Coffee Grounds-Derived CNPs for Efficient Cr(VI) Water Remediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Methods of CNPs
2.3. CNPs Characterization
3. Results
4. Discussion
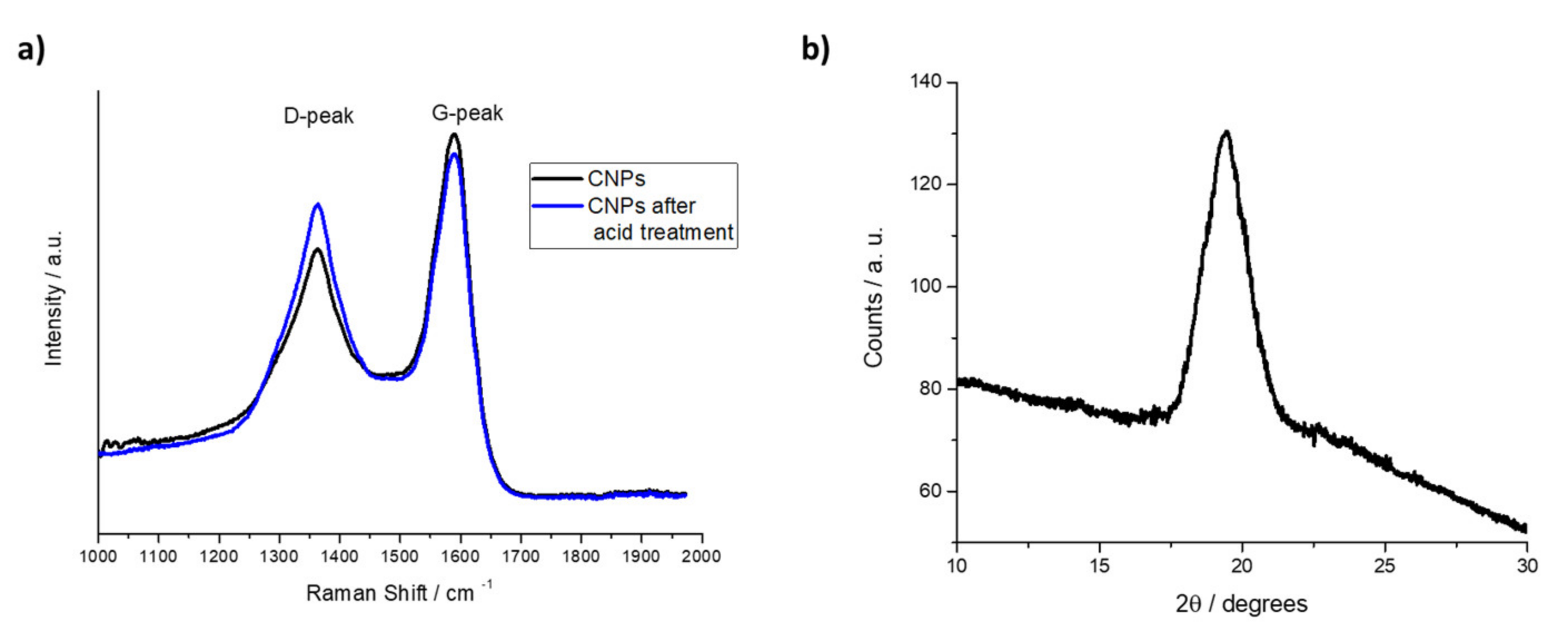
4.1. CNPs Spectroscopic Characterization
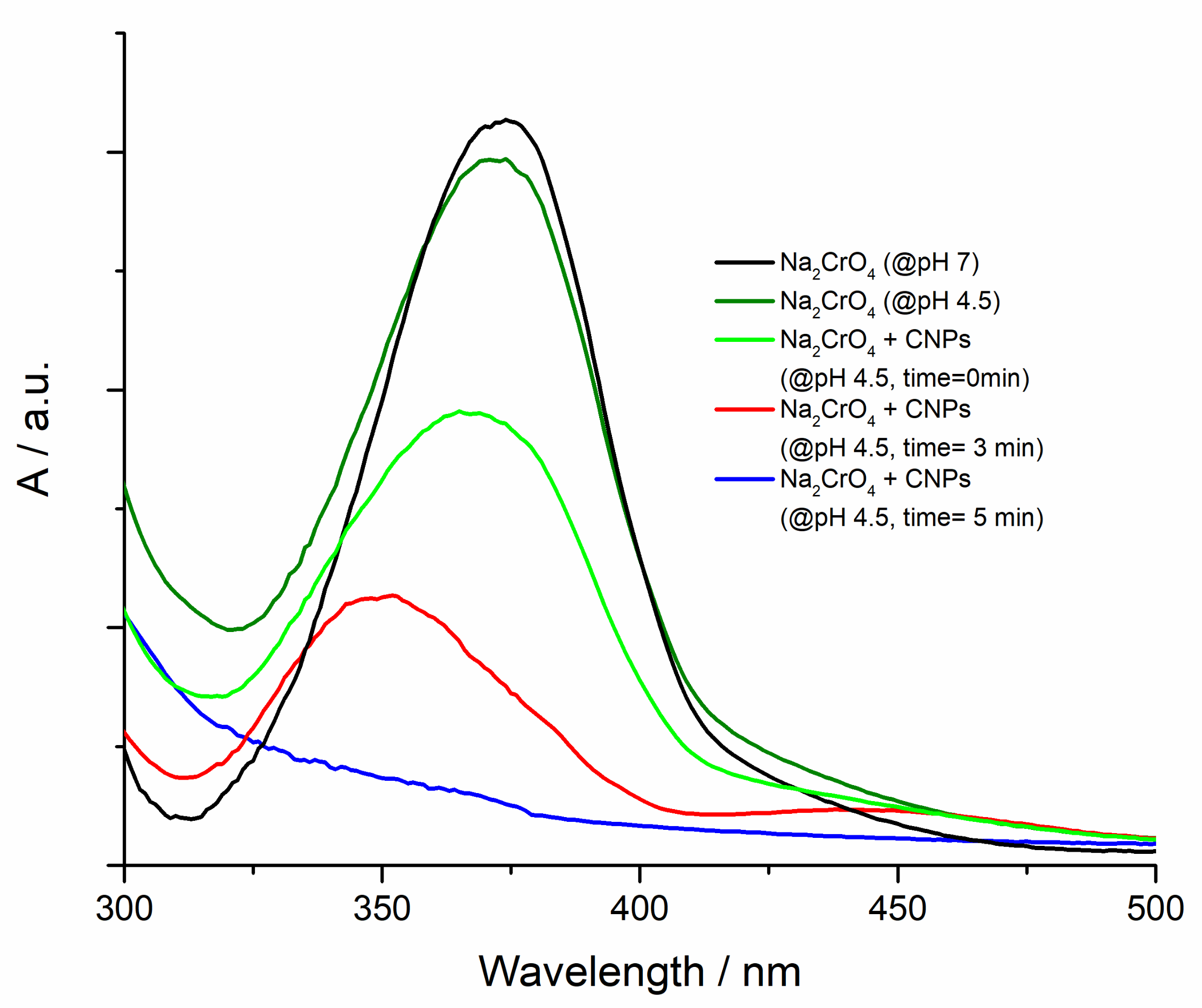
4.2. Remediation Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vishnu, D.; Dhandapani, B.; Kannappan Panchamoorthy, G.; Vo, D.V.N.; Ramakrishnan, S.R. Comparison of surface-engineered superparamagnetic nanosorbents with low-cost adsorbents of cellulose, zeolites and biochar for the removal of organic and inorganic pollutants: A review. Environ. Chem. Lett. 2021, 1, 3. [Google Scholar]
- Gheju, M. Progress in Understanding the Mechanism of CrVI Removal in Fe0-Based Filtration Systems. Water 2018, 10, 651. [Google Scholar] [CrossRef] [Green Version]
- Eivazihollagh, A.; Tejera, J.; Svanedal, I.; Edlund, H.; Blanco, A.; Norgren, M. Removal of Cd2+, Zn2+, and Sr2+ by Ion Flotation, Using a Surface-Active Derivative of DTPA (C12-DTPA). Ind. Eng. Chem. Res. 2017, 56, 10605–10614. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.N.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. A macroalgae-based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J. Environ. Manag. 2017, 191, 275–289. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Hammouda, S.B.; Doshi, B.; Guijarro, N.; Min, X.; Tang, C.J.; Sillanpää, M.; Sivula, K.; Wang, S. MIL-101(Fe)/g-C3N4 for enhanced visible-light-driven photocatalysis toward simultaneous reduction of Cr(VI) and oxidation of bisphenol A in aqueous media. Appl. Catal. B Environ. 2020, 272, 119033. [Google Scholar] [CrossRef]
- Peng, D.; Qiao, S.; Luo, Y.; Ma, H.; Zhang, L.; Hou, S.; Wu, B.; Xu, H. Performance of microbial induced carbonate precipitation for immobilizing Cd in water and soil. J. Hazard. Mater. 2020, 400. [Google Scholar] [CrossRef]
- Masindi, V. Recovery of drinking water and valuable minerals from acid mine drainage using an integration of magnesite, lime, soda ash, CO2 and reverse osmosis treatment processes. J. Environ. Chem. Eng. 2017, 5, 3136–3142. [Google Scholar] [CrossRef]
- Pagano, R.; Quarta, A.; Pal, S.; Licciulli, A.; Valli, L.; Bettini, S. Enhanced Solar-Driven Applications of ZnO@Ag Patchy Nanoparticles. J. Phys. Chem. C 2017, 121, 27199–27206. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, 1904362. [Google Scholar] [CrossRef]
- Sgobba, V.; Giancane, G.; Cannoletta, D.; Operamolla, A.; Hassan Omar, O.; Farinola, G.M.; Guldi, D.M.; Valli, L. Langmuir-schaefer films for aligned carbon nanotubes functionalized with a conjugate polymer and photoelectrochemical response enhancement. ACS Appl. Mater. Interfaces 2014, 6, 153–158. [Google Scholar] [CrossRef]
- Giancane, G.; Bettini, S.; Valli, L. State of art in the preparation, characterisation and applications of Langmuir-Blodgett films of carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 81–90. [Google Scholar] [CrossRef]
- Zieleniewska, A.; Lodermeyer, F.; Roth, A.; Guldi, D.M. Fullerenes-how 25 years of charge transfer chemistry have shaped our understanding of (interfacial) interactions. Chem. Soc. Rev. 2018, 47, 702–714. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, S.; Feng, T.; Xia, C.; Song, Y.; Yang, B. The polymeric characteristics and photoluminescence mechanism in polymer carbon dots: A review. Mater. Today Chem. 2017, 6, 13–25. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response. C J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Sawalha, S.; Carbone, L.; Giancane, G.; Prato, M.; Valli, L. Carbon nanodot-based heterostructures for improving the charge separation and the photocurrent generation. Nanoscale 2019, 11, 7414–7423. [Google Scholar] [CrossRef] [PubMed]
- Testa, C.; Zammataro, A.; Pappalardo, A.; Trusso Sfrazzetto, G. Catalysis with carbon nanoparticles. RSC Adv. 2019, 9, 27659–27664. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Boakye-Yiadom, K.O.; Kesse, S.; Opoku-Damoah, Y.; Filli, M.S.; Aquib, M.; Joelle, M.M.B.; Farooq, M.A.; Mavlyanova, R.; Raza, F.; Bavi, R.; et al. Carbon dots: Applications in bioimaging and theranostics. Int. J. Pharm. 2019, 564, 308–317. [Google Scholar] [CrossRef]
- Benetti, D.; Jokar, E.; Yu, C.H.; Fathi, A.; Zhao, H.; Vomiero, A.; Wei-Guang Diau, E.; Rosei, F. Hole-extraction and photostability enhancement in highly efficient inverted perovskite solar cells through carbon dot-based hybrid material. Nano Energy 2019, 62, 781–790. [Google Scholar] [CrossRef]
- Essner, J.B.; Baker, G.A. The emerging roles of carbon dots in solar photovoltaics: A critical review. Environ. Sci. Nano 2017, 4, 1216–1263. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, R.; Chen, H.; Zhang, J.; Wu, P. Highly efficient oxygen photosensitization of carbon dots: The role of nitrogen doping. Nanoscale 2020, 12, 5543–5553. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Ibarbia, A.; Sánchez-Abella, L.; Lezama, L.; Grande, H.J.; Ruiz, V. Graphene quantum dot-based hydrogels for photocatalytic degradation of organic dyes. Appl. Surf. Sci. 2020, 527, 146937. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Graphene quantum dots decorated rutile TiO2 nanoflowers for water splitting application. J. Energy Chem. 2018, 27, 306–310. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A.; Tian, Y.; Wang, X.; Gong, D.; Guo, Y.; Iqbal, K.; Wang, Z.; Liu, W.; Qin, W. Carbon dots prepared by solid state method via citric acid and 1,10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sensors Actuators, B Chem. 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Ji, C.; Zhou, Y.; Leblanc, R.M.; Peng, Z. Recent Developments of Carbon Dots in Biosensing: A Review. ACS Sensors 2020, 5, 2724–2741. [Google Scholar] [CrossRef]
- Yarur, F.; Macairan, J.R.; Naccache, R. Ratiometric detection of heavy metal ions using fluorescent carbon dots. Environ. Sci. Nano 2019, 6, 1121–1130. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small molecules derived carbon dots: Synthesis and applications in sensing, catalysis, imaging, and biomedicine. J. Nanobiotechnol. 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Zhi, L.; Müllen, K. A bottom-up approach from molecular nanographenes to unconventional carbon materials. J. Mater. Chem. 2008, 18, 1472–1484. [Google Scholar] [CrossRef]
- Sawalha, S.; Silvestri, A.; Criado, A.; Bettini, S.; Prato, M.; Valli, L. Tailoring the sensing abilities of carbon nanodots obtained from olive solid wastes. Carbon N. Y. 2020, 167, 696–708. [Google Scholar] [CrossRef]
- Kübel, C.; Eckhardt, K.; Enkelmann, V.; Wegner, G.; Müllen, K. Synthesis and crystal packing of large polycyclic aromatic hydrocarbons: Hexabenzo[bc,ef,hi,kl,no,qr]coronene and dibenzo[fg,ij]phenanthro[9,10,1,2,3- pqrst]pentaphene. J. Mater. Chem. 2000, 10, 879–886. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; Prato, M. Preparation, functionalization and characterization of engineered carbon nanodots. Nat. Protoc. 2019, 14, 2931–2953. [Google Scholar] [CrossRef]
- Jovanović, S.P.; Syrgiannis, Z.; Budimir, M.D.; Milivojević, D.D.; Jovanovic, D.J.; Pavlović, V.B.; Papan, J.M.; Bartenwerfer, M.; Mojsin, M.M.; Stevanović, M.J.; et al. Graphene quantum dots as singlet oxygen producer or radical quencher—The matter of functionalization with urea/thiourea. Mater. Sci. Eng. C 2020, 109, 110539. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; Tian, T.; Lin, S.; Xu, P.; Zhou, L.; Dai, C.; Hao, Q.; Wu, Y.; Zhai, Z.; et al. The effect of hexavalent chromium on the incidence and mortality of human cancers: A meta-analysis based on published epidemiological cohort studies. Front. Oncol. 2019, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Alammar, A.; Fulop, Z.; Pulido, B.A.; Nunes, S.P.; Szekely, G. Hydrophobic thin film composite nanofiltration membranes derived solely from sustainable sources. Green Chem. 2021, 23, 1175–1184. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Deepak, A.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Yang Nilsson, T.; Ulmefors, H.; Köhnke, T. Wet Spinning of Chitosan Fibers: Effect of Sodium Dodecyl Sulfate Adsorption and Enhanced Dope Temperature. ACS Appl. Polym. Mater. 2020, 2, 3867–3875. [Google Scholar] [CrossRef]
- Rincón-Iglesias, M.; Lizundia, E.; Costa, C.M.; Lanceros-Méndez, S. Tailoring Electrical and Mechanical Properties of All-Natural Polymer Composites for Environmentally Friendlier Electronics. ACS Appl. Polym. Mater. 2020, 2, 1448–1457. [Google Scholar] [CrossRef]
- Joe-Wong, C.; Brown, G.E.; Maher, K. Kinetics and Products of Chromium(VI) Reduction by Iron(II/III)-Bearing Clay Minerals. Environ. Sci. Technol. 2017, 51, 9817–9825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Chen, Z. Influence of pH on Cr(VI) reduction by organic reducing substances from sugarcane molasses. Appl. Water Sci. 2019, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Ahemad, M. Bacterial mechanisms for Cr(VI) resistance and reduction: An overview and recent advances. Folia Microbiol. 2014, 59, 321–332. [Google Scholar] [CrossRef]
- Resta, V.; Calcagnile, L.; Quarta, G.; Maruccio, L.; Cola, A.; Farella, I.; Giancane, G.; Valli, L. Optical and electrical properties of polycarbonate layers implanted by high energy Cu ions. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 312, 42–47. [Google Scholar] [CrossRef]
- Arumugam, N.; Kim, J. Synthesis of carbon quantum dots from Broccoli and their ability to detect silver ions. Mater. Lett. 2018, 219, 37–40. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Commun. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Sharma, A.; Gadly, T.; Gupta, A.; Ballal, A.; Ghosh, S.K.; Kumbhakar, M. Origin of Excitation Dependent Fluorescence in Carbon Nanodots. J. Phys. Chem. Lett. 2016, 7, 3695–3702. [Google Scholar] [CrossRef]
- Zhi, B.; Gallagher, M.J.; Frank, B.P.; Lyons, T.Y.; Qiu, T.A.; Da, J.; Mensch, A.C.; Hamers, R.J.; Rosenzweig, Z.; Fairbrother, D.H.; et al. Investigation of phosphorous doping effects on polymeric carbon dots: Fluorescence, photostability, and environmental impact. Carbon N. Y. 2018, 129, 438–449. [Google Scholar] [CrossRef]
- Bhaisare, M.L.; Talib, A.; Khan, M.S.; Pandey, S.; Wu, H.F. Synthesis of fluorescent carbon dots via microwave carbonization of citric acid in presence of tetraoctylammonium ion, and their application to cellular bioimaging. Microchim. Acta 2015, 182, 2173–2181. [Google Scholar] [CrossRef]
- Bajpai, S.K.; D’Souza, A.; Suhail, B. Blue light-emitting carbon dots (CDs) from a milk protein and their interaction with Spinacia oleracea leaf cells. Int. Nano Lett. 2019, 9, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Discrimination between roasted coffee, roasted corn and coffee husks by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. LWT Food Sci. Technol. 2013, 50, 715–722. [Google Scholar] [CrossRef] [Green Version]
- Figueiró, S.D.; Góes, J.C.; Moreira, R.A.; Sombra, A.S.B. On the physico-chemical and dielectric properties of glutaraldehyde crosslinked galactomannan-collagen films. Carbohydr. Polym. 2004, 56, 313–320. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Chou, W.L.; Wang, C.T.; Huang, K.Y.; Chang, Y.C.; Shu, C.M. Investigation of indium ions removal from aqueous solutions using spent coffee grounds. Int. J. Phys. Sci. 2012, 7. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational spectral investigation on xanthine and its derivatives—Theophylline, caffeine and theobromine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chemie Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Sun, L.; Teng, T.H.; Rashid, M.H.; Krysmann, M.; Dallas, P.; Wang, Y.; Hyun, B.R.; Bartnik, A.C.; Malliaras, G.G.; Wise, F.W.; et al. Electrogenerated Chemiluminescence from Carbon Dots. MRS Online Proc. Libr. (OPL) 2010, 1284, 131–136. [Google Scholar] [CrossRef]
- Papaioannou, N.; Titirici, M.M.; Sapelkin, A. Investigating the Effect of Reaction Time on Carbon Dot Formation, Structure, and Optical Properties. ACS Omega 2019, 4, 21658–21665. [Google Scholar] [CrossRef] [Green Version]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-Doped Carbon Quantum Dots as Fluorometric Probes for Iron Detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Kang, Z.; Lee, S.L. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Kim, S.; Hee Shin, D.; Oh Kim, C.; Seok Kang, S.; Sin Joo, S.; Choi, S.H.; Won Hwang, S.; Sone, C. Size-dependence of Raman scattering from graphene quantum dots: Interplay between shape and thickness. Appl. Phys. Lett. 2013, 102, 53108. [Google Scholar] [CrossRef]
- Kim, S.; Hwang, S.W.; Kim, M.K.; Shin, D.Y.; Shin, D.H.; Kim, C.O.; Yang, S.B.; Park, J.H.; Hwang, E.; Choi, S.H.; et al. Anomalous behaviors of visible luminescence from graphene quantum dots: Interplay between size and shape. ACS Nano 2012, 6, 8203–8208. [Google Scholar] [CrossRef]
- Vinci, J.C.; Ferrer, I.M.; Guterry, N.W.; Colón, V.M.; Destino, J.F.; Bright, F.V.; Colón, L.A. Spectroscopic characteristics of carbon dots (C-dots) derived from carbon fibers and conversion to sulfur-bridged C-dots nanosheets. Appl. Spectrosc. 2015, 69, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571–16581. [Google Scholar] [CrossRef] [PubMed]




| Elements | Coffee Grounds | CNPs * | ||
|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | |
| Ca | 47.10 | 44.31 | 10.40 | 7.73 |
| S | 10.88 | 12.79 | 0.21 | 0.20 |
| Mg | 8.53 | 13.23 | 20.67 | 25.35 |
| Fe | 8.13 | 5.49 | 0.53 | 0.28 |
| K | 8.02 | 7.73 | 7.00 | 5.33 |
| P | 4.48 | 5.45 | 42.60 | 41.00 |
| Al | 3.86 | 5.39 | 17.86 | 19.73 |
| Zn | 3.85 | 2.22 | 0.40 | 0.18 |
| Mn | 2.26 | 1.55 | 0.13 | 0.07 |
| Cu | 2.19 | 1.3 | 0.03 | 0.01 |
| Ni | 0.52 | 0.33 | 0.12 | 0.06 |
| Si | 0.10 | 0.13 | -- | -- |
| Ti | 0.09 | 0.07 | 0.06 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettini, S.; Ottolini, M.; Pagano, R.; Pal, S.; Licciulli, A.; Valli, L.; Giancane, G. Coffee Grounds-Derived CNPs for Efficient Cr(VI) Water Remediation. Nanomaterials 2021, 11, 1064. https://doi.org/10.3390/nano11051064
Bettini S, Ottolini M, Pagano R, Pal S, Licciulli A, Valli L, Giancane G. Coffee Grounds-Derived CNPs for Efficient Cr(VI) Water Remediation. Nanomaterials. 2021; 11(5):1064. https://doi.org/10.3390/nano11051064
Chicago/Turabian StyleBettini, Simona, Michela Ottolini, Rosanna Pagano, Sudipto Pal, Antonio Licciulli, Ludovico Valli, and Gabriele Giancane. 2021. "Coffee Grounds-Derived CNPs for Efficient Cr(VI) Water Remediation" Nanomaterials 11, no. 5: 1064. https://doi.org/10.3390/nano11051064
APA StyleBettini, S., Ottolini, M., Pagano, R., Pal, S., Licciulli, A., Valli, L., & Giancane, G. (2021). Coffee Grounds-Derived CNPs for Efficient Cr(VI) Water Remediation. Nanomaterials, 11(5), 1064. https://doi.org/10.3390/nano11051064











