S.; Navarro Pacheco, N.I.; Prinelli, A.; et al. Addressing Nanomaterial Immunosafety by Evaluating Innate Immunity across Living Species. Small 2020, 16, 2000598. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar] [CrossRef]
- Dolar, A.; Kostanjšek, R.; Mayall, C.; Drobne, D.; Kokalj, A.J. Modulations of immune parameters caused by bacterial and viral infections in the terrestrial crustacean Porcellio scaber: Implications for potential markers in environmental research. Dev. Comp. Immunol. 2020, 113. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Chen, F.Y.; Thilagam, H.; Qiao, K.; Xu, W.F.; Wang, K.J. Modulation and interaction of immune-associated parameters with antioxidant in the immunocytes of crab Scylla paramamosain challenged with lipopolysaccharides. Evidence-based Complement. Altern. Med. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Kostanjšek, R.; Pirc Marolt, T. Pathogenesis, tissue distribution and host response to Rhabdochlamydia porcellionis infection in rough woodlouse Porcellio scaber. J. Invertebr. Pathol. 2015, 125, 56–67. [Google Scholar] [CrossRef]
- Chevalier, F.; Herbinière-Gaboreau, J.; Bertaux, J.; Raimond, M.; Morel, F.; Bouchon, D.; Grève, P.; Braquart-Varnier, C. The immune cellular effectors of terrestrial isopod armadillidium vulgare: Meeting with their invaders, wolbachia. PLoS ONE 2011, 6, e18531. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Herbinière-Gaboreau, J.; Charif, D.; Mitta, G.; Gavory, F.; Wincker, P.; Grève, P.; Braquart-Varnier, C.; Bouchon, D. Feminizing Wolbachia: A transcriptomics approach with insights on the immune response genes in Armadillidium vulgare. BMC Microbiol. 2012, 12, S1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Li, W.; Xu, M.; Zhu, Y.; Zhou, Y.; Wang, Q. Transcriptome-wide analysis of immune responses in Eriocheir sinensis hemocytes after challenge with different microbial derivatives. Dev. Comp. Immunol. 2019, 101. [Google Scholar] [CrossRef] [PubMed]
- Snyman, R.G.; Odendaal, J.P. Effect of cadmium on haemocyte viability of the woodlouse Porcellio laevis (Isopoda, Crustacea). Bull. Environ. Contam. Toxicol. 2009, 83, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Hornung, E. Evolutionary adaptation of oniscidean isopods to terrestrial life: Structure, physiology and behavior. Terr. Arthropod Rev. 2011, 4, 95–130. [Google Scholar] [CrossRef]
- Moret, Y.; Moreau, J. The immune role of the arthropod exoskeleton. Invertebr. Surviv. J. 2012, 9, 200–206. [Google Scholar]
- Mount, A.S.; Wheeler, A.P.; Paradkar, R.P.; Snider, D. Hemocyte-Mediated Shell Mineralization in the Eastern Oyster. Science 2004, 304, 297–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Liu, Y.; Liu, C.; Huang, J.; Zheng, G.; Xie, L.; Zhang, R. Hemocytes participate in calcium carbonate crystal formation, transportation and shell regeneration in the pearl oyster Pinctada fucata. Fish Shellfish Immunol. 2016, 51, 263–270. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Rebelo, M.; de Souza Figueiredo, E.; Mariante, R.M.; Nóbrega, A.; de Barros, C.M.; Allodi, S. New Insights from the Oyster Crassostrea rhizophorae on Bivalve Circulating Hemocytes. PLoS ONE 2013, 8, e57384. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, P.G.C.; De Abreu, I.S.; Cavalcante, L.A.; De Barros, C.M.; Allodi, S. Role of hemocytes in invertebrate adult neurogenesis and brain repair. Invertebr. Surviv. J. 2015, 12, 142–154. [Google Scholar]
- Russo, J.; Brehélin, M.; Carton, Y. Haemocyte changes in resistant and susceptible strains of D. melanogaster caused by virulent and avirulent strains of the parasitic wasp Leptopilina boulardi. J. Insect Physiol. 2001, 47, 167–172. [Google Scholar] [CrossRef]
- Du, M.H.; Yan, Z.W.; Hao, Y.J.; Yan, Z.T.; Si, F.L.; Chen, B.; Qiao, L. Suppression of Laccase 2 severely impairs cuticle tanning and pathogen resistance during the pupal metamorphosis of Anopheles sinensis (Diptera: Culicidae). Parasites Vectors 2017, 10, 171. [Google Scholar] [CrossRef] [Green Version]
- Jussila, J.; Jago, J.; Tsvetnenko, E.; Dunstan, B.; Evans, L.H. Total and differential haemocyte counts in western rock lobsters (Panulirus cygnus George) under post-harvest stress. Mar. Freshw. Res. 1997, 48, 863–867. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Qin, Q.; Qin, S.; Wang, L.; Lei, W. Immune responses and ultrastructural changes of hemocytes in freshwater crab Sinopotamon henanense exposed to elevated cadmium. Aquat. Toxicol. 2012, 106–107, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, J.C. Effects of intrinsic and extrinsic factors on the haemocyte profile of the prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2001, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.W.; Keyser, P.; Sritunyalucksana, K.; Söderhäll, K. Crustacean haemocytes and haematopoiesis. Aquaculture 2000, 191, 45–52. [Google Scholar] [CrossRef]
- Alikhan, M.A.; Naich, M. Changes in counts of haemocytes and in their physicochemical properties during the moult cycle in Porcellio spinicornis Say (Porcellionidae, Isopoda). Can. J. Zool. 1987, 65, 1685–1688. [Google Scholar] [CrossRef]
- Jiang, H.; Kanost, M.R. The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol. 2000, 30, 95–105. [Google Scholar] [CrossRef]
- Stevenson, J.R.; Murphy, J.C. Mucopolysaccharide glands in the isopod crustacean Armadillidium vulgare. Trans. Am. Microsc. Soc. 1967, 86, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase Activity Acts as a Mosquito Innate Immune Response against Infection with Semliki Forest Virus. PLoS Pathog. 2012, 8, e1002977. [Google Scholar] [CrossRef] [Green Version]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Santiago, P.B.; De Araújo, C.N.; Motta, F.N.; Praça, Y.R.; Charneau, S.; Bastos, I.M.D.; Santana, J.M. Proteases of haematophagous arthropod vectors are involved in blood-feeding, yolk formation and immunity—A review. Parasites Vectors 2017, 10, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; Zhang, X.; Yang, L.; Pan, S. Effects of Vibro harveyi and Staphyloccocus aureus infection on hemocyanin synthesis and innate immune responses in white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 93, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Jaenicke, E.; Fraune, S.; May, S.; Irmak, P.; Augustin, R.; Meesters, C.; Decker, H.; Zimmer, M. Is activated hemocyanin instead of phenoloxidase involved in immune response in woodlice? Dev. Comp. Immunol. 2009, 33, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Soderhall, K. Developmental and comparative immunology: Editorial. Dev. Comp. Immunol. 1999, 23, 263–266. [Google Scholar]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A key component of the insect immune system. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- Castillo, J.C.; Robertson, A.E.; Strand, M.R. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem. Mol. Biol. 2006, 36, 891–903. [Google Scholar] [CrossRef] [Green Version]
- Le Moullac, G.; Le Groumellec, M.; Ansquer, D.; Froissard, S.; Levy, P. Haematological and phenoloxidase activity changes in the shrimp Penaeus stylirostris in relation with the moult cycle: Protection against vibriosis. Fish Shellfish Immunol. 1997, 7, 227–234. [Google Scholar] [CrossRef]
- Lai-Fook, J. The repair of wounds in the integument of insects. J. Insect Physiol. 1966, 12, 195–226. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide Biol. Chem. 2000, 4, 423–430. [Google Scholar] [CrossRef]
- Radomski, M.W.; Martin, J.F.; Moncada, S. Synthesis of nitric oxide by the haemocytes of the American horseshoe crab (Limulus polyphemus). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1991, 334, 129–133. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Wang, L.; Jiang, S.; Sun, M.; Wang, M.; Yi, Q.; Song, L. Functional characterization of hemocytes from Chinese mitten crab Eriocheir sinensis by flow cytometry. Fish Shellfish Immunol. 2017, 69, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Raman, T.; Arumugam, M.; Mullainadhan, P. Agglutinin-mediated phagocytosis-associated generation of superoxide anion and nitric oxide by the hemocytes of the giant freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol. 2008, 24, 337–345. [Google Scholar] [CrossRef]
- Davidson, S.K.; Koropatnick, T.A.; Kossmehl, R.; Sycuro, L.; McFall-Ngai, M.J. NO means “yes” in the squid-vibrio symbiosis: Nitric oxide (NO) during the initial stages of a beneficial association. Cell. Microbiol. 2004, 6, 1139–1151. [Google Scholar] [CrossRef]
- Van De Braak, C.B.T.; Botterblom, M.H.A.; Liu, W.; Taverne, N.; Van Der Knaap, W.P.W.; Rombout, J.H.W.M. The role of the haematopoietic tissue in haemocyte production and maturation in the black tiger shrimp (Penaeus monodon). Fish Shellfish Immunol. 2002, 12, 253–272. [Google Scholar] [CrossRef]
- Traifalgar, R.F.M.; Corre, V.L.; Serrano, A.E. Efficacy of dietary immunostimulants to enhance the immunological responses and vibriosis resistance of juvenile Penaeus monodon. J. Fish. Aquat. Sci. 2013, 8, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, W.; Dankert, J.R.; Jenkins, J.A. Flow cytometric analysis of crayfish haemocytes activated by lipopolysaccharides. Fish Shellfish Immunol. 2004, 17, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Smith, V.J.; Brown, J.H.; Hauton, C. Immunostimulation in crustaceans: Does it really protect against infection? Fish Shellfish Immunol. 2003, 15, 71–90. [Google Scholar] [CrossRef]
- Roach, K.A.; Anderson, S.E.; Stefaniak, A.B.; Shane, H.L.; Boyce, G.R.; Roberts, J.R. Evaluation of the skin-sensitizing potential of gold nanoparticles and the impact of established dermal sensitivity on the pulmonary immune response to various forms of gold. Nanotoxicology 2020, 14, 1096–1117. [Google Scholar] [CrossRef]
- Suchomel, P.; Kvitek, L.; Prucek, R.; Panacek, A.; Halder, A.; Vajda, S.; Zboril, R. Simple size-controlled synthesis of Au nanoparticles and their size-dependent catalytic activity. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Škarková, P.; Romih, T.; Kos, M.; Novak, S.; Kononenko, V.; Jemec, A.; Vávrová, M.; Drobne, D. Gold nanoparticles do not induce adverse effects on terrestrial isopods Porcellio scaber after 14-day exposure Nanodelci zlata nimajo negativnih učinkov na kopenske rake vrste Porcellio scaber po 14-dnevni izpostavitvi. Acta Biol. Slov. 2016, 59, 2016. [Google Scholar]
- Bourdineaud, J.P.; Štambuk, A.; Šrut, M.; Radić Brkanac, S.; Ivanković, D.; Lisjak, D.; Sauerborn Klobučar, R.; Dragun, Z.; Bačić, N.; Klobučar, G.I.V. Gold and silver nanoparticles effects to the earthworm Eisenia fetida –the importance of tissue over soil concentrations. Drug Chem. Toxicol. 2021, 44, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Hornos Carneiro, M.F.; Barbosa, F. Gold nanoparticles: A critical review of therapeutic applications and toxicological aspects. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2016, 19, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, M.; Dudek, M.; Knutelska, J.; Nowiński, L.; Sapa, J.; Zygmunt, M.; Nowak, G.; Luty-Błocho, M.; Wojnicki, M.; Fitzner, K.; et al. The influence of the route of administration of gold nanoparticles on their tissue distribution and basic biochemical parameters: In vivo studies. Pharmacol. Rep. 2015, 67, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Paula, M.M.S.; Petronilho, F.; Vuolo, F.; Ferreira, G.K.; De Costa, L.; Santos, G.P.; Effting, P.S.; Dal-Pizzol, F.; Dal-Bõ, A.G.; Frizon, T.E.; et al. Gold nanoparticles and/or N-acetylcysteine mediate carrageenan-induced inflammation and oxidative stress in a concentration-dependent manner. J. Biomed. Mater. Res. Part A 2015, 103, 3323–3330. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H.; Wei, M.; Chen, X.; Zhang, Y.; Cao, L.; Yuan, P.; Wang, F.; Yang, G.; Ma, J. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int. J. Nanomed. 2017, 12, 4963–4979. [Google Scholar] [CrossRef] [Green Version]
- Sendra, M.; Blasco, J.; Araújo, C.V.M. Is the cell wall of marine phytoplankton a protective barrier or a nanoparticle interaction site? Toxicological responses of Chlorella autotrophica and Dunaliella salina to Ag and CeO2 nanoparticles. Ecol. Indic. 2018, 95, 1053–1067. [Google Scholar] [CrossRef]
- Malev, O.; Trebše, P.; Piecha, M.; Novak, S.; Budič, B.; Dramićanin, M.D.; Drobne, D. Effects of CeO2 Nanoparticles on Terrestrial Isopod Porcellio scaber: Comparison of CeO2 Biological Potential with Other Nanoparticles. Arch. Environ. Contam. Toxicol. 2017, 72, 303–311. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Karakoti, A.S.; Munusamy, P.; Hostetler, K.; Kodali, V.; Kuchibhatla, S.; Orr, G.; Pounds, J.G.; Teeguarden, J.G.; Thrall, B.D.; Baer, D.R. Preparation and characterization challenges to understanding environmental and biological impacts of ceria nanoparticles. Surf. Interface Anal. 2012, 44, 882–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakoti, A.S.; Monteiro-Riviere, N.A.; Aggarwal, R.; Davis, J.P.; Narayan, R.J.; Seif, W.T.; McGinnis, J.; Seal, S. Nanoceria as antioxidant: Synthesis and biomedical applications. JOM 2008, 60, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauton, C.; Hudspith, M.; Gunton, L. Future prospects for prophylactic immune stimulation in crustacean aquaculture - the need for improved metadata to address immune system complexity. Dev. Comp. Immunol. 2015, 48, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Alijagic, A.; Barbero, F.; Gaglio, D.; Napodano, E.; Benada, O.; Kofroňová, O.; Puntes, V.F.; Bastús, N.G.; Pinsino, A. Gold nanoparticles coated with polyvinylpyrrolidone and sea urchin extracellular molecules induce transient immune activation. J. Hazard. Mater. 2021, 402, 123793. [Google Scholar] [CrossRef]
- Kos, M.; Jemec Kokalj, A.; Glavan, G.; Marolt, G.; Zidar, P.; Božič, J.; Novak, S.; Drobne, D. Cerium(IV) oxide nanoparticles induce sublethal changes in honeybees after chronic exposure. Environ. Sci. Nano 2017, 4, 2297–2310. [Google Scholar] [CrossRef] [Green Version]
- Jemec Kokalj, A.; Horvat, P.; Skalar, T.; Kržan, A. Plastic bag and facial cleanser derived microplastic do not affect feeding behaviour and energy reserves of terrestrial isopods. Sci. Total Environ. 2018, 615, 761–766. [Google Scholar] [CrossRef]
- Praetorius, A.; Gundlach-Graham, A.; Goldberg, E.; Fabienke, W.; Navratilova, J.; Gondikas, A.; Kaegi, R.; Günther, D.; Hofmann, T.; Von Der Kammer, F. Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ. Sci. Nano 2017, 4, 307–314. [Google Scholar] [CrossRef]
- Mahapatra, I.; Sun, T.Y.; Clark, J.R.A.; Dobson, P.J.; Hungerbuehler, K.; Owen, R.; Nowack, B.; Lead, J. Probabilistic modelling of prospective environmental concentrations of gold nanoparticles from medical applications as a basis for risk assessment. J. Nanobiotechnology 2015, 13, 93. [Google Scholar] [CrossRef] [Green Version]
- Keller, A.A.; Lazareva, A. Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local. Environ. Sci. Technol. Lett. 2013, 1, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.Y.; Bornhöft, N.A.; Hungerbühler, K.; Nowack, B. Dynamic Probabilistic Modeling of Environmental Emissions of Engineered Nanomaterials. Environ. Sci. Technol. 2016, 50, 4701–4711. [Google Scholar] [CrossRef]
- Faraldo, A.C.; Sá-Nunes, A.; Del Bel, E.A.; Faccioli, L.H.; Lello, E. Nitric oxide production in blowfly hemolymph after yeast inoculation. Nitric Oxide Biol. Chem. 2005, 13, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Charles, H.M.; Killian, K.A. Response of the insect immune system to three different immune challenges. J. Insect Physiol. 2015, 81, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Setyawan, A.; Isnansetyo, A.; Murwantoko; Indarjulianto, S.; Handayani, C.R. Comparative immune response of dietary fucoidan from three indonesian brown algae in white shrimp Litopenaeus vannamei. AACL Bioflux 2018, 11, 1707–1723. [Google Scholar]
- Contreras-Garduño, J.; Lanz-Mendoza, H.; Córdoba-Aguilar, A. The expression of a sexually selected trait correlates with different immune defense components and survival in males of the American rubyspot. J. Insect Physiol. 2007, 53, 612–621. [Google Scholar] [CrossRef]
- Stewart, J.E.; Cornick, J.W.; Dingle, J.R. An electronic method for counting lobster (Homarus americanus Milne-Edwards) hemocytes and the influence of diet on hemocyte numbers and hemolymph proteins. Can. J. Zool. 1967, 45, 291–304. [Google Scholar] [CrossRef]
- Pascual, C.; Zenteno, E.; Cuzon, G.; Sánchez, A.; Gaxiola, G.; Taboada, G.; Suárez, J.; Maldonado, T.; Rosas, C. Litopenaeus vannamei juveniles energetic balance and immunological response to dietary protein. Aquaculture 2004, 236, 431–450. [Google Scholar] [CrossRef]
- Rosenberger, C.R.; Jones, J.C. Studies on Total Blood Cell Counts of the Southern Armyworm Larva, Prodenia Eridania (Lepidoptera). Ann. Entomol. Soc. Am. 1960, 53, 351–355. [Google Scholar] [CrossRef]
- Shapiro, M. Pathologic changes in the blood of the greater wax moth, Galleria mellonella, during the course of nucleopolyhedrosis and starvation. I. Total hemocyte count. J. Invertebr. Pathol. 1967, 9, 111–113. [Google Scholar] [CrossRef]
- Matozzo, V.; Gallo, C.; Marin, M.G. Can starvation influence cellular and biochemical parameters in the crab Carcinus aestuarii? Mar. Environ. Res. 2011, 71, 207–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sequeira, T.; Tavares, D.; Arala-Chaves, M. Evidence for circulating hemocyte proliferation in the shrimp Penaeus japonicus. Dev. Comp. Immunol. 1996, 20, 97–104. [Google Scholar] [CrossRef]
- Muralisankar, T.; Bhavan, P.S.; Radhakrishnan, S.; Seenivasan, C.; Manickam, N.; Srinivasan, V. Dietary supplementation of zinc nanoparticles and its influence on biology, physiology and immune responses of the freshwater prawn, Macrobrachium rosenbergii. Biol. Trace Elem. Res. 2014, 160, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Durstewitz, G.; Terwilliger, N.B. Developmental changes in hemocyanin expression in the dungeness crab, Cancer magister. J. Biol. Chem. 1997, 272, 4347–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.; Wang, R.; Van Antwerpen, R.; Sottrup-Jensen, L.; Söderhäll, K. The crayfish plasma clotting protein: A vitellogenin-related protein responsible for clot formation in crustacean blood. Proc. Natl. Acad. Sci. USA 1999, 96, 1965–1970. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.; Grinstein, S.; Marques, P.E. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front. Immunol. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Parle, E.; Dirks, J.H.; Taylor, D. Damage, repair and regeneration in insect cuticle: The story so far, and possibilities for the future. Arthropod Struct. Dev. 2017, 46, 49–55. [Google Scholar] [CrossRef]
- Tümer, C.; Bilgin, H.M.; Obay, B.D.; Diken, H.; Atmaca, M.; Kelle, M. Effect of nitric oxide on phagocytic activity of lipopolysaccharide-induced macrophages: Possible role of exogenous l-arginine. Cell Biol. Int. 2007, 31, 565–569. [Google Scholar] [CrossRef]
- Giese, B.; Klaessig, F.; Park, B.; Kaegi, R.; Steinfeldt, M.; Wigger, H.; Von Gleich, A.; Gottschalk, F. Risks, Release and Concentrations of Engineered Nanomaterial in the Environment. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef]
- Victor, B.; Narayanan, M.; Nelson, D.J. Gill pathology and hemocyte response in mercury exposed Macrobrachium idae heller. J. Environ. Biol. 1990, 11, 61–66. [Google Scholar]
- Johnson, N.G.; Burnett, L.E.; Burnett, K.G. Properties of bacteria that trigger hemocytopenia in the Atlantic Blue Crab, Callinectes sapidus. Biol. Bull. 2011, 221, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Matozzo, V.; Gallo, C.; Marin, M.G. Effects of temperature on cellular and biochemical parameters in the crab Carcinus aestuarii (Crustacea, Decapoda). Mar. Environ. Res. 2011, 71, 351–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnett, L.E.; Holman, J.D.; Jorgensen, D.D.; Ikerd, J.L.; Burnett, K.G. Immune defense reduces respiratory fitness in Callinectes sapidus, the Atlantic blue crab. Biol. Bull. 2006, 211, 50–57. [Google Scholar] [CrossRef]
- Truscott, R.; White, K.N. The Influence of Metal and Temperature Stress on the Immune System of Crabs. Funct. Ecol. 1990, 4, 455. [Google Scholar] [CrossRef]
- Singaram, G.; Harikrishnan, T.; Chen, F.Y.; Bo, J.; Giesy, J.P. Modulation of immune-associated parameters and antioxidant responses in the crab (Scylla serrata) exposed to mercury. Chemosphere 2013, 90, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Hauton, C.; Hawkins, L.E.; Williams, J.A. In situ variability in phenoloxidase activity in the shore crab, Carcinus maenas (L.). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 117, 267–271. [Google Scholar] [CrossRef]
- Raduolovic, K.; Mak’Anyengo, R.; Kaya, B.; Steinert, A.; Niess, J.H. Injections of lipopolysaccharide into mice to mimic entrance of microbial-derived products after intestinal barrier breach. J. Vis. Exp. 2018, 2018, 57610. [Google Scholar] [CrossRef]
- Goarant, C.; Boglio, E. Changes in Hemocyte Counts in Litopenaeus stylirostris Subjected to Sublethal Infection and to Vaccination. J. World Aquac. Soc. 2000, 31, 123–129. [Google Scholar] [CrossRef]
- Lorenzon, S.; de Guarrini, S.; Smith, V.J.; Ferrero, E.A. Effects of LPS injection on circulating haemocytes in crustaceansin vivo. Fish Shellfish Immunol. 1999, 9, 31–50. [Google Scholar] [CrossRef]
- Chiaramonte, M.; Inguglia, L.; Vazzana, M.; Deidun, A.; Arizza, V. Stress and immune response to bacterial LPS in the sea urchin Paracentrous lividus (Lamarck, 1816). Fish Shellfish Immunol. 2019, 92, 384–394. [Google Scholar] [CrossRef]
- Xu, H.-S.; Lyu, S.-J.; Xu, J.-H.; Lu, B.-J.; Zhao, J.; Li, S.; Li, Y.-Q.; Chen, Y.-Y. Effect of lipopolysaccharide on the hemocyte apoptosis of Eriocheir sinensis. J. Zhejiang Univ. Sci. B 2015, 16, 971–979. [Google Scholar] [CrossRef] [Green Version]
- Yeh, F.C.; Wu, S.H.; Lai, C.Y.; Lee, C.Y. Demonstration of nitric oxide synthase activity in crustacean hemocytes and anti-microbial activity of hemocyte-derived nitric oxide. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Salawu, M.O.; Oloyede, H.O.B.; Oladiji, T.A.; Yakubu, M.T.; Amuzat, A.O. Hemolymph coagulation and phenoloxidase activity in Uca tangeri induced by Escherichia coli endotoxin. J. Immunotoxicol. 2016, 13, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, K.W. Dead cell phagocytosis and innate immune checkpoint. BMB Rep. 2017, 50, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Hammond, J.A.; Smith, V.J. Lipopolysaccharide induces DNA-synthesis in a sub-population of hemocytes from the swimming crab, Liocarcinus depurator. Dev. Comp. Immunol. 2002, 26, 227–236. [Google Scholar] [CrossRef]
- Smith, V.J.; Swindlehurst, R.J.; Johnston, P.A.; Vethaak, A.D. Disturbance of host defence capability in the common shrimp, Crangon crangon, by exposure to harbour dredge spoils. Aquat. Toxicol. 1995, 32, 43–58. [Google Scholar] [CrossRef]
- Le Moullac, G.; Soyez, C.; Saulnier, D.; Ansquer, D.; Avarre, J.C.; Levy, P. Effect of hypoxic stress on the immune response and the resistance to vibriosis of the shrimp Penaeus stylirostris. Fish Shellfish Immunol. 1998, 8, 621–629. [Google Scholar] [CrossRef]
- Tello-Olea, M.; Rosales-Mendoza, S.; Campa-Córdova, A.I.; Palestino, G.; Luna-González, A.; Reyes-Becerril, M.; Velazquez, E.; Hernandez-Adame, L.; Angulo, C. Gold nanoparticles (AuNP) exert immunostimulatory and protective effects in shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immunol. 2019, 84, 756–767. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayall, C.; Dolar, A.; Jemec Kokalj, A.; Novak, S.; Razinger, J.; Barbero, F.; Puntes, V.; Drobne, D. Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio scaber: A Focus on Nanomaterials. Nanomaterials 2021, 11, 934. https://doi.org/10.3390/nano11040934
Mayall C, Dolar A, Jemec Kokalj A, Novak S, Razinger J, Barbero F, Puntes V, Drobne D. Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio scaber: A Focus on Nanomaterials. Nanomaterials. 2021; 11(4):934. https://doi.org/10.3390/nano11040934
Chicago/Turabian StyleMayall, Craig, Andraz Dolar, Anita Jemec Kokalj, Sara Novak, Jaka Razinger, Francesco Barbero, Victor Puntes, and Damjana Drobne. 2021. "Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio scaber: A Focus on Nanomaterials" Nanomaterials 11, no. 4: 934. https://doi.org/10.3390/nano11040934
APA StyleMayall, C., Dolar, A., Jemec Kokalj, A., Novak, S., Razinger, J., Barbero, F., Puntes, V., & Drobne, D. (2021). Stressor-Dependant Changes in Immune Parameters in the Terrestrial Isopod Crustacean, Porcellio scaber: A Focus on Nanomaterials. Nanomaterials, 11(4), 934. https://doi.org/10.3390/nano11040934







