Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection
Abstract
1. Introduction
2. Applications of Magnetofection In Vivo
2.1. Magnetopolyplexes
2.2. Magnetolipoplexes
2.3. “Unusual” Examples of Magnetic Carriers
3. Conclusions and Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Tregubov, A.A.; Nikitin, P.I.; Nikitin, M.P. Advanced Smart Nanomaterials with Integrated Logic-Gating and Biocomputing: Dawn of Theranostic Nanorobots. Chem. Rev. 2018, 118, 10294–10348. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Shipunova, V.O.; Deyev, S.M.; Nikitin, P.I. Biocomputing based on particle disassembly. Nat. Nanotechnol. 2014, 9, 716–722. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Zelepukin, I.V.; Shipunova, V.O.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat. Biomed. Eng. 2020, 4, 717–731. [Google Scholar] [CrossRef]
- Rozhkova, E.A. Nanoscale Materials for Tackling Brain Cancer: Recent Progress and Outlook. Adv. Mater. 2011, 23, H136–H150. [Google Scholar] [CrossRef] [PubMed]
- Vitol, E.A.; Novosad, V.; Rozhkova, E.A. Microfabricated magnetic structures for future medicine: From sensors to cell actuators. Nanomedicine 2012, 7, 1611–1624. [Google Scholar] [CrossRef]
- Rueda-Gensini, L.; Cifuentes, J.; Castellanos, M.C.; Puentes, P.R.; Serna, J.A.; Muñoz-Camargo, C.; Cruz, J.C. Tailoring Iron Oxide Nanoparticles for Efficient Cellular Internalization and Endosomal Escape. Nanomaterials 2020, 10, 1816. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Higuchi, Y.; Hashida, M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J. Pharm. Sci. 2008, 97, 726–745. [Google Scholar] [CrossRef]
- Riley, M.K.; Vermerris, W. Recent Advances in Nanomaterials for Gene Delivery—A Review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Ten years of magnetofection—Progress and prospects. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef]
- Luo, D.; Saltzman, W.M. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat. Biotechnol. 2000, 18, 893–895. [Google Scholar] [CrossRef]
- Li, Y.; Humphries, B.; Yang, C.; Wang, Z. Nanoparticle-Mediated Therapeutic Agent Delivery for Treating Metastatic Breast Cancer—Challenges and Opportunities. Nanomaterials 2018, 8, 361. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 1–7. [Google Scholar] [CrossRef]
- Ramasamy, T.; Munusamy, S.; Ruttala, H.B.; Kim, J.O. Smart Nanocarriers for the Delivery of Nucleic Acid-Based Therapeutics: A Comprehensive Review. Biotechnol. J. 2021, 16. [Google Scholar] [CrossRef]
- Jiang, Z.; Thayumanavan, S. Noncationic Material Design for Nucleic Acid Delivery. Adv. Ther. 2020, 3, 1900206. [Google Scholar] [CrossRef]
- Palfi, S.; Gurruchaga, J.M.; Ralph, G.S.; Lepetit, H.; Lavisse, S.; Buttery, P.C.; Watts, C.; Miskin, J.; Kelleher, M.; Deeley, S.; et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: A dose escalation, open-label, phase 1/2 trial. Lancet 2014, 383, 1138–1146. [Google Scholar] [CrossRef]
- Crystal, R.G. Adenovirus: The First EffectiveIn Vivo Gene Delivery Vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.M.; Black, G.C.M.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [CrossRef]
- Marshall, E. Gene Therapy Death Prompts Review of Adenovirus Vector. Science 1999, 286, 2244–2245. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; von Kalle, C.; Schmidt, M.; Le Deist, F.; Wulffraat, N.; McIntyre, E.; Radford, I.; Villeval, J.-L.; Fraser, C.C.; Cavazzana-Calvo, M.; et al. A Serious Adverse Event after Successful Gene Therapy for X-Linked Severe Combined Immunodeficiency. N. Engl. J. Med. 2003, 348, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy- An overview. J. Clin. Diagn. Res. 2015, 9, GE01–GE06. [Google Scholar] [CrossRef]
- Mintzer, M.A.; Simanek, E.E. Nonviral Vectors for Gene Delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.A.; Saber, M.; Aggag, M.; El-Shahawy, A.; Shokier, H.A. Magnetic nanoparticle-induced hyperthermia treatment under magnetic resonance imaging. Magn. Reson. Imaging 2011, 29, 272–280. [Google Scholar] [CrossRef]
- Loh, X.J.; Lee, T.-C.; Dou, Q.; Deen, G.R. Utilising inorganic nanocarriers for gene delivery. Biomater. Sci. 2015, 4, 70–86. [Google Scholar] [CrossRef]
- Saiyed, Z.M.; Telang, S.D.; Ramchand, C.N. Application of magnetic techniques in the field of drug discovery and biomedicine. Biomagn. Res. Technol. 2003. [Google Scholar] [CrossRef]
- Gigante, A.; Li, M.; Junghänel, S.; Hirschhäuser, C.; Knauer, S.; Schmuck, C. Non-viral transfection vectors: Are hybrid materials the way forward? Medchemcomm 2019, 10, 1692–1718. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.C.; Curtis, A.S.G. Functionalisation of magnetic nanoparticles for applications in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R198–R206. [Google Scholar] [CrossRef]
- Haimov-Talmoud, E.; Harel, Y.; Schori, H.; Motiei, M.; Atkins, A.; Popovtzer, R.; Lellouche, J.P.; Shefi, O. Magnetic Targeting of mTHPC to Improve the Selectivity and Efficiency of Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 45368–45380. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.N.; Kanev, I.L.; Mikheev, A.Y.; Shlyapnikova, E.A.; Shlyapnikov, Y.M.; Nikitin, M.P.; Nikitin, P.I.; Nwabueze, A.O.; van Hoek, M.L. Generation and delivery of nanoaerosols from biological and biologically active substances. J. Aerosol Sci. 2014, 69, 48–61. [Google Scholar] [CrossRef]
- Namiki, Y.; Namiki, T.; Yoshida, H.; Ishii, Y.; Tsubota, A.; Koido, S.; Nariai, K.; Mitsunaga, M.; Yanagisawa, S.; Kashiwagi, H.; et al. A novel magnetic crystal–lipid nanostructure for magnetically guided in vivo gene delivery. Nat. Nanotechnol. 2009, 4, 598–606. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Yoon, T.-J.; Jang, E.-S.; Hong, K.S.; Lee, S.Y.; Kim, O.R.; Park, C.; Kim, Y.-J.; Yi, G.-C.; Chang, K. Cetuximab-conjugated magneto-fluorescent silica nanoparticles for in vivo colon cancer targeting and imaging. Cancer Lett. 2010, 299, 63–71. [Google Scholar] [CrossRef]
- Tregubov, A.A.; Sokolov, I.L.; Babenyshev, A.V.; Nikitin, P.I.; Cherkasov, V.R.; Nikitin, M.P. Magnetic hybrid magnetite/metal organic framework nanoparticles: Facile preparation, post-synthetic biofunctionalization and tracking in vivo with magnetic methods. J. Magn. Magn. Mater. 2018, 449, 590–596. [Google Scholar] [CrossRef]
- Liu, G.; Xie, J.; Zhang, F.; Wang, Z.; Luo, K.; Zhu, L.; Quan, Q.; Niu, G.; Lee, S.; Ai, H.; et al. N-Alkyl-PEI-functionalized iron oxide nanoclusters for efficient siRNA delivery. Small 2011, 7, 2742–2749. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-One Target-Cell-Specific Magnetic Nanoparticles for Simultaneous Molecular Imaging and siRNA Delivery. Angew. Chem. Int. Ed. 2009, 48, 4174–4179. [Google Scholar] [CrossRef]
- Motiei, M.; Dreifuss, T.; Sadan, T.; Omer, N.; Blumenfeld-Katzir, T.; Fragogeorgi, E.; Loudos, G.; Popovtzer, R.; Ben-Eliezer, N. Trimodal Nanoparticle Contrast Agent for CT, MRI and SPECT Imaging: Synthesis and Characterization of Radiolabeled Core/Shell Iron Oxide@Gold Nanoparticles. Chem. Lett. 2019, 48, 291–294. [Google Scholar] [CrossRef]
- Lepeltier, E.; Rijo, P.; Rizzolio, F.; Popovtzer, R.; Petrikaite, V.; Assaraf, Y.G.; Passirani, C. Nanomedicine to target multidrug resistant tumors. Drug Resist. Updates 2020, 52, 100704. [Google Scholar] [CrossRef]
- Medarova, Z.; Pham, W.; Farrar, C.; Petkova, V.; Moore, A. In vivo imaging of siRNA delivery and silencing in tumors. Nat. Med. 2007, 13, 372–377. [Google Scholar] [CrossRef]
- Kumar, M.; Yigit, M.; Dai, G.; Moore, A.; Medarova, Z. Image-Guided Breast Tumor Therapy Using a Small Interfering RNA Nanodrug. Cancer Res. 2010, 70, 7553–7561. [Google Scholar] [CrossRef]
- Nikitin, M.P.; Orlov, A.V.; Znoyko, S.L.; Bragina, V.A.; Gorshkov, B.G.; Ksenevich, T.I.; Cherkasov, V.R.; Nikitin, P. Multiplex biosensing with highly sensitive magnetic nanoparticle quantification method. J. Magn. Magn. Mater. 2018, 459, 260–264. [Google Scholar] [CrossRef]
- Znoyko, S.L.; Orlov, A.V.; Pushkarev, A.V.; Mochalova, E.N.; Guteneva, N.V.; Lunin, A.V.; Nikitin, M.P.; Nikitin, P.I. Ultrasensitive quantitative detection of small molecules with rapid lateral-flow assay based on high-affinity bifunctional ligand and magnetic nanolabels. Anal. Chim. Acta 2018, 1034, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Orlov, A.V.; Sokolov, I.L.; Minakov, A.A.; Nikitin, P.I.; Ding, J.; Bader, S.D.; Rozhkova, E.A.; Novosad, V. Ultrasensitive detection enabled by nonlinear magnetization of nanomagnetic labels. Nanoscale 2018, 10, 11642–11650. [Google Scholar] [CrossRef]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-Coated Iron Oxide Nanoparticles for Efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Li, L.; Wang, C.; Su, Z.; Li, J. Multifunctional fluorescent-magnetic polyethyleneimine functionalized Fe3O4–mesoporous silica yolk–shell nanocapsules for siRNA delivery. Chem. Commun. 2012, 48, 8706–8708. [Google Scholar] [CrossRef] [PubMed]
- Krötz, F.; Sohn, H.-Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef]
- Mah, C.; Fraites, T.J.; Zolotukhin, I.; Song, S.; Flotte, T.R.; Dobson, J.; Batich, C.D.; Byrne, B.J. Improved Method of Recombinant AAV2 Delivery for Systemic Targeted Gene Therapy. Mol. Ther. 2002, 6, 106–112. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Plank, C.; Schillinger, U.; Scherer, F.; Bergemann, C.; Rémy, J.-S.; Krötz, F.; Anton, M.; Lausier, J.; Rosenecker, J. The Magnetofection Method: Using Magnetic Force to Enhance Gene Delivery. Biol. Chem. 2003, 384, 737–747. [Google Scholar] [CrossRef]
- Song, H.P.; Yang, J.Y.; Lo, S.L.; Wang, Y.; Fan, W.M.; Tang, X.S.; Xue, J.M.; Wang, S. Gene transfer using self-assembled ternary complexes of cationic magnetic nanoparticles, plasmid DNA and cell-penetrating Tat peptide. Biomaterials 2010, 31, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Rosenecker, J. Magnetofection: The Use of Magnetic Nanoparticles for Nucleic Acid Delivery. Cold Spring Harb. Protoc. 2009, 2009. [Google Scholar] [CrossRef] [PubMed]
- Castellani, S.; Orlando, C.; Carbone, A.; Di Gioia, S.; Conese, M. Magnetofection Enhances Lentiviral-Mediated Transduction of Airway Epithelial Cells through Extracellular and Cellular Barriers. Genes 2016, 7, 103. [Google Scholar] [CrossRef]
- Adijanto, J.; Naash, M.I. Nanoparticle-based technologies for retinal gene therapy. Eur. J. Pharm. Biopharm. 2015, 95, 353–367. [Google Scholar] [CrossRef]
- Schillinger, U.; Brill, T.; Rudolph, C.; Huth, S.; Gersting, S.; Krötz, F.; Hirschberger, J.; Bergemann, C.; Plank, C. Advances in magnetofection—magnetically guided nucleic acid delivery. J. Magn. Magn. Mater. 2005, 293, 501–508. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Antequera, Y.S.; Vlaskou, D.; Plank, C. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat. Protoc. 2007, 2, 2391–2411. [Google Scholar] [CrossRef]
- Schwerdt, J.I.; Goya, G.F.; Calatayud, M.P.; Herenu, C.B.; Reggiani, P.C.; Goya, R.G. Magnetic field-assisted gene delivery: Achievements and therapeutic potential. Curr. Gene Ther. 2012, 12, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Laurent, N.; Sapet, C.; Le Gourriérec, L.; Bertosio, E.; Zelphati, O. Nucleic acid delivery using magnetic nanoparticles: The Magnetofection™ technology. Ther. Deliv. 2011, 2, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Acosta, J.R.; Iriarte-Mesa, C.; Ortega, G.A.; Díaz-García, A.M. DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications. Top. Curr. Chem. 2020, 378, 1–29. [Google Scholar] [CrossRef]
- Dobson, J.M. Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Sicard, F.; Sapet, C.; Laurent, N.; Bertosio, E.; Bertuzzi, M.; Zelphati, O. Magnetofection of Minicircle DNA Vectors. In Minicircle and Miniplasmid DNA Vectors; Wiley: Hoboken, NJ, USA, 2013; pp. 165–176. [Google Scholar]
- Nie, X.; Zhang, Z.; Wang, C.-H.; Fan, Y.-S.; Meng, Q.-Y.; You, Y.-Z. Interactions in DNA Condensation: An Important Factor for Improving the Efficacy of Gene Transfection. Bioconjug. Chem. 2019, 30, 284–292. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef]
- Zhang, Y.; Satterlee, A.; Huang, L. In Vivo Gene Delivery by Nonviral Vectors: Overcoming Hurdles? Mol. Ther. 2012, 20, 1298–1304. [Google Scholar] [CrossRef]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of Magnetic Nanoparticles to Gene Delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef] [PubMed]
- Massadeh, S.; Al-Aamery, M.; Bawazeer, S.; Alahmad, O.; Alsubai, R.; Barker, S.; Craig, D. Nano-materials for Gene Therapy: An Efficient Way in Overcoming Challenges of Gene Delivery. J. Biosens. Bioelectron. 2016, 7. [Google Scholar] [CrossRef]
- He, C.-X.; Tabata, Y.; Gao, J.-Q. Non-viral gene delivery carrier and its three-dimensional transfection system. Int. J. Pharm. 2010, 386, 232–242. [Google Scholar] [CrossRef]
- Namvar, A.; Bolhassani, A.; Khairkhah, N.; Motevalli, F. Physicochemical properties of polymers: An important system to overcome the cell barriers in gene transfection. Biopolymers 2015, 103, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Feng, Y.; Zhang, L. Biodegradable carrier/gene complexes to mediate the transfection and proliferation of human vascular endothelial cells. Polym. Adv. Technol. 2015, 26, 1370–1377. [Google Scholar] [CrossRef]
- Gulce-Iz, S.; Saglam-Metiner, P. Current State of the Art in DNA Vaccine Delivery and Molecular Adjuvants: Bcl-xL Anti-Apoptotic Protein as a Molecular Adjuvant. In Immune Response Activation and Immunomodulation; IntechOpen Limited: London, UK, 2019. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, X.; Liu, M.; Deng, Y.; He, N. Current Progress in Gene Delivery Technology Based on Chemical Methods and Nano-carriers. Theranostics 2014, 4, 240–255. [Google Scholar] [CrossRef]
- Tian, H.; Chen, J.; Chen, X. Nanoparticles for Gene Delivery. Small 2013, 9, 2034–2044. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Jiao, Y.; Xia, Z.L.; Ze, L.J.; Jing, H.; Xin, B.; Fu, S. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed. Microdevices 2020, 22, 16. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Ruedel, A.; Bosserhoff, A.K. Transfection Methods Overview. Methods Cell Biol. 2012, 112, 163–182. [Google Scholar] [CrossRef]
- Kaestner, L.; Scholz, A.; Lipp, P. Conceptual and technical aspects of transfection and gene delivery. Bioorg. Med. Chem. Lett. 2015, 25, 1171–1176. [Google Scholar] [CrossRef]
- Unciti-Broceta, A.; Bacon, M.N.; Bradley, M. Strategies for the preparation of synthetic transfection vectors. Top. Curr. Chem. 2010, 296, 15–49. [Google Scholar] [CrossRef] [PubMed]
- Agi, E.; Mosaferi, Z.; Khatamsaz, S.; Cheraghi, P.; Samadian, N.; Bolhassani, A. Different strategies of gene delivery for treatment of cancer and other disorders. J. Solid Tumors 2016, 6. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Mantovani, D.; Candiani, G. Non-Viral in Vitro Gene Delivery: It is Now Time to Set the Bar! Pharmaceutics 2020, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Lee, S.J.; Cherukula, K.; Cho, C.-S.; Park, I.-K. Polysaccharide-Coated Magnetic Nanoparticles for Imaging and Gene Therapy. BioMed Res. Int. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Q.; Hao, X.; Ren, X.; Guo, J.; Feng, Y.; Shi, C. Multi-targeting peptides for gene carriers with high transfection efficiency. J. Mater. Chem. B 2017, 5, 8035–8051. [Google Scholar] [CrossRef]
- Mykhaylyk, O.; Zelphati, O.; Hammerschmid, E.; Anton, M.; Rosenecker, J.; Plank, C. Recent Advances in Magnetofection and Its Potential to Deliver siRNAs In Vitro. Methods Mol. Biol. 2008, 487, 1–36. [Google Scholar] [CrossRef]
- Buerli, T.; Pellegrino, C.; Baer, K.; Lardi-Studler, B.; Chudotvorova, I.; Fritschy, J.-M.; Medina, I.; Fuhrer, C. Efficient transfection of DNA or shRNA vectors into neurons using magnetofection. Nat. Protoc. 2007, 2, 3090–3101. [Google Scholar] [CrossRef]
- Krötz, F.; de Wit, C.; Sohn, H.-Y.; Zahler, S.; Gloe, T.; Pohl, U.; Plank, C. Magnetofection—A highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol. Ther. 2003, 7, 700–710. [Google Scholar] [CrossRef]
- Cui, Y.; Li, X.; Zeljic, K.; Shan, S.; Qiu, Z.; Wang, Z. Effect of PEGylated Magnetic PLGA-PEI Nanoparticles on Primary Hippocampal Neurons: Reduced Nanoneurotoxicity and Enhanced Transfection Efficiency with Magnetofection. ACS Appl. Mater. Interfaces 2019, 11, 38190–38204. [Google Scholar] [CrossRef]
- Soto-Sánchez, C.; Martínez-Navarrete, G.; Humphreys, L.; Puras, G.; Zarate, J.; Pedraz, J.L.; Fernández, E. Enduring high-efficiency in vivo transfection of neurons with non-viral magnetoparticles in the rat visual cortex for optogenetic applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 835–843. [Google Scholar] [CrossRef]
- Singh, J.; Mohanty, I.; Rattan, S. In vivo magnetofection: A novel approach for targeted topical delivery of nucleic acids for rectoanal motility disorders. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G109–G118. [Google Scholar] [CrossRef]
- Brett, E.; Zielins, E.R.; Luan, A.; Ooi, C.C.; Shailendra, S.; Atashroo, D.; Menon, S.; Blackshear, C.; Flacco, J.; Quarto, N.; et al. Magnetic Nanoparticle-Based Upregulation of B-Cell Lymphoma 2 Enhances Bone Regeneration. Stem Cells Transl. Med. 2016, 6, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.; Oyane, A.; Nakamura, M.; Puentes, S.; Marushima, A.; Tsurushima, H. Rapid one-pot fabrication of magnetic calcium phosphate nanoparticles immobilizing DNA and iron oxide nanocrystals using injection solutions for magnetofection and magnetic targeting. Mater. Today Chem. 2017, 6, 51–61. [Google Scholar] [CrossRef]
- Shubhra, Q.T.; Oyane, A.; Nakamura, M.; Puentes, S.; Marushima, A.; Tsurushima, H. Preliminary in vivo magnetofection data using magnetic calcium phosphate nanoparticles immobilizing DNA and iron oxide nanocrystals. Data Brief 2018, 18, 1696–1701. [Google Scholar] [CrossRef]
- Prosen, L.; Markelc, B.; Dolinsek, T.; Music, B.; Cemazar, M.; Sersa, G. Mcam Silencing With RNA Interference Using Magnetofection has Antitumor Effect in Murine Melanoma. Mol. Ther. Nucleic Acids 2014, 3, e205. [Google Scholar] [CrossRef] [PubMed]
- Prosen, L.; Hudoklin, S.; Čemažar, M.; Stimac, M.; Tratar, U.L.; Ota, M.; Ščančar, J.; Romih, R.; Sersa, G. Magnetic field contributes to the cellular uptake for effective therapy with magnetofection using plasmid DNA encoding against Mcam in B16F10 melanoma in vivo. Nanomedicine 2016, 11, 627–641. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, W.; Nie, Y.; He, Y.; Jiang, Q.; Lan, F.; Wu, Y.; Gu, Z. Low aggregation magnetic polyethyleneimine complexes with different saturation magnetization for efficient gene transfection in vitro and in vivo. RSC Adv. 2013, 3, 23571–23581. [Google Scholar] [CrossRef]
- Hüttinger, C.; Hirschberger, J.; Jahnke, A.; Köstlin, R.; Brill, T.; Plank, C.; Küchenhoff, H.; Krieger, S.; Schillinger, U. Neoadjuvant gene delivery of feline granulocyte-macrophage colony-stimulating factor using magnetofection for the treatment of feline fibrosarcomas: A phase I trial. J. Gene Med. 2008, 10, 655–667. [Google Scholar] [CrossRef]
- Jahnke, A.; Hirschberger, J.; Fischer, C.; Brill, T.; Köstlin, R.; Plank, C.; Küchenhoff, H.; Krieger, S.; Kamenica, K.; Schillinger, U. Intra-tumoral Gene Delivery of feIL-2, feIFN-γ and feGM-CSF using Magnetofection as a Neoadjuvant Treatment Option for Feline Fibrosarcomas: A Phase-I Study. J. Veter Med. Ser. A Physiol. Pathol. Clin. Med. 2007, 54, 599–606. [Google Scholar] [CrossRef]
- Li, W.; Ma, N.; Ong, L.-L.; Kaminski, A.; Skrabal, C.; Ugurlucan, M.; Lorenz, P.; Gatzen, H.-H.; Lützow, K.; Lendlein, A.; et al. Enhanced thoracic gene delivery by magnetic nanobead-mediated vector. J. Gene Med. 2008, 10, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, K.; Qiao, C.; Jin, X.; Zheng, C.; Yang, B.; Sun, H. Antitumor effect of human TRAIL on adenoid cystic carcinoma using magnetic nanoparticle–mediated gene expression. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 141–150. [Google Scholar] [CrossRef]
- Prijic, S.; Prosen, L.; Cemazar, M.; Scancar, J.; Romih, R.; Lavrencak, J.; Bregar, V.B.; Coer, A.; Krzan, M.; Žnidaršič, A.; et al. Surface modified magnetic nanoparticles for immuno-gene therapy of murine mammary adenocarcinoma. Biomaterials 2012, 33, 4379–4391. [Google Scholar] [CrossRef]
- Xenariou, S.; Griesenbach, U.; Ferrari, S.; Dean, P.; Scheule, R.K.; Cheng, S.H.; Geddes, D.M.; Plank, C.; Alton, E.W.F.W. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006, 13, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, Z.; Zhou, G.; Ling, J.; Tian, A.; Sun, N. Silencing Bag-1 gene via magnetic gold nanoparticle-delivered siRNA plasmid for colorectal cancer therapy in vivo and in vitro. Tumor Biol. 2016, 37, 10365–10374. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, X.; Li, M.; Huang, H.; Kang, Q.; Zhang, X.; Hui, H.; Zhang, X.; Chen, C.; Luo, Y.; et al. A novel strategy for in vivo angiogenesis and osteogenesis: Magnetic micro-movement in a bone scaffold. Artif. Cells Nanomed. Biotechnol. 2018, 46, 636–645. [Google Scholar] [CrossRef]
- Holzbach, T.; Vlaskou, D.; Neshkova, I.; Konerding, M.A.; Wörtler, K.; Mykhaylyk, O.; Gänsbacher, B.; Machens, H.; Plank, C.; Giunta, R.E. Non-viral VEGF165 gene therapy – magnetofection of acoustically active magnetic lipospheres (‘magnetobubbles’) increases tissue survival in an oversized skin flap model. J. Cell. Mol. Med. 2010, 14, 587–599. [Google Scholar] [CrossRef]
- Xiang, L.; Bin, W.; Huali, J.; Wei, J.; Jiesheng, T.; Feng, G.; Ying, L. Bacterial magnetic particles (BMPs)-PEI as a novel and efficient non-viral gene delivery system. J. Gene Med. 2007, 9, 679–690. [Google Scholar] [CrossRef]
- Zhou, X.-F.; Liu, B.; Yu, X.-H.; Zha, X.; Zhang, X.-Z.; Wang, X.-Y.; Jin, Y.-H.; Wu, Y.-G.; Jiang, C.-L.; Chen, Y.; et al. Using Magnetic Force to Enhance Immune Response to DNA Vaccine. Small 2007, 3, 1707–1713. [Google Scholar] [CrossRef]
- De Almeida, S.S.T.; Horst, C.H.; Soto-Sánchez, C.; Fernandez, E.; De Almeida, R.T. Delivery of miRNA-Targeted Oligonucleotides in the Rat Striatum by Magnetofection with Neuromag®. Molecules 2018, 23, 1825. [Google Scholar] [CrossRef]
- Tang, Y.-S.; Wang, D.; Zhou, C.; Ma, W.; Zhang, Y.-Q.; Liu, B.; Zhang, S. Bacterial magnetic particles as a novel and efficient gene vaccine delivery system. Gene Ther. 2011, 19, 1187–1195. [Google Scholar] [CrossRef]
- Lübbe, A.S.; Bergemann, C.; Brock, J.; McClure, D.G. Physiological aspects in magnetic drug-targeting. J. Magn. Magn. Mater. 1999, 194, 149–155. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Wang, B.; Qiao, W.; Liu, D.; Li, Z. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J. Control. Release 2004, 100, 165–180. [Google Scholar] [CrossRef]
- Noske, S.; Karimov, M.; Aigner, A.; Ewe, A. Tyrosine-Modification of Polypropylenimine (PPI) and Polyethylenimine (PEI) Strongly Improves Efficacy of siRNA-Mediated Gene Knockdown. Nanomaterials 2020, 10, 1809. [Google Scholar] [CrossRef]
- Puente-Massaguer, E.; Strobl, F.; Grabherr, R.; Striedner, G.; Lecina, M.; Gòdia, F. PEI-Mediated Transient Transfection of High Five Cells at Bioreactor Scale for HIV-1 VLP Production. Nanomaterials 2020, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- Blömer, U.; Naldini, L.; Kafri, T.; Trono, D.; Verma, I.M.; Gage, F.H. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 1997, 71, 6641–6649. [Google Scholar] [CrossRef]
- Kordower, J.H.; Bloch, J.; Ma, S.Y.; Chu, Y.; Palfi, S.; Roitberg, B.Z.; Emborg, M.; Hantraye, P.; Déglon, N.; Aebischer, P. Lentiviral Gene Transfer to the Nonhuman Primate Brain. Exp. Neurol. 1999, 160, 1–16. [Google Scholar] [CrossRef]
- Riban, V.; Fitzsimons, H.L.; During, M.J. Gene therapy in epilepsy. Epilepsia 2009, 50, 24–32. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-Soluble Iron Oxide Nanoparticles with High Stability and Selective Surface Functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef] [PubMed]
- Stayton, P.; El-Sayed, M.; Murthy, N.; Bulmuş, V.; Lackey, C.; Cheung, C.; Hoffman, A. ’Smart’ delivery systems for biomolecular therapeutics. Orthod. Craniofac. Res. 2005, 8, 219–225. [Google Scholar] [CrossRef]
- Trubetskoy, V.S.; Wong, S.C.; Subbotin, V.; Budker, V.G.; Loomis, A.; Hagstrom, J.E.; Wolff, J.A. Recharging cationic DNA complexes with highly charged polyanions for in vitro and in vivo gene delivery. Gene Ther. 2003, 10, 261–271. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4(M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, J.; Song, X.; Wang, G.; Yang, W. Facile synthesis of Fe3O4/SiO2 composite nanoparticles from primary silica particles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 317, 450–456. [Google Scholar] [CrossRef]
- Pan, S.; Cao, D.; Huang, H.; Yi, W.; Qin, L.; Feng, M. A Serum-Resistant Low-Generation Polyamidoamine with PEI 423 Outer Layer for Gene Delivery Vector. Macromol. Biosci. 2013, 13, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef]
- Kumar, R.; Kulkarni, A.; Nabulsi, J.; Nagesha, D.K.; Cormack, R.; Makrigiorgos, M.G.; Sridhar, S. Facile synthesis of PEGylated PLGA nanoparticles encapsulating doxorubicin and its in vitro evaluation as potent drug delivery vehicle. Drug Deliv. Transl. Res. 2013, 3, 299–308. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-Targeting Magnetic PLGA Nanoparticles for Codelivery of Paclitaxel and Curcumin for Brain Tumor Therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Chen, Z.; Xie, X.; Zhang, H.; Feng, Y.; Li, S.; Qin, X.; Yang, H.; Wu, C.; et al. NIR-Light-Triggered Anticancer Strategy for Dual-Modality Imaging-Guided Combination Therapy via a Bioinspired Hybrid PLGA Nanoplatform. Mol. Pharm. 2019, 16, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Didier, J.E.; Ingber, D.E.; Weitz, D.A. Collective Shape Actuation of Polymer Double Emulsions by Solvent Evaporation. ACS Appl. Mater. Interfaces 2018, 10, 31865–31869. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Tong, S.; Lee, C.M.; Deshmukh, H.; Bao, G. Spatial control of in vivo CRISPR–Cas9 genome editing via nanomagnets. Nat. Biomed. Eng. 2019, 3, 126–136. [Google Scholar] [CrossRef]
- Deveza, L.; Choi, J.; Imanbayev, G.; Yang, F. Paracrine Release from Nonviral Engineered Adipose-Derived Stem Cells Promotes Endothelial Cell Survival and Migration In Vitro. Stem Cells Dev. 2013, 22, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Keeney, M.; Ong, S.-G.; Padilla, A.; Yao, Z.; Goodman, S.; Wu, J.C.; Yang, F. Development of Poly(β-amino ester)-Based Biodegradable Nanoparticles for Nonviral Delivery of Minicircle DNA. ACS Nano 2013, 7, 7241–7250. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Grova, M.; Nejadnik, H.; Lo, D.; Morrison, S.; Montoro, D.; Chung, M.; Zimmermann, A.; Walmsley, G.G.; Lee, M.; et al. Enhancing In Vivo Survival of Adipose-Derived Stromal Cells Through Bcl-2 Overexpression Using a Minicircle Vector. Stem Cells Transl. Med. 2013, 2, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Levi, B.; James, A.W.; Nelson, E.R.; Vistnes, D.; Wu, B.; Lee, M.; Gupta, A.; Longaker, M.T. Human Adipose Derived Stromal Cells Heal Critical Size Mouse Calvarial Defects. PLoS ONE 2010, 5, e11177. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Wang, M.-J.; Pai, N.-S.; Yen, S.-K. Preparation and characterization of gelatin–hydroxyapatite composite microspheres for hard tissue repair. Mater. Sci. Eng. C 2015, 57, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.Z.; Farkhani, S.M.; Goloujeh, M.S.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotechnol. 2014, 44, 722–734. [Google Scholar] [CrossRef]
- Guo, L.; Liu, G.; Hong, R.-Y.; Li, H.-Z. Preparation and Characterization of Chitosan Poly(acrylic acid) Magnetic Microspheres. Mar. Drugs 2010, 8, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Giunta, R.E.; Holzbach, T.; Taskov, C.; Holm, P.S.; Konerding, M.A.; Schams, D.; Biemer, E.; Gänsbacher, B. AdVEGF165gene transfer increases survival in overdimensioned skin flaps. J. Gene Med. 2004, 7, 297–306. [Google Scholar] [CrossRef]
- Vlaskou, D.; Mykhaylyk, O.; Krötz, F.; Hellwig, N.; Renner, R.; Schillinger, U.; Gleich, B.; Heidsieck, A.; Schmitz, G.; Hensel, K.; et al. Magnetic and Acoustically Active Lipospheres for Magnetically Targeted Nucleic Acid Delivery. Adv. Funct. Mater. 2010, 20, 3881–3894. [Google Scholar] [CrossRef]
- Graham, F.; Van der Eb, A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Herbaj, S.; Redmond, J.; McCarthy, H.O.; Dunne, N.J. Calcium Phosphate Nanoparticles-Based Systems for RNAi Delivery: Applications in Bone Tissue Regeneration. Nanomater. 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Mostaghaci, B.; Loretz, B.; Lehr, C.-M. Calcium Phosphate System for Gene Delivery: Historical Background and Emerging Opportunities. Curr. Pharm. Des. 2016, 22, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Shubhra, Q.T.H.; Oyane, A.; Araki, H.; Nakamura, M.; Tsurushima, H. Calcium phosphate nanoparticles prepared from infusion fluids for stem cell transfection: Process optimization and cytotoxicity analysis. Biomater. Sci. 2017, 5, 972–981. [Google Scholar] [CrossRef]
- Lo, S.L.; Wang, S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials 2008, 29, 2408–2414. [Google Scholar] [CrossRef]
- Blakemore, R.P. Magnetotactic bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Fang, K.; Liu, P.; Dong, S.; Guo, Y.; Cui, X.; Zhu, X.; Li, X.; Jiang, L.; Liu, T.; Wu, Y. Magnetofection based on superparamagnetic iron oxide nanoparticle-mediated low lncRNA HOTAIR expression decreases the proliferation and invasion of glioma stem cells. Int. J. Oncol. 2016, 49, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Huang, Y.; Qian, H.; Du, X.; Qin, W.; Liu, T. Superparamagnetic iron oxide nanoparticles drive miR-485-5p inhibition in glioma stem cells by silencing Tie1 expression. Int. J. Biol. Sci. 2020, 16, 1274–1287. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Shi, Z.; Wei, H.; Sun, F.; Song, J.; Huang, Y.; Liu, T.; Mao, Y. Magnetofection Based on Superparamagnetic Iron Oxide Nanoparticles Weakens Glioma Stem Cell Proliferation and Invasion by Mediating High Expression of MicroRNA-374a. J. Cancer 2016, 7, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, W.; Park, S.-J.; Larson, A.C.; Kim, D.-H.; Park, K.-H. Multimodal Magnetic Nanoclusters for Gene Delivery, Directed Migration, and Tracking of Stem Cells. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Muthana, M.; Scott, S.D.; Farrow, N.; Morrow, F.; Murdoch, C.; Grubb, S.; Brown, N.; Dobson, J.; E Lewis, C. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther. 2008, 15, 902–910. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Yuryev, M.V.; Mirkasymov, A.B.; Sokolov, I.L.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Fast processes of nanoparticle blood clearance: Comprehensive study. J. Control. Release 2020, 326, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Mirkasymov, A.B.; Zelepukin, I.V.; Nikitin, P.I.; Nikitin, M.P.; Deyev, S.M. In vivo blockade of mononuclear phagocyte system with solid nanoparticles: Efficiency and affecting factors. J. Control. Release 2021, 330, 111–118. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef]
- Ringaci, A.; Yaremenko, A.V.; Shevchenko, K.G.; Zvereva, S.D.; Nikitin, M.P. Metal-organic frameworks for simultaneous gene and small molecule delivery in vitro and in vivo. Chem. Eng. J. 2021, 418, 129386. [Google Scholar] [CrossRef]
- Cherkasov, V.R.; Mochalova, E.N.; Babenyshev, A.V.; Vasilyeva, A.V.; Nikitin, P.I.; Nikitin, M.P. Nanoparticle Beacons: Supersensitive Smart Materials with On/Off-Switchable Affinity to Biomedical Targets. ACS Nano 2020, 14, 1792–1803. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Lunov, O.; Kubinova, S.; Polyakova, T.; Sykova, E.; Dejneka, A. Effects of high-gradient magnetic fields on living cell machinery. J. Phys. D Appl. Phys. 2016, 49, 493003. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Syková, E.; Kubinová, Š. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef] [PubMed]

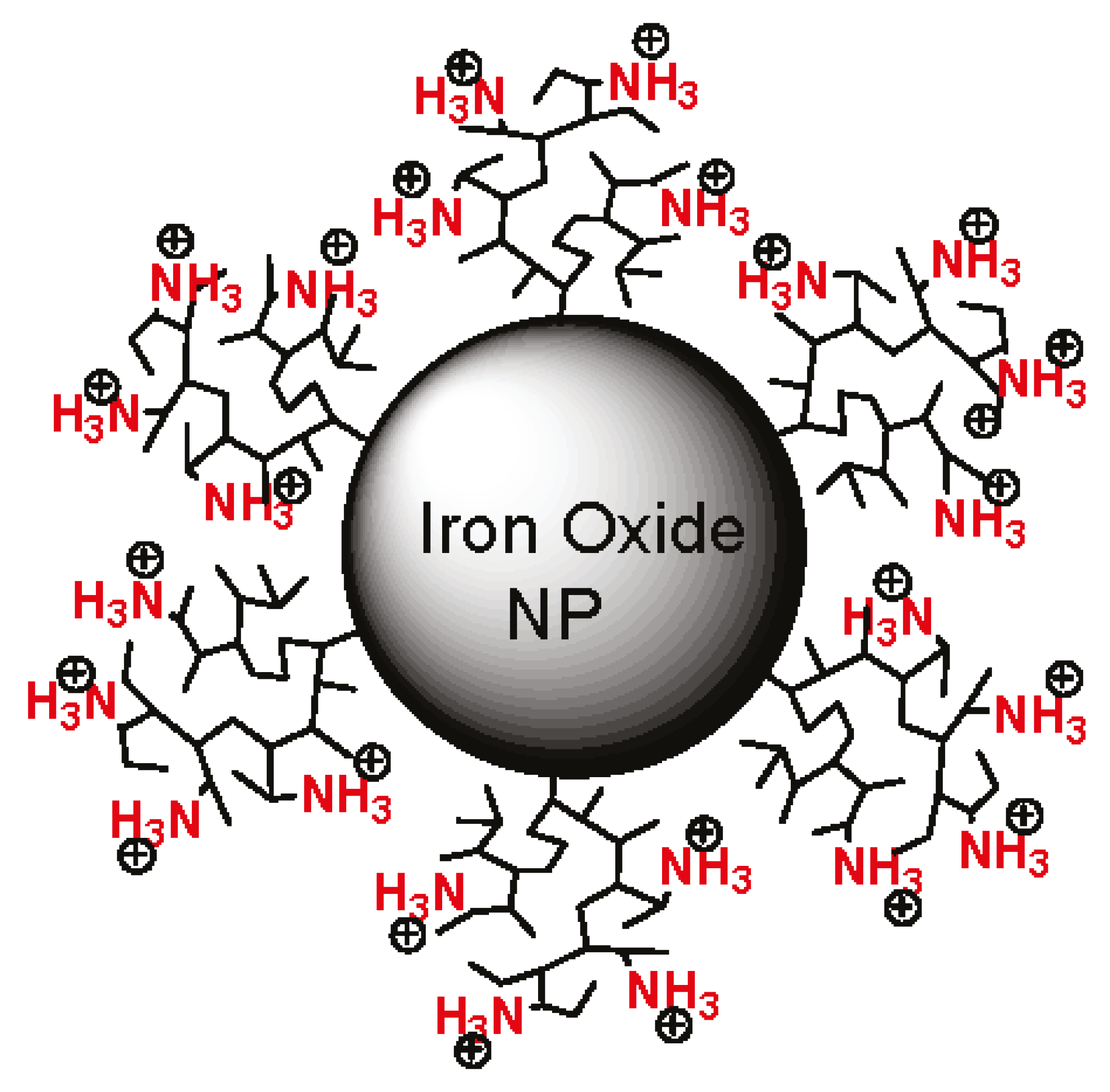
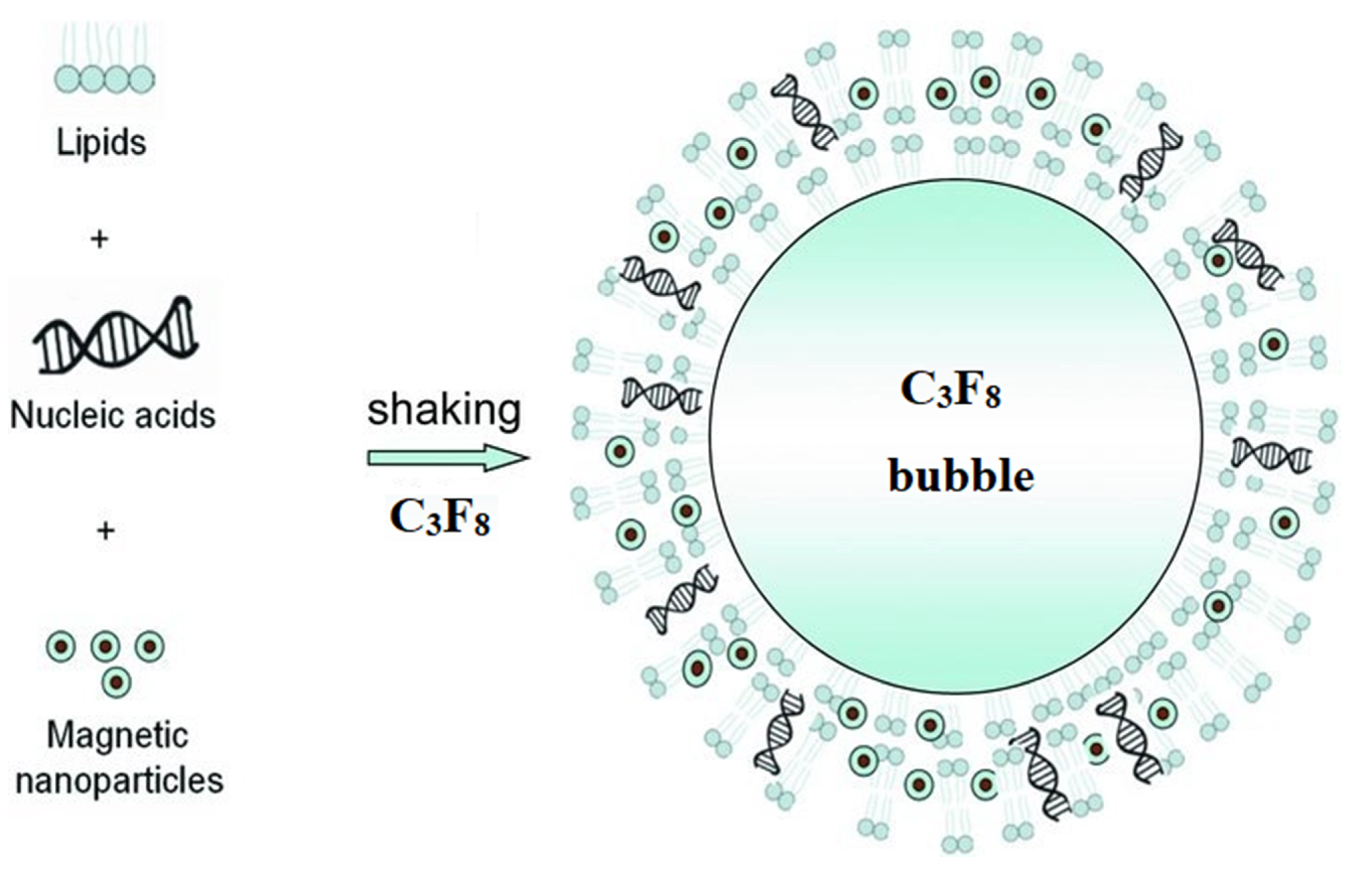
| Target (Tissue/Organ) | Animals | Nucleic Acid Type a | Cell Lines Tested in Vitro b | Magnetic Nanoparticle Composition c | Comparing Results with and Without Magnetic Field | Ref. | Magnetic Field (Gradient), T (T/m) |
| hippocampus | mouse | pDNA | 293T, PHNC | MNP + PLGA + PEI + PEG | + | [83] | - |
| cerebral cortex | rat | pDNA | - | NeuroMag (OzBiosciences) | + | [84] | - |
| circular smooth of perianal region | rat | siRNA miRNA | - | PolyMag (Oz Biosciences) | - | [85] | - |
| skull | mouse | pDNA | ASCs | MNP + PEI + PBAE | + | [86] | 1.2 |
| brain | mouse | pDNA | CHO-K1 | γ-Fe2O3 + CaP | - | [87,88] | 0.24 |
| subcutaneous tumor | mouse | pDNA | B16F1, B16F10, 2H-11 | SPIONs + PAA + PEI | + | [89,90] | 0.4 (38) |
| subcutaneoustumor | mouse | pDNA | B16F10, HepG2 | Fe3O4@SiO2-COOH + PEI | + | [91] | - |
| scapular region/thoracic wall (subcutaneous tumor) | cat | pDNA | - | transMAGPEI (Chemicell) | - | [92,93] | - |
| Lungs/heart | mouse | pDNA | NIH3T3, HEK293, COS7 | MNBs (MiltenyiBiotec)-PEI | + | [94] | - |
| dorsalflank (subcutaneous tumor) | mouse | pDNA | SACC-83 | Fe3O4 -PEI | + | [95] | - |
| rightflank (subcutaneous tumor) | mouse | pDNA | B16F1, SK-MEL-28, MeT-5A, L929 | SPIONs-PAA-PEI | + | [96] | - |
| ileum (rat), ear veins (pig) | rat, pig | pDNA | K562, PBL | transMAGPEI (Chemicell) | + | [46] | - |
| spinal cord | rat | pDNA | U87, H4, T98G, NT2 (Ntera-2/D1), U251 | PolyMag(Chemicell)-Tat | - | [48] | 1.21 |
| nasal epithelium | mouse | pDNA | C127 | transMAGPEI + GL67 | + | [97] | 1.08–1.15 |
| rightflank (subcutaneous tumor) | mouse | pDNA | LoVo | MGN (GodMag) | + | [98] | 0.5 |
| radial bone defect | rabbit | pDNA | HUVEC-1, MG-63 | Fe3O4 + Chitosan | + | [99] | 0.2/0.8 |
| skin | rat | pDNA | - | fluidMAG-Tween60 (Chemicell) + magnetobubbles | - | [100] | - |
| thigh muscle | mouse | pDNA | BHK-21, Hela, CHO | BMPs + PEI | + | [101] | 0.5 |
| tibialisanterior muscle | mouse/rabbit | pDNA | COS-7 | transMAGPEI (Chemicell) | + | [102] | 0.4 |
| striatum | rat | AntisenseODN | - | NeuroMag (OzBiosciences) | - | [103] | - |
| subcutaneoustumor/lungs | mouse | pDNA | B16F10, LLC1 | BMPs | + | [104] | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sizikov, A.A.; Kharlamova, M.V.; Nikitin, M.P.; Nikitin, P.I.; Kolychev, E.L. Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection. Nanomaterials 2021, 11, 1078. https://doi.org/10.3390/nano11051078
Sizikov AA, Kharlamova MV, Nikitin MP, Nikitin PI, Kolychev EL. Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection. Nanomaterials. 2021; 11(5):1078. https://doi.org/10.3390/nano11051078
Chicago/Turabian StyleSizikov, Artem A., Marianna V. Kharlamova, Maxim P. Nikitin, Petr I. Nikitin, and Eugene L. Kolychev. 2021. "Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection" Nanomaterials 11, no. 5: 1078. https://doi.org/10.3390/nano11051078
APA StyleSizikov, A. A., Kharlamova, M. V., Nikitin, M. P., Nikitin, P. I., & Kolychev, E. L. (2021). Nonviral Locally Injected Magnetic Vectors for In Vivo Gene Delivery: A Review of Studies on Magnetofection. Nanomaterials, 11(5), 1078. https://doi.org/10.3390/nano11051078








