Recent Advances in Sequential Infiltration Synthesis (SIS) of Block Copolymers (BCPs)
Abstract
:1. Introduction
2. SIS Processing and Mechanism
2.1. Polymer Selectivity
2.2. Diffusion
3. Characterization Techniques
3.1. Phenomenology of the Infiltration Process
3.2. Characterization of the Infiltrated Materials’ Properties
4. Control of the Materials’ Functional Properties by SIS
4.1. Optical Properties
4.2. Electrical Properties
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Suh, H.S.; Moni, P.; Xiong, S.; Ocola, L.E.; Zaluzec, N.J.; Gleason, K.K.; Nealey, P.F. Sub-10-nm patterning via directed self-assembly of block copolymer films with a vapour-phase deposited topcoat. Nat. Nanotechnol. 2017, 12, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Cummins, C.; Pino, G.; Mantione, D.; Fleury, G. Engineering block copolymer materials for patterning ultra-low dimensions. Mol. Syst. Des. Eng. 2020, 5, 1642–1657. [Google Scholar] [CrossRef]
- Ding, Y.; Gadelrab, K.R.; Rodriguez, K.M.; Huang, H.; Ross, C.A.; Alexander-Katz, A. Emergent symmetries in block copolymer epitaxy. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Stein, A.; Wright, G.; Yager, K.G.; Doerk, G.S.; Black, C.T. Selective directed self-assembly of coexisting morphologies using block copolymer blends. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stefik, M.; Guldin, S.; Vignolini, S.; Wiesner, U.; Steiner, U. Block copolymer self-assembly for nanophotonics. Chem. Soc. Rev. 2015, 44, 5076–5091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasen, W.; Dong, R.; Aini, A.; Zhu, X. Recent advances in supramolecular block copolymers for biomedical applications. J. Mater. Chem. B 2020, 8, 8219–8231. [Google Scholar] [CrossRef]
- Shiohara, A.; Prieto-Simon, B.; Voelcker, N.H. Porous polymeric membranes: Fabrication techniques and biomedical applications. J. Mater. Chem. B 2021, 9, 2129–2154. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef]
- Guo, C.; Lin, Y.H.; Witman, M.D.; Smith, K.A.; Wang, C.; Hexemer, A.; Strzalka, J.; Gomez, E.D.; Verduzco, R. Conjugated block copolymer photovoltaics with near 3% efficiency through microphase separation. Nano Lett. 2013, 13, 2957–2963. [Google Scholar] [CrossRef]
- Liu, C.C.; Franke, E.; Mignot, Y.; Xie, R.; Yeung, C.W.; Zhang, J.; Chi, C.; Zhang, C.; Farrell, R.; Lai, K.; et al. Directed self-assembly of block copolymers for 7 nanometre FinFET technology and beyond. Nat. Electron. 2018, 1, 562–569. [Google Scholar] [CrossRef]
- Jacobberger, R.M.; Thapar, V.; Wu, G.P.; Chang, T.H.; Saraswat, V.; Way, A.J.; Jinkins, K.R.; Ma, Z.; Nealey, P.F.; Hur, S.M.; et al. Boundary-directed epitaxy of block copolymers. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Wu, M.L.; Wang, D.; Wan, L.J. Directed block copolymer self-assembly implemented via surface-embedded electrets. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ferrarese Lupi, F.; Giammaria, T.; Seguini, G.; Laus, M.; Enrico, E.; De Leo, N.; Boarino, L.; Ober, C.; Perego, M. Thermally induced orientational flipping of cylindrical phase diblock copolymers. J. Mater. Chem. C 2014, 2, 2175–2182. [Google Scholar] [CrossRef]
- Giammaria, T.J.; Ferrarese Lupi, F.; Seguini, G.; Perego, M.; Vita, F.; Francescangeli, O.; Wenning, B.; Ober, C.K.; Sparnacci, K.; Antonioli, D.; et al. Micrometer-scale ordering of silicon-containing block copolymer thin films via high-temperature thermal treatments. ACS Appl. Mater. Interfaces 2016, 8, 9897–9908. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese Lupi, F.; Giammaria, T.J.; Miti, A.; Zuccheri, G.; Carignano, S.; Sparnacci, K.; Seguini, G.; De Leo, N.; Boarino, L.; Perego, M.; et al. Hierarchical order in dewetted block copolymer thin films on chemically patterned surfaces. ACS Nano 2018, 12, 7076–7085. [Google Scholar] [CrossRef] [PubMed]
- Leniart, A.A.; Pula, P.; Sitkiewicz, A.; Majewski, P.W. Macroscopic Alignment of Block Copolymers on Silicon Substrates by Laser Annealing. ACS Nano 2020, 14, 4805–4815. [Google Scholar] [CrossRef]
- Rahman, A.; Majewski, P.W.; Doerk, G.; Black, C.T.; Yager, K.G. Non-native three-dimensional block copolymer morphologies. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jacobs, A.G.; Wenning, B.; Liedel, C.; Thompson, M.O.; Ober, C.K. Ultrafast Self-Assembly of Sub-10 nm Block Copolymer Nanostructures by Solvent-Free High-Temperature Laser Annealing. ACS Appl. Mater. Interfaces 2017, 9, 31317–31324. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese Lupi, F.; Giammaria, T.J.; Seguini, G.; Vita, F.; Francescangeli, O.; Sparnacci, K.; Antonioli, D.; Gianotti, V.; Laus, M.; Perego, M. Fine tuning of lithographic masks through thin films of PS-b-PMMA with different molar mass by rapid thermal processing. ACS Appl. Mater. Interfaces 2014, 6, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Berggren, K.; Xia, Q.; Likharev, K.K.; Strukov, D.B.; Jiang, H.; Mikolajick, T.; Querlioz, D.; Salinga, M.; Erickson, J.R.; Pi, S.; et al. Roadmap on emerging hardware and technology for machine learning. Nanotechnology 2020, 32, 012002. [Google Scholar] [CrossRef]
- Frascaroli, J.; Brivio, S.; Ferrarese Lupi, F.; Seguini, G.; Boarino, L.; Perego, M.; Spiga, S. Resistive switching in high-density nanodevices fabricated by block copolymer self-assembly. ACS Nano 2015, 9, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Murataj, I.; Channab, M.; Cara, E.; Pirri, C.F.; Boarino, L.; Angelini, A.; Ferrarese Lupi, F. Hyperbolic Metamaterials via Hierarchical Block Copolymer Nanostructures. Adv. Opt. Mater. 2020, 2001933. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.; Kim, B.H.; Chang, T.; Lim, J.; Jin, H.M.; Mun, J.H.; Choi, Y.J.; Chung, K.; Shin, J.; et al. Highly tunable refractive index visible-light metasurface from block copolymer self-assembly. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Y.; Lee, J.; Diroll, B.T.; Lee, B.; Aouadi, S.; Shevchenko, E.V.; Berman, D. Rapid synthesis of nanoporous conformal coatings via plasma-enhanced sequential infiltration of a polymer template. ACS Omega 2017, 2, 7812–7819. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Tseng, Y.C.; Darling, S.B.; Elam, J.W. A route to nanoscopic materials via sequential infiltration synthesis on block copolymer templates. ACS Nano 2011, 5, 4600–4606. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Berg, A.I.; Noheda, B.; Loos, K. Progress and perspective on polymer templating of multifunctional oxide nanostructures. J. Appl. Phys. 2020, 128, 190903. [Google Scholar] [CrossRef]
- Waldman, R.Z.; Mandia, D.J.; Yanguas-Gil, A.; Martinson, A.B.; Elam, J.W.; Darling, S.B. The chemical physics of sequential infiltration synthesis—A thermodynamic and kinetic perspective. J. Chem. Phys. 2019, 151, 190901. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.F.; Jur, J.S. Properties and applications of vapor infiltration into polymeric substrates. JOM 2019, 71, 238–245. [Google Scholar] [CrossRef]
- McGuinness, E.K.; Zhang, F.; Ma, Y.; Lively, R.P.; Losego, M.D. Vapor phase infiltration of metal oxides into nanoporous polymers for organic solvent separation membranes. Chem. Mater. 2019, 31, 5509–5518. [Google Scholar] [CrossRef]
- Leng, C.Z.; Losego, M.D. Vapor phase infiltration (VPI) for transforming polymers into organic–inorganic hybrid materials: A critical review of current progress and future challenges. Mater. Horiz. 2017, 4, 747–771. [Google Scholar] [CrossRef]
- Subramanian, A.; Doerk, G.; Kisslinger, K.; Daniel, H.Y.; Grubbs, R.B.; Nam, C.Y. Three-dimensional electroactive ZnO nanomesh directly derived from hierarchically self-assembled block copolymer thin films. Nanoscale 2019, 11, 9533–9546. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Pippel, E.; Gösele, U.; Dresbach, C.; Qin, Y.; Chandran, C.V.; Bräuniger, T.; Hause, G.; Knez, M. Greatly increased toughness of infiltrated spider silk. Science 2009, 324, 488–492. [Google Scholar] [CrossRef]
- Peng, Q.; Tseng, Y.C.; Long, Y.; Mane, A.U.; DiDona, S.; Darling, S.B.; Elam, J.W. Effect of nanostructured domains in self-assembled block copolymer films on sequential infiltration synthesis. Langmuir 2017, 33, 13214–13223. [Google Scholar] [CrossRef]
- Dandley, E.C.; Needham, C.D.; Williams, P.S.; Brozena, A.H.; Oldham, C.J.; Parsons, G.N. Temperature-dependent reaction between trimethylaluminum and poly (methyl methacrylate) during sequential vapor infiltration: Experimental and ab initio analysis. J. Mater. Chem. C 2014, 2, 9416–9424. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. New insight into the mechanism of sequential infiltration synthesis from infrared spectroscopy. Chem. Mater. 2014, 26, 6135–6141. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. Kinetics for the sequential infiltration synthesis of alumina in poly (methyl methacrylate): An infrared spectroscopic study. J. Phys. Chem. C 2015, 119, 14585–14592. [Google Scholar] [CrossRef]
- Caligiore, F.E.; Nazzari, D.; Cianci, E.; Sparnacci, K.; Laus, M.; Perego, M.; Seguini, G. Effect of the Density of Reactive Sites in P(S-r-MMA) Film during Al2O3 Growth by Sequential Infiltration Synthesis. Adv. Mater. Interfaces 2019, 6, 1900503. [Google Scholar] [CrossRef]
- Liapis, A.C.; Subramanian, A.; Cho, S.; Kisslinger, K.; Nam, C.Y.; Yun, S.H. Conformal Coating of Freestanding Particles by Vapor-Phase Infiltration. Adv. Mater. Interfaces 2020, 7, 2001323. [Google Scholar] [CrossRef] [PubMed]
- Hill, G.T.; Lee, D.T.; Williams, P.S.; Needham, C.D.; Dandley, E.C.; Oldham, C.J.; Parsons, G.N. Insight on the Sequential Vapor Infiltration Mechanisms of Trimethylaluminum with Poly (methyl methacrylate), Poly (vinylpyrrolidone), and Poly (acrylic acid). J. Phys. Chem. C 2019, 123, 16146–16152. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. Polycaprolactone: A Promising Addition to the Sequential Infiltration Synthesis Polymer Family Identified through In Situ Infrared Spectroscopy. ACS Appl. Polym. Mater. 2020, 2, 5501–5510. [Google Scholar] [CrossRef]
- Ocola, L.E.; Connolly, A.; Gosztola, D.J.; Schaller, R.D.; Yanguas-Gil, A. Infiltrated zinc oxide in poly (methyl methacrylate): An atomic cycle growth study. J. Phys. Chem. C 2017, 121, 1893–1903. [Google Scholar] [CrossRef]
- Kim, J.J.; Suh, H.S.; Zhou, C.; Mane, A.U.; Lee, B.; Kim, S.; Emery, J.D.; Elam, J.W.; Nealey, P.F.; Fenter, P.; et al. Mechanistic understanding of tungsten oxide in-plane nanostructure growth via sequential infiltration synthesis. Nanoscale 2018, 10, 3469–3479. [Google Scholar] [CrossRef] [PubMed]
- Barick, B.K.; Simon, A.; Weisbord, I.; Shomrat, N.; Segal-Peretz, T. Tin oxide nanostructure fabrication via sequential infiltration synthesis in block copolymer thin films. J. Colloid Interface Sci. 2019, 557, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.H.; Nam, C.Y.; Doerk, G.; Black, C.T.; Grubbs, R.B. Infiltration synthesis of diverse metal oxide nanostructures from epoxidized diene–styrene block copolymer templates. ACS Appl. Polym. Mater. 2019, 1, 672–683. [Google Scholar] [CrossRef]
- Pilz, J.; Coclite, A.M.; Losego, M.D. Vapor phase infiltration of zinc oxide into thin films of cis-polyisoprene rubber. Mater. Adv. 2020, 1, 1695–1704. [Google Scholar] [CrossRef]
- She, Y.; Lee, J.; Lee, B.; Diroll, B.; Scharf, T.; Shevchenko, E.V.; Berman, D. Effect of the micelle opening in self-assembled amphiphilic block Co-polymer films on the infiltration of inorganic precursors. Langmuir 2019, 35, 796–803. [Google Scholar] [CrossRef]
- She, Y.; Goodman, E.D.; Lee, J.; Diroll, B.T.; Cargnello, M.; Shevchenko, E.V.; Berman, D. Block-co-polymer-assisted synthesis of all inorganic highly porous heterostructures with highly accessible thermally stable functional centers. ACS Appl. Mater. Interfaces 2019, 11, 30154–30162. [Google Scholar] [CrossRef]
- Peng, Q.; Tseng, Y.C.; Darling, S.B.; Elam, J.W. Nanoscopic patterned materials with tunable dimensions via atomic layer deposition on block copolymers. Adv. Mater. 2010, 22, 5129–5133. [Google Scholar] [CrossRef]
- Waldman, R.Z.; Jeon, N.; Mandia, D.J.; Heinonen, O.; Darling, S.B.; Martinson, A.B. Sequential infiltration synthesis of electronic materials: Group 13 oxides via metal alkyl precursors. Chem. Mater. 2019, 31, 5274–5285. [Google Scholar] [CrossRef]
- Berman, D.; Shevchenko, E. Design of functional composite and all-inorganic nanostructured materials via infiltration of polymer templates with inorganic precursors. J. Mater. Chem. C 2020, 8, 10604–10627. [Google Scholar] [CrossRef]
- Azoulay, R.; Shomrat, N.; Weisbord, I.; Atiya, G.; Segal-Peretz, T. Metal oxide heterostructure array via spatially controlled–growth within block copolymer templates. Small 2019, 15, 1904657. [Google Scholar] [CrossRef]
- Weisbord, I.; Shomrat, N.; Azoulay, R.; Kaushansky, A.; Segal-Peretz, T. Understanding and Controlling Polymer–Organometallic Precursor Interactions in Sequential Infiltration Synthesis. Chem. Mater. 2020, 32, 4499–4508. [Google Scholar] [CrossRef]
- Leng, C.Z.; Losego, M.D. A physiochemical processing kinetics model for the vapor phase infiltration of polymers: Measuring the energetics of precursor-polymer sorption, diffusion, and reaction. Phys. Chem. Chem. Phys. 2018, 20, 21506–21514. [Google Scholar] [CrossRef] [PubMed]
- Padbury, R.P.; Jur, J.S. Systematic study of trimethyl aluminum infiltration in polyethylene terephthalate and its effect on the mechanical properties of polyethylene terephthalate fibers. J. Vac. Sci. Technol. A 2015, 33, 01A112. [Google Scholar] [CrossRef]
- Young, M.J.; Choudhury, D.; Letourneau, S.; Mane, A.; Yanguas-Gil, A.; Elam, J.W. Molecular Layer Etching of Metalcone Films Using Lithium Organic Salts and Trimethylaluminum. Chem. Mater. 2020, 32, 992–1001. [Google Scholar] [CrossRef]
- Cianci, E.; Nazzari, D.; Seguini, G.; Perego, M. Trimethylaluminum diffusion in PMMA thin films during sequential infiltration synthesis: In situ dynamic spectroscopic ellipsometric investigation. Adv. Mater. Interfaces 2018, 5, 1801016. [Google Scholar] [CrossRef]
- Aprile, G.; Ferrarese Lupi, F.; Fretto, M.; Enrico, E.; De Leo, N.; Boarino, L.; Volpe, F.G.; Seguini, G.; Sparnacci, K.; Gianotti, V.; et al. Toward Lateral Length Standards at the Nanoscale Based on Diblock Copolymers. ACS Appl. Mater. Interfaces 2017, 9, 15685–15697. [Google Scholar] [CrossRef]
- Ishchenko, O.M.; Krishnamoorthy, S.; Valle, N.; Guillot, J.; Turek, P.; Fechete, I.; Lenoble, D. Investigating sequential vapor infiltration synthesis on block-copolymer-templated titania nanoarrays. J. Phys. Chem. C 2016, 120, 7067–7076. [Google Scholar] [CrossRef]
- Ozaki, Y.; Ito, S.; Hiroshiba, N.; Nakamura, T.; Nakagawa, M. Elemental depth profiles and plasma etching rates of positive-tone electron beam resists after sequential infiltration synthesis of alumina. Jpn. J. Appl. Phys. 2018, 57, 06HG01. [Google Scholar] [CrossRef] [Green Version]
- Segal-Peretz, T.; Winterstein, J.; Doxastakis, M.; Ramirez-Hernandez, A.; Biswas, M.; Ren, J.; Suh, H.S.; Darling, S.B.; Liddle, J.A.; Elam, J.W.; et al. Characterizing the three-dimensional structure of block copolymers via sequential infiltration synthesis and scanning transmission electron tomography. ACS Nano 2015, 9, 5333–5347. [Google Scholar] [CrossRef]
- Lee, S.; Subramanian, A.; Tiwale, N.; Kisslinger, K.; Mumtaz, M.; Shi, L.Y.; Aissou, K.; Nam, C.Y.; Ross, C.A. Resolving Triblock Terpolymer Morphologies by Vapor-Phase Infiltration. Chem. Mater. 2020, 32, 5309–5316. [Google Scholar] [CrossRef]
- McGuinness, E.K.; Leng, C.Z.; Losego, M.D. Increased Chemical Stability of Vapor-Phase Infiltrated AlO x–Poly (methyl methacrylate) Hybrid Materials. ACS Appl. Polym. Mater. 2020, 2, 1335–1344. [Google Scholar] [CrossRef]
- Ito, S.; Ozaki, Y.; Nakamura, T.; Nakagawa, M. Depth profiles of aluminum component in sequential infiltration synthesis-treated electron beam resist films analyzed by time-of-flight secondary ion mass spectrometry. Jpn. J. Appl. Phys. 2020, 59, SIIC03. [Google Scholar] [CrossRef]
- He, X.; Waldman, R.Z.; Mandia, D.J.; Jeon, N.; Zaluzec, N.J.; Borkiewicz, O.J.; Ruett, U.; Darling, S.B.; Martinson, A.B.; Tiede, D.M. Resolving the Atomic Structure of Sequential Infiltration Synthesis Derived Inorganic Clusters. ACS Nano 2020, 14, 14846–14860. [Google Scholar] [CrossRef]
- Marneffe, J.F.d.; Chan, B.T.; Spieser, M.; Vereecke, G.; Naumov, S.; Vanhaeren, D.; Wolf, H.; Knoll, A.W. Conversion of a patterned organic resist into a high performance inorganic hard mask for high resolution pattern transfer. ACS Nano 2018, 12, 11152–11160. [Google Scholar] [CrossRef]
- Ozaki, Y.; Ito, S.; Nakamura, T.; Nakagawa, M. Sequential infiltration synthesis-and solvent annealing-induced morphological changes in positive-tone e-beam resist patterns evaluated by atomic force microscopy. Jpn. J. Appl. Phys. 2019, 58, SDDJ04. [Google Scholar] [CrossRef]
- Lorenzoni, M.; Evangelio, L.; Fernandez-Regulez, M.; Nicolet, C.; Navarro, C.; Perez-Murano, F. Sequential infiltration of self-assembled block copolymers: A study by atomic force microscopy. J. Phys. Chem. C 2017, 121, 3078–3086. [Google Scholar] [CrossRef]
- Massonnet, P.; Heeren, R.M. A concise tutorial review of TOF-SIMS based molecular and cellular imaging. J. Anal. At. Spectrom. 2019, 34, 2217–2228. [Google Scholar] [CrossRef]
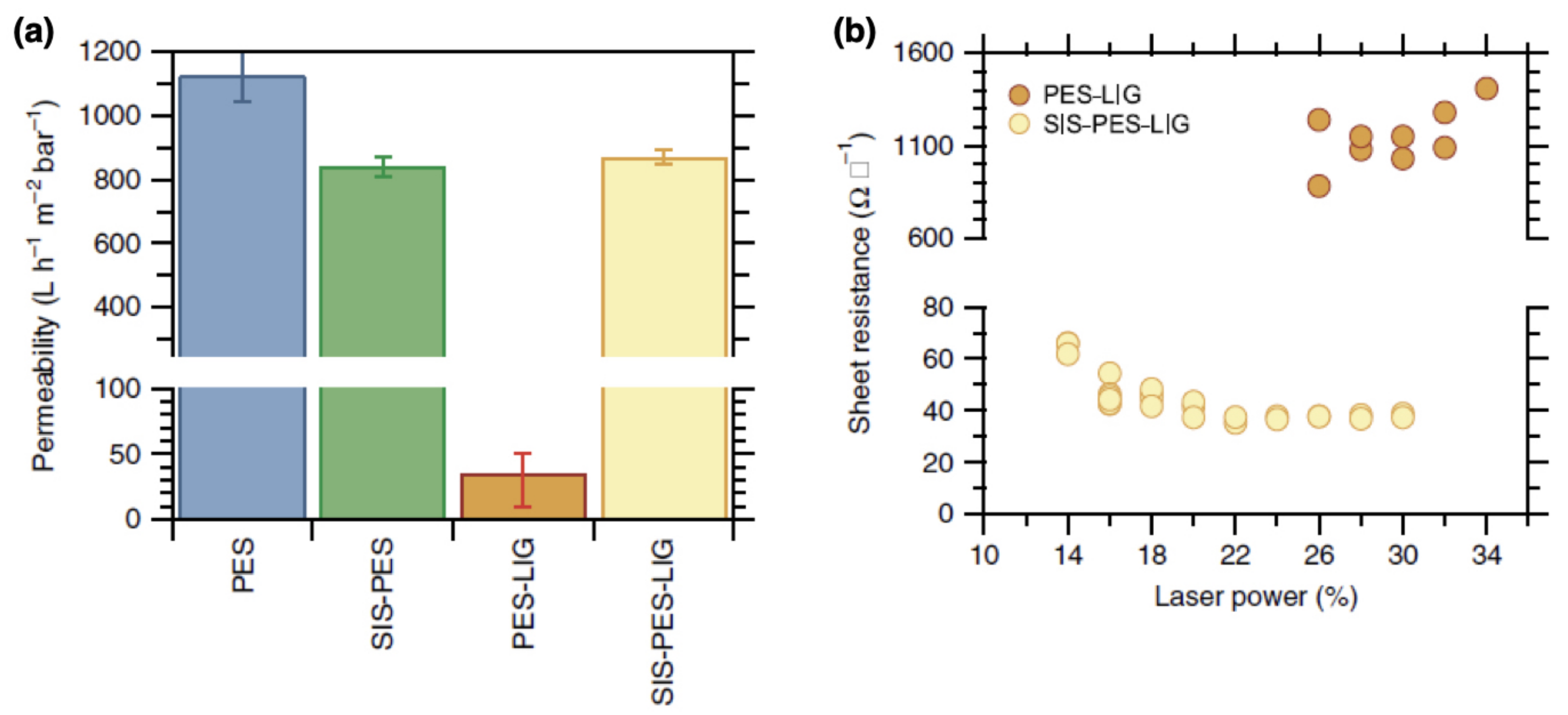
- Bergsman, D.S.; Getachew, B.A.; Cooper, C.B.; Grossman, J.C. Preserving nanoscale features in polymers during laser induced graphene formation using sequential infiltration synthesis. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferrarese Lupi, F.; Giammaria, T.J.; Seguini, G.; Laus, M.; Dubček, P.; Pivac, B.; Bernstorff, S.; Perego, M. GISAXS analysis of the in-depth morphology of thick PS-b-PMMA films. ACS Appl. Mater. Interfaces 2017, 9, 11054–11063. [Google Scholar] [CrossRef] [PubMed]
- Dialameh, M.; Ferrarese Lupi, F.; Hönicke, P.; Kayser, Y.; Beckhoff, B.; Weimann, T.; Fleischmann, C.; Vandervorst, W.; Dubček, P.; Pivac, B.; et al. Development and Synchrotron-Based Characterization of Al and Cr Nanostructures as Potential Calibration Samples for 3D Analytical Techniques. Phys. Status Solidi A 2018, 215, 1700866. [Google Scholar] [CrossRef]
- Tiede, D.M.; Kwon, G.; He, X.; Mulfort, K.L.; Martinson, A.B. Characterizing electronic and atomic structures for amorphous and molecular metal oxide catalysts at functional interfaces by combining soft X-ray spectroscopy and high-energy X-ray scattering. Nanoscale 2020, 12, 13276–13296. [Google Scholar] [CrossRef]
- Vora, A.; Schmidt, K.; Alva, G.; Arellano, N.; Magbitang, T.; Chunder, A.; Thompson, L.E.; Lofano, E.; Pitera, J.W.; Cheng, J.Y.; et al. Orientation control of block copolymers using surface active, phase-preferential additives. ACS Appl. Mater. Interfaces 2016, 8, 29808–29817. [Google Scholar] [CrossRef]
- Elam, J.W.; Biswas, M.; Darling, S.; Yanguas-Gil, A.; Emery, J.D.; Martinson, A.B.; Nealey, P.F.; Segal-Peretz, T.; Peng, Q.; Winterstein, J.; et al. New insights into sequential infiltration synthesis. ECS Trans. 2015, 69, 147. [Google Scholar] [CrossRef] [Green Version]
- Mokarian-Tabari, P.; Senthamaraikannan, R.; Glynn, C.; Collins, T.W.; Cummins, C.; Nugent, D.; O’Dwyer, C.; Morris, M.A. Large block copolymer self-assembly for fabrication of subwavelength nanostructures for applications in optics. Nano Lett. 2017, 17, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Lee, H.J.; Kim, H.G.; Jo, G.C.; Park, W.I.; Ryu, S.W.; Lee, H.B.R.; Kwon, S.H. Circular Double-Patterning Lithography Using a Block Copolymer Template and Atomic Layer Deposition. Adv. Mater. Interfaces 2018, 5, 1800054. [Google Scholar] [CrossRef]
- Guldin, S.; Kohn, P.; Stefik, M.; Song, J.; Divitini, G.; Ecarla, F.; Ducati, C.; Wiesner, U.; Steiner, U. Self-cleaning antireflective optical coatings. Nano Lett. 2013, 13, 5329–5335. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Guha, S.; Lee, B.; Elam, J.W.; Darling, S.B.; Shevchenko, E.V. Sequential infiltration synthesis for the design of low refractive index surface coatings with controllable thickness. ACS Nano 2017, 11, 2521–2530. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, R.; Yu, M.; Bai, F.; Li, C.; Wang, Z.L. Cellular level biocompatibility and biosafety of ZnO nanowires. J. Phys. Chem. C 2008, 112, 20114–20117. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.M.; Ho, C.L.; Chang, H.J.; Wu, M.C. Fabrication of inverted zinc oxide photonic crystal using sol–gel solution by spin coating method. Nanoscale Res. Lett. 2013, 8, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size tunable ZnO nanoparticles to enhance electron injection in solution processed QLEDs. ACS Photonics 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.; Abdulkareem, A.; Tijani, J.; Shuaib, D.; Ajala, A.; Mohammed, A. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Fang, B.; Zhang, C.; Wang, G.; Wang, M.; Ji, Y. A glucose oxidase immobilization platform for glucose biosensor using ZnO hollow nanospheres. Sens. Actuators B Chem. 2011, 155, 304–310. [Google Scholar] [CrossRef]
- Kamcev, J.; Germack, D.S.; Nykypanchuk, D.; Grubbs, R.B.; Nam, C.Y.; Black, C.T. Chemically enhancing block copolymers for block-selective synthesis of self-assembled metal oxide nanostructures. ACS Nano 2013, 7, 339–346. [Google Scholar] [CrossRef]
- Ocola, L.E.; Gosztola, D.J.; Yanguas-Gil, A.; Suh, H.S.; Connolly, A. Photoluminescence of sequential infiltration synthesized ZnO nanostructures. In Quantum Sensing and Nano Electronics and Photonics XIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; Volume 9755, p. 97552C. [Google Scholar]
- Choi, S.; Berhane, A.M.; Gentle, A.; Ton-That, C.; Phillips, M.R.; Aharonovich, I. Electroluminescence from localized defects in zinc oxide: Toward electrically driven single photon sources at room temperature. ACS Appl. Mater. Interfaces 2015, 7, 5619–5623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berggren, M.; Nilsson, D.; Robinson, N.D. Organic materials for printed electronics. Nat. Mater. 2007, 6, 3–5. [Google Scholar] [CrossRef]
- Dimitrakopoulos, C.D.; Malenfant, P.R. Organic thin film transistors for large area electronics. Adv. Mater. 2002, 14, 99–117. [Google Scholar] [CrossRef]
- Gross, M.; Müller, D.C.; Nothofer, H.G.; Scherf, U.; Neher, D.; Bräuchle, C.; Meerholz, K. Improving the performance of doped π-conjugated polymers for use in organic light-emitting diodes. Nature 2000, 405, 661–665. [Google Scholar] [CrossRef]
- Hains, A.W.; Liang, Z.; Woodhouse, M.A.; Gregg, B.A. Molecular semiconductors in organic photovoltaic cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef]
- Muench, S.; Wild, A.; Friebe, C.; Häupler, B.; Janoschka, T.; Schubert, U.S. Polymer-based organic batteries. Chem. Rev. 2016, 116, 9438–9484. [Google Scholar] [CrossRef]
- Gregorczyk, K.; Knez, M. Hybrid nanomaterials through molecular and atomic layer deposition: Top down, bottom up, and in-between approaches to new materials. Prog. Mater. Sci. 2016, 75, 1–37. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Tollan, C.; Yang, F.; Beltran, M.; Qin, Y.; Knez, M. Conductive Polymer–Inorganic Hybrid Materials through Synergistic Mutual Doping of the Constituents. ACS Appl. Mater. Interfaces 2017, 9, 27964–27971. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Z.; Wang, Y.; Gong, S.; Wang, X. Sequential infiltration synthesis of doped polymer films with tunable electrical properties for efficient triboelectric nanogenerator development. Adv. Mater. 2015, 27, 4938–4944. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Dev. 2016, 1, 225–255. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Yang, F.; Chen, C.; Zhang, L.; Qin, Y.; Knez, M. Tuning the conductivity of polyaniline through doping by means of single precursor vapor phase infiltration. Adv. Mater. Interfaces 2017, 4, 1600806. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Tollan, C.; Yang, F.; Qin, Y.; Knez, M. Efficient and controllable vapor to solid doping of the polythiophene P3HT by low temperature vapor phase infiltration. J. Mater. Chem. C 2017, 5, 2686–2694. [Google Scholar] [CrossRef]
- Ocola, L.E.; Wang, Y.; Divan, R.; Chen, J. Multifunctional UV and gas sensors based on vertically nanostructured zinc oxide: Volume versus surface effect. Sensors 2019, 19, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
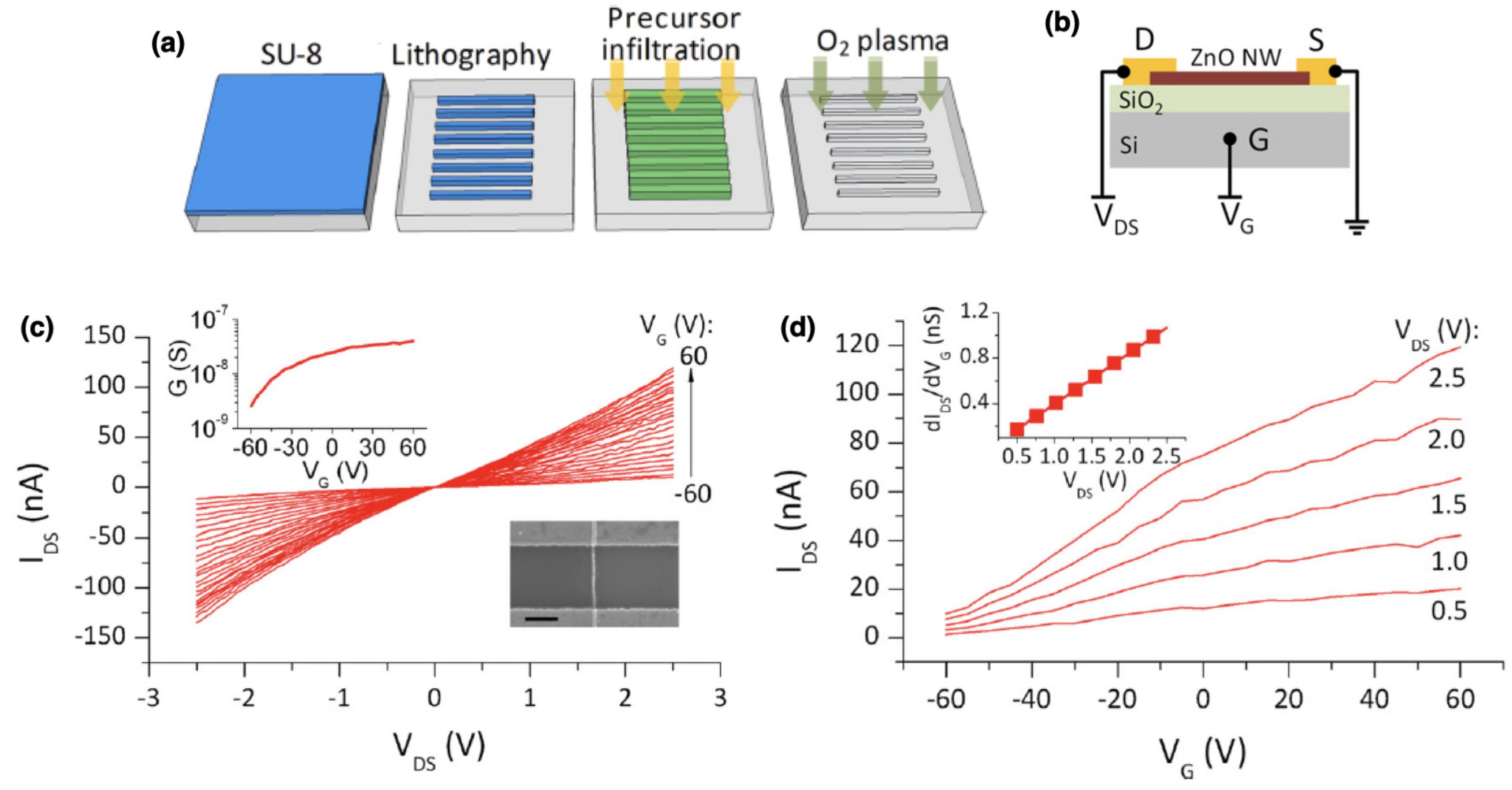
- Nam, C.Y.; Stein, A.; Kisslinger, K.; Black, C.T. Electrical and structural properties of ZnO synthesized via infiltration of lithographically defined polymer templates. Appl. Phys. Lett. 2015, 107, 203106. [Google Scholar] [CrossRef] [Green Version]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.P.; Lin, Y.H.; Lin, J.J. Electrical conduction mechanisms in natively doped ZnO nanowires. Nanotechnology 2008, 20, 015203. [Google Scholar] [CrossRef]
- Milano, G.; D’Ortenzi, L.; Bejtka, K.; Ciubini, B.; Porro, S.; Boarino, L.; Ricciardi, C. Metal-insulator transition in single crystalline ZnO nanowires. Nanotechnology 2021, 32, 185202. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, B.; Chan, H.; Alam, K.; Koneru, A.; Gage, T.E.; Ocola, L.E.; Divan, R.; Rosenmann, D.; Khanna, A.; Grisafe, B.; et al. Nanoporous Dielectric Resistive Memories Using Sequential Infiltration Synthesis. ACS Nano 2021, 15, 4155–4164. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Brede, J.; Ablat, H.; Abadia, M.; Zhang, L.; Rogero, C.; Elliott, S.D.; Knez, M. Reversible and irreversible reactions of trimethylaluminum with common organic functional groups as a model for molecular layer deposition and vapor phase infiltration. Adv. Mater. Interfaces 2017, 4, 1700237. [Google Scholar] [CrossRef] [Green Version]
- Waszkowska, K.; Krupka, O.; Kharchenko, O.; Figà, V.; Smokal, V.; Kutsevol, N.; Sahraoui, B. Influence of ZnO nanoparticles on nonlinear optical properties. Appl. Nanosci. 2020, 10, 4977–4982. [Google Scholar] [CrossRef]
- Huo, P.; Zhang, S.; Liang, Y.; Lu, Y.; Xu, T. Hyperbolic Metamaterials: Hyperbolic Metamaterials and Metasurfaces: Fundamentals and Applications. Adv. Opt. Mater. 2019, 7, 1970054. [Google Scholar] [CrossRef] [Green Version]
- Prezhdo, O.V. Advancing Physical Chemistry with Machine Learning. J. Phys. Chem. Lett. 2020, 11, 9656–9658. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef]
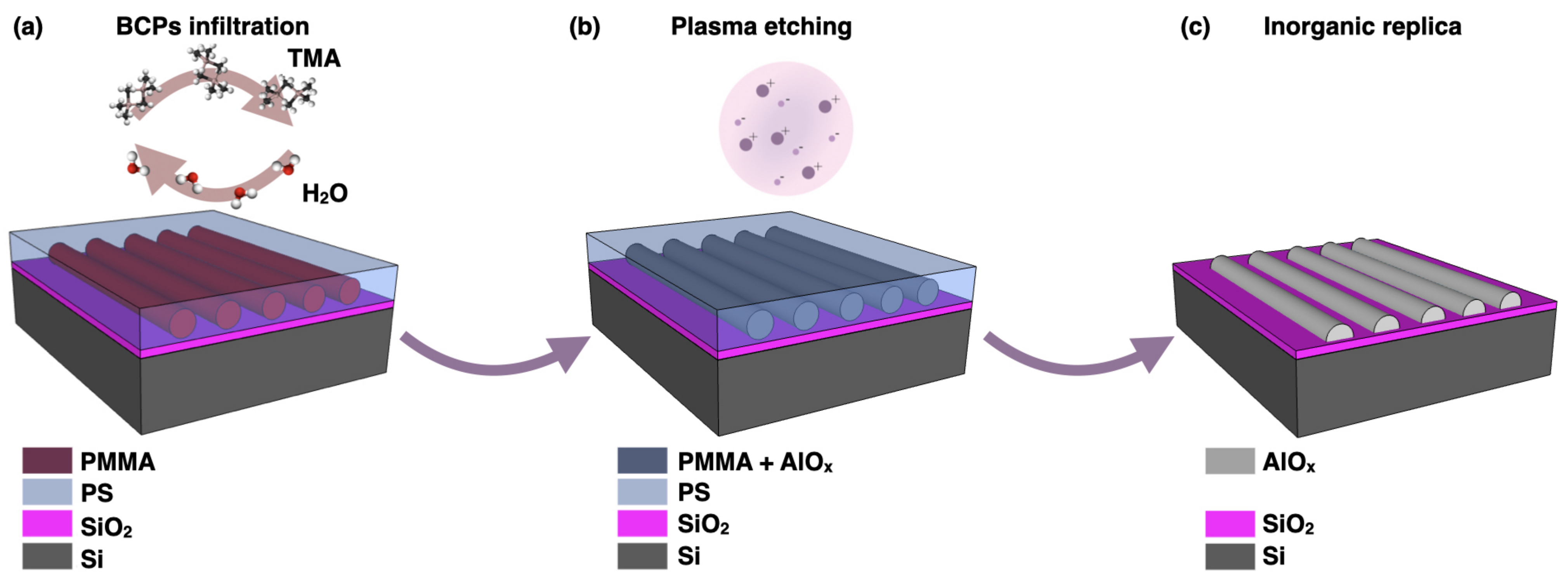

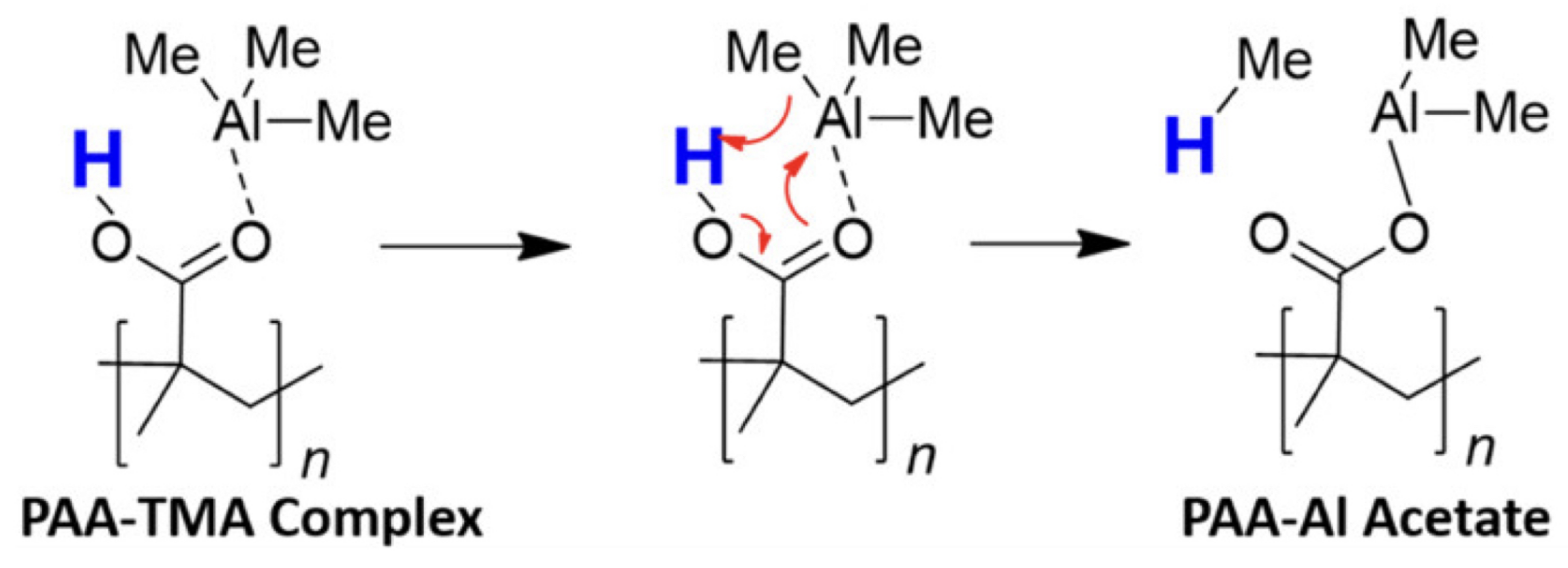




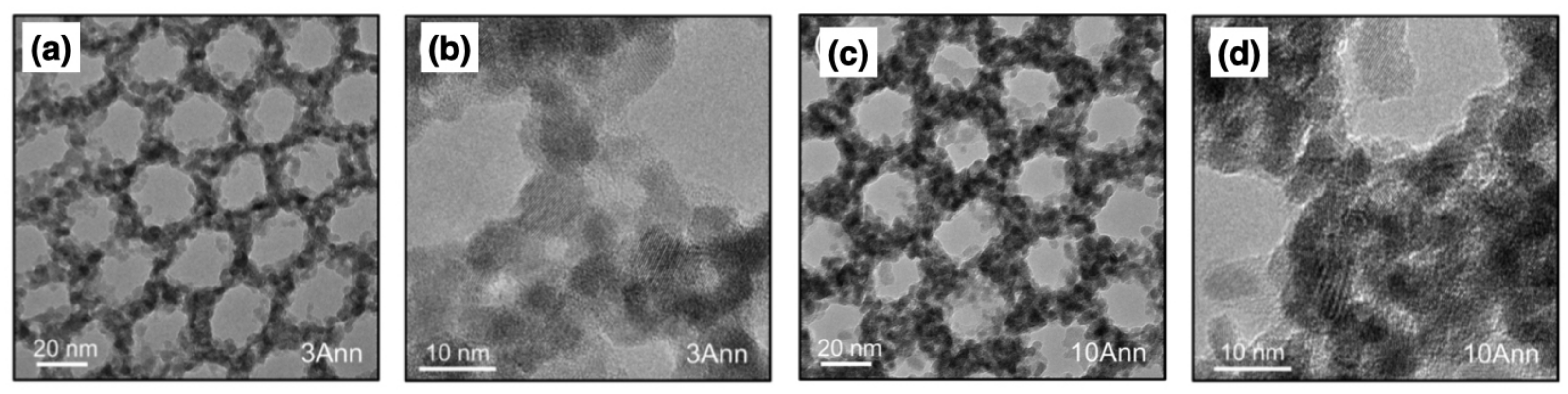
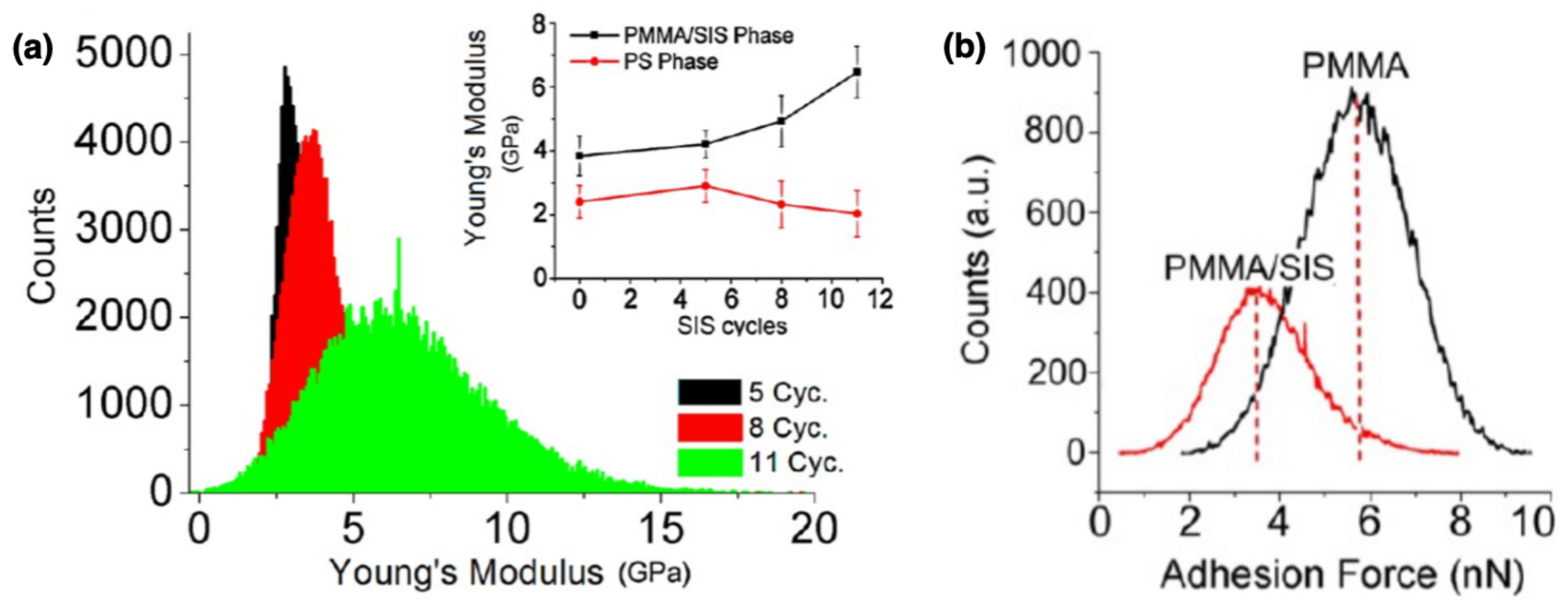
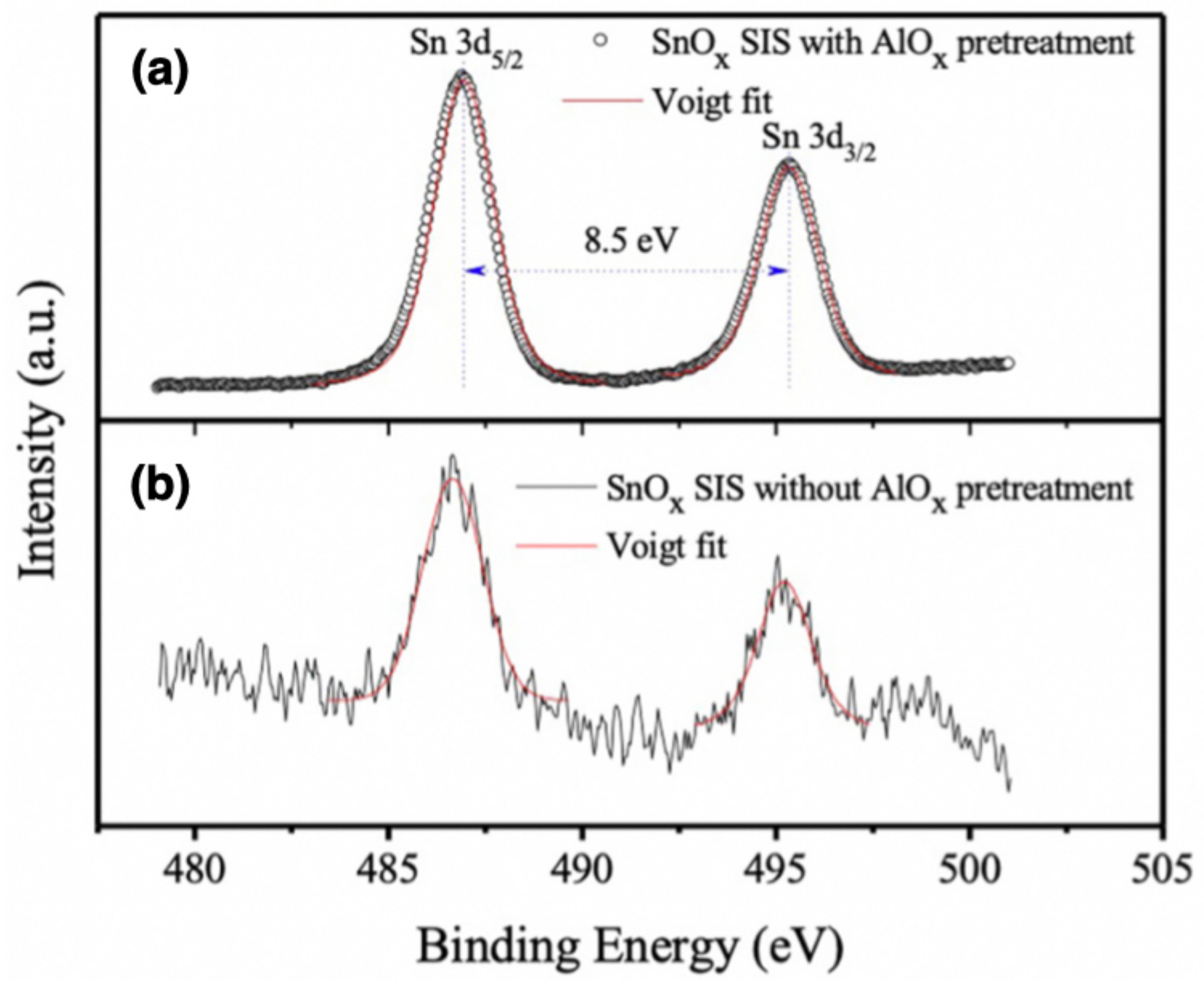
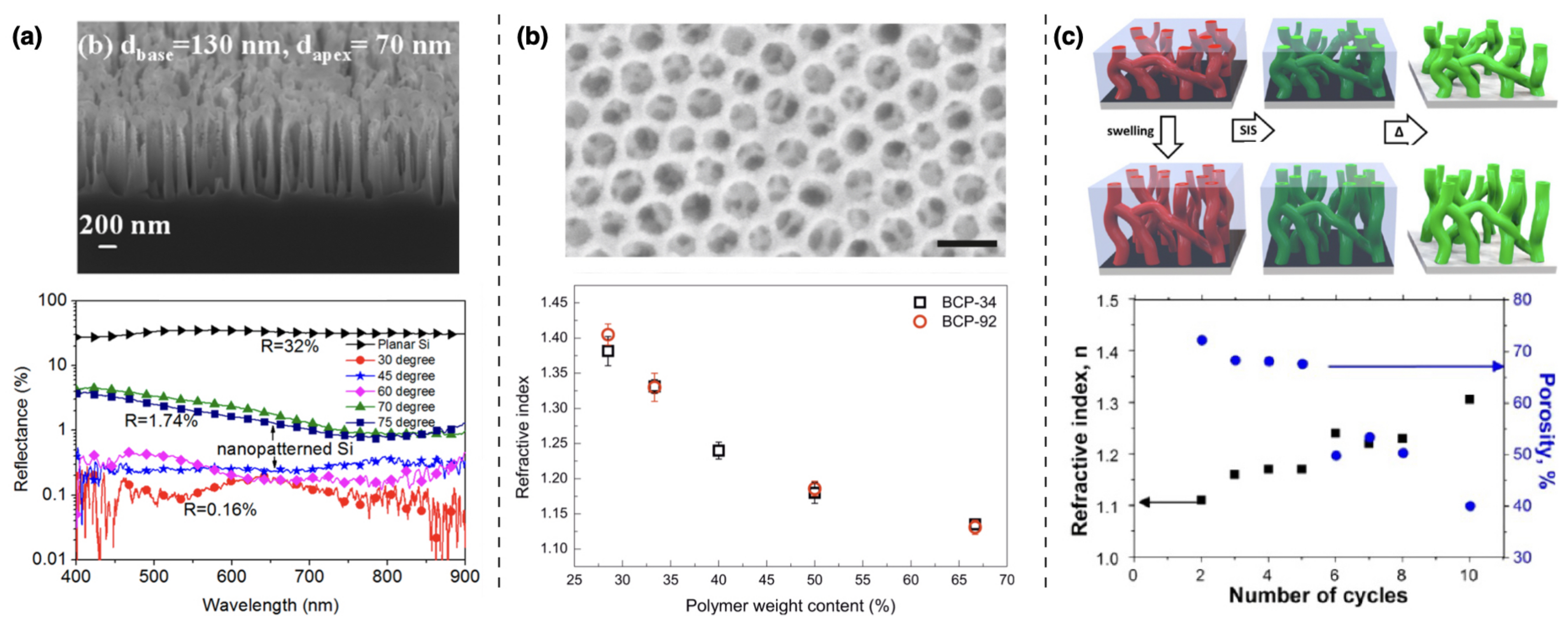
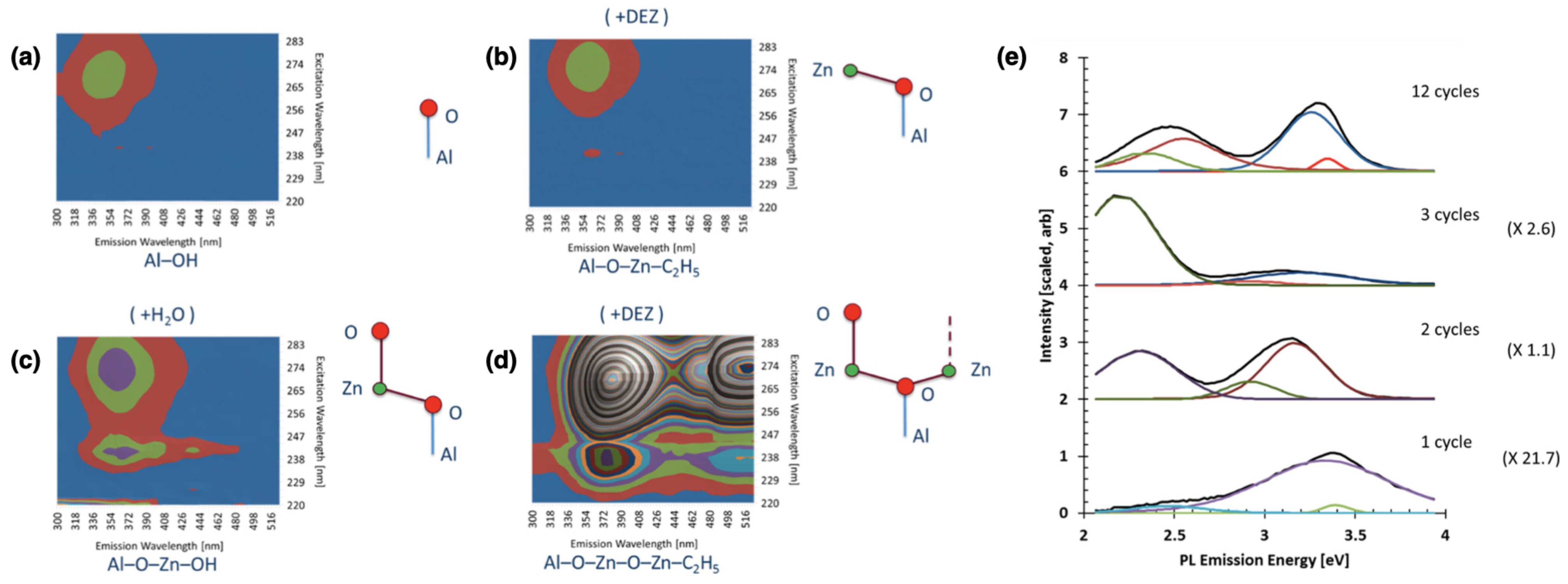
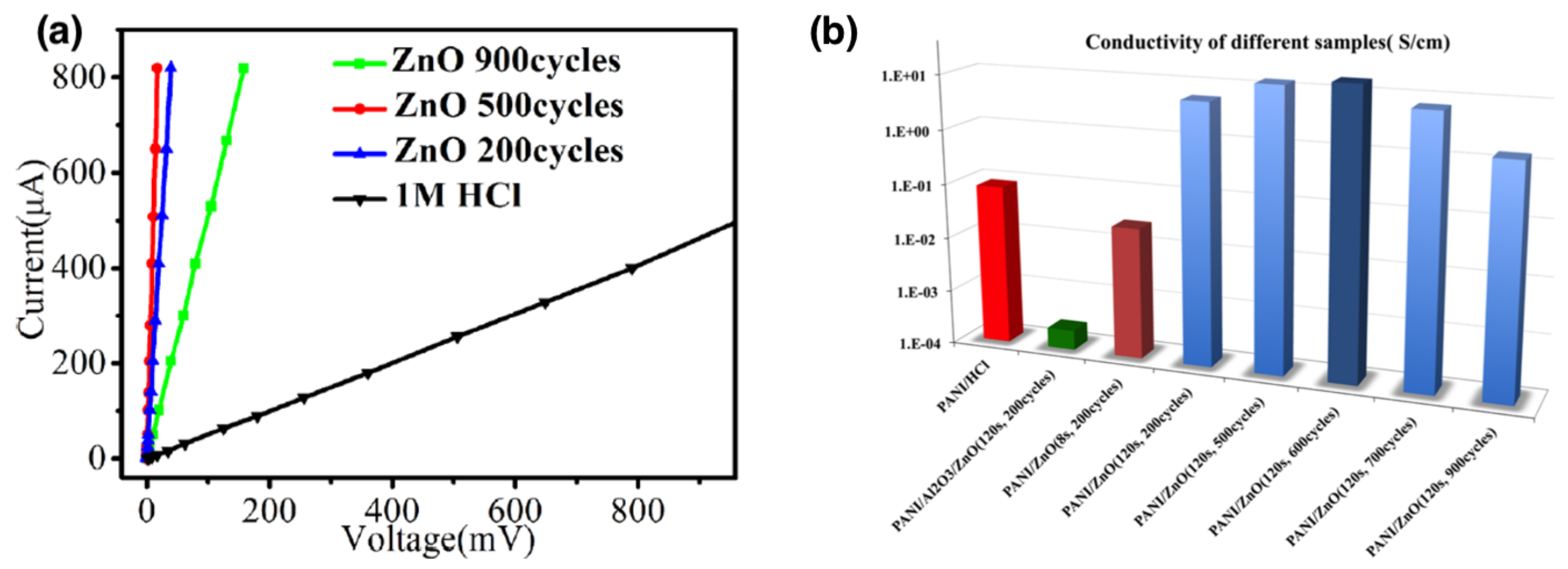
| Functional Groups | Polymers | References |
|---|---|---|
| Alkenes | PS-b-PI | [44,45] |
| Amides | PVP | [39] |
| Carboxylic acids | PAA | [39] |
| Esters | PS-b-PMMA PCL | [25,33,34,35,36] [40] |
| Epoxydes | PS-b-PIO | [44] |
| Pyridines | PS-b-P2VP PS-b-P4VP | [31,40] [46,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cara, E.; Murataj, I.; Milano, G.; De Leo, N.; Boarino, L.; Ferrarese Lupi, F. Recent Advances in Sequential Infiltration Synthesis (SIS) of Block Copolymers (BCPs). Nanomaterials 2021, 11, 994. https://doi.org/10.3390/nano11040994
Cara E, Murataj I, Milano G, De Leo N, Boarino L, Ferrarese Lupi F. Recent Advances in Sequential Infiltration Synthesis (SIS) of Block Copolymers (BCPs). Nanomaterials. 2021; 11(4):994. https://doi.org/10.3390/nano11040994
Chicago/Turabian StyleCara, Eleonora, Irdi Murataj, Gianluca Milano, Natascia De Leo, Luca Boarino, and Federico Ferrarese Lupi. 2021. "Recent Advances in Sequential Infiltration Synthesis (SIS) of Block Copolymers (BCPs)" Nanomaterials 11, no. 4: 994. https://doi.org/10.3390/nano11040994






