Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models
Abstract
:1. Introduction
2. Medical Applications of Manganese and Manganese Oxide Nanoparticles
2.1. Biological Imaging
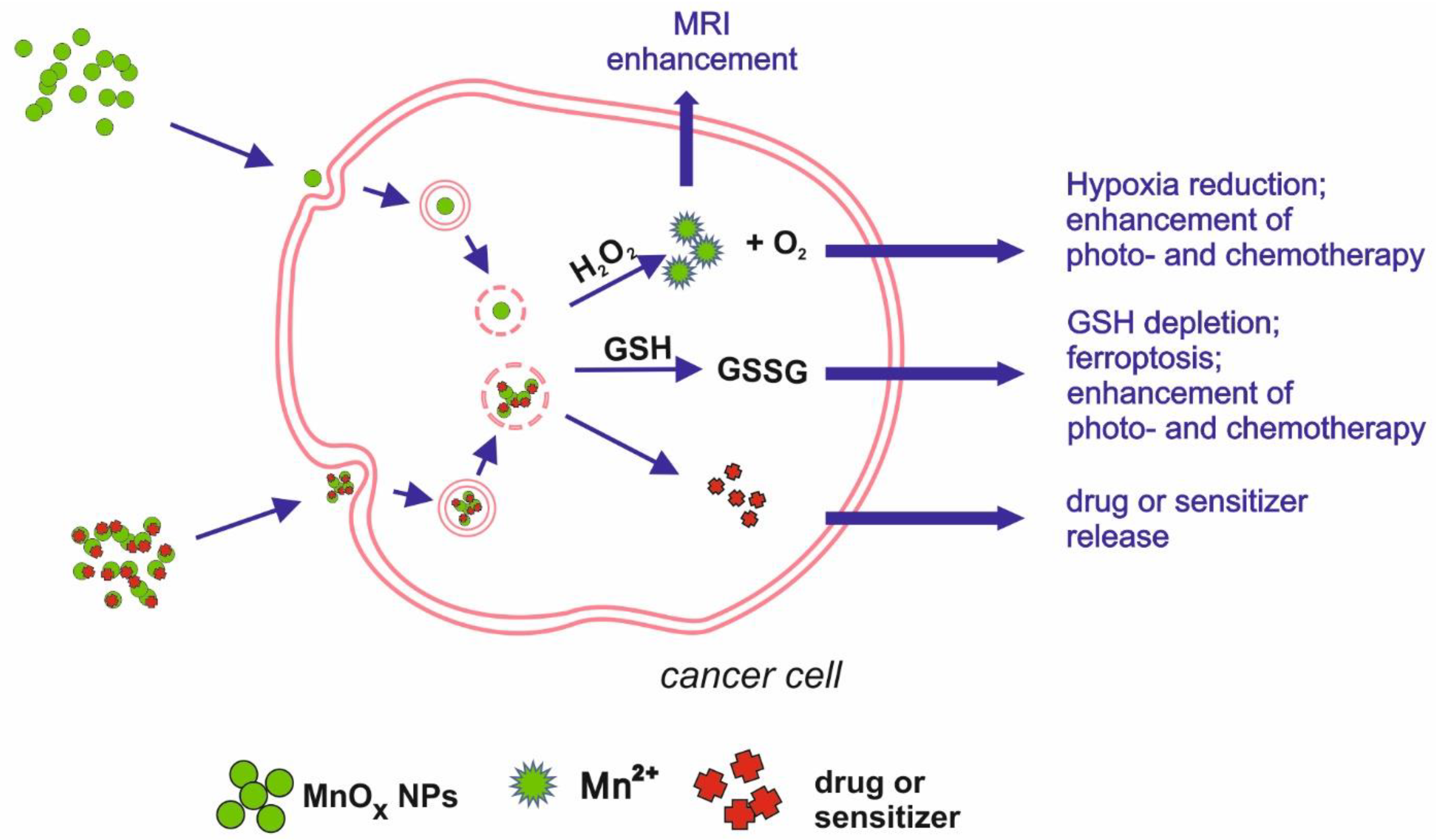
2.2. Modulating Tumour Microenvironment, Drug Delivery/Chemotherapy, Photo- and Radiotherapy
2.3. Theranostic Nanoplatforms
| Mn/Mn Oxide Nanoform | Modification | Application | Comments | Research Model | Reference |
|---|---|---|---|---|---|
| Mn3O4 | - | MRI contrasting agent | - | Balb/c nude mice with nasopharyngeal carcinoma (NPC)2 xenografted tumour | Xiao et al. 2013 [23] |
| MnO | PVP | MRI contrasting agent | - | Human lung carcinoma cell line (SPCA-1 cells) KM mice | Hu et al. 2013 [24] |
| Mn3O4 | PEG, Cy7.5 | Dual modality contrasting agent (MRI + fluorescence) | - | BALB/c mice | Zhan et al. 2017 [25] |
| Fe3O4/MnO nanocrystals | - | MRI contrasting agent | T1 and T2 mode | BALB/c nude mice | Im et al. 2013 [26] |
| Mn | Doped on silica NPs | Cancer treatment + drug delivery | induce ferroptosis via GSH depletion; might be loaded with drugs, e.g., sorafenib | Human hepatocellular carcinoma cell line (HepG2) | Tang et al. 2019 [27] |
| MnO2 | Ce6, PEG-cRGD | Photosensitizer delivery for PTT and PDT | - | Human prostate adenocarcinoma cell line (PC3) | Zeng et al. 2019 [29] |
| MnO2 | BSA, IR780, doxorubicin | Combined photo- and chemotherapy for cancer treatment | MnO2 degradation leading to red-ox imbalance as additional anti-cancer mechanism | Human breast adenocarcinoma (MCF-7), Balb/c nude mice inoculated with MCF-7 tumor | Yuan et al. 2019 [30] |
| MnO2 | OA | Radiosensitizer delivery | Mn-induced O2 release as additional anti-cancer mechanism | Human non-small cell lung cancer cell line (H1299), human head and neck squamous cell carcinoma cell line (SCC7), athymic female nude mice inoculated with H1299 cells | Liu et al. 2020 [31] |
| MnO2 | BSA, ICG | Combined photothermal and photodynamic for cancer treatment | Mn-induced O2 release as additional anti-cancer mechanism | Nude mice inoculated with murine melanoma (B16F10) cells | Wen et al. 2020 [32] |
| MnO | PEG, Cy5.5 | MRI contrasting agent + drug delivery for targeted therapy | Good retention and selectiveness | Sprague–Dawley rats with surgically developed myocardial ischemia | Zheng et al. 2018 [33] |
| MnO2 | BPD | Drug delivery for targeted therapy + MRI contrasting agent | Mn-induced O2 release as additional anti-cancer mechanism | HepG2 orthotopic mice | Wang et al. 2020 [37] |
| MnO2 | captopril–stabilized Au nanoclusters, DSP | Sensitizer (PDT) and drug delivery for targeted therapy +MRI contrasting agent | Mn ion-related depletion of GSH as mechanism supporting the effects of PDT | Mice inoculated with mouse cervical carcinoma (U14) cells | Bi et al. 2018 [38] |
| MnO | Loaded into LCN with BA | Chemodynamic therapy + fluorescent imaging | Mn ions catalyse Fenton-like reaction, triggering apoptosis | Balb/c mice with 4T1 (breast cancer) xenografted tumour | Urandur et al. 2020 [39] |
| MnO2 | GOx | Starvation/hyperthermia therapy+ MRI and PA contrasting agent | Mn-dependent reaction releases O2 necessary for GOx activity | Human melanoma (A375 cells), nude mice inoculated with A375 cells | He et al. 2020 [40] |
3. Analysis of the Biological Impact of Nano-Sized Manganese Compounds
3.1. In Vitro Studies
3.1.1. Studies Suggesting Cytotoxicity and Investigating Cytotoxicity Mechanisms
3.1.2. Studies Showing No Cytotoxicity of the Nanoparticles of Mn Oxides
3.2. Studies on In Vivo Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Chng, E.L.; Sofer, Z.; Pumera, M. MoS2 exhibits stronger toxicity with increased exfoliation. Nanoscale 2014, 6, 14412–14418. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [Green Version]
- Pfalzer, A.C.; Bowman, A.B. Relationships Between Essential Manganese Biology and Manganese Toxicity in Neurological Disease. Curr. Environ. Health Rep. 2017, 4, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Reidies, A.H. Manganese Compounds. Ullmann’s Encycl. Chem. Technol. 2007, 22. [Google Scholar] [CrossRef]
- Horning, K.J.; Caito, S.W.; Tipps, K.G.; Bowman, A.B.; Aschner, M. Manganese Is Essential for Neuronal Health. Annu. Rev. Nutr. 2015, 35, 71–108. [Google Scholar] [CrossRef]
- Park, R.M.; Bouchard, M.F.; Baldwin, M.; Bowler, R.; Mergler, D. Respiratory manganese particle size, time-course and neurobehavioral outcomes in workers at a manganese alloy production plant. Neurotoxicology 2014, 45, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Erikson, K.M.; Aschner, M. Manganese: Its Role in Disease and Health. Met. Ions. Life Sci. 2019, 19. [Google Scholar] [CrossRef]
- Michálková, Z.; Komárek, M.; Veselská, V.; Číhalová, S. Selected Fe and Mn (nano)oxides as perspective amendments for the stabilization of as in contaminated soils. Environ. Sci. Pollut. Res. Int. 2016, 23, 10841–10854. [Google Scholar] [CrossRef]
- Xie, W.; Liang, Q.; Qian, T.; Zhao, D. Immobilization of selenite in soil and groundwater using stabilized Fe-Mn binary oxide nanoparticles. Water Res. 2015, 70, 485–494. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, C.S.; Chang, Y.Y.; Chang, Y.S. Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl. Mater. Interfaces 2013, 5, 9628–9634. [Google Scholar] [CrossRef]
- Huangfu, X.; Ma, C.; Ma, J.; He, Q.; Yang, C.; Jiang, J.; Wang, Y.; Wu, Z. Significantly improving trace thallium removal from surface waters during coagulation enhanced by nanosized manganese dioxide. Chemosphere 2017, 168, 264–271. [Google Scholar] [CrossRef]
- Sabna, V.; Thampi, S.G.; Chandrakaran, S. Degradation of rhodamine B with manganese dioxide nanorods. J. Water Health 2018, 16, 846–856. [Google Scholar] [CrossRef]
- Oliveira, L.V.F.; Bennici, S.; Josien, L.; Limousy, L.; Bizeto, M.A.; Camilo, F.F. Free-standing cellulose film containing manganese dioxide nanoparticles and its use in discoloration of indigo carmine dye. Carbohydr. Polym. 2020, 230, 115621. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, M.; Zhao, D.; Feng, Y. Degradation of aqueous and soil-sorbed estradiol using a new class of stabilized manganese oxide nanoparticles. Water Res. 2015, 70, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, M.; Zhao, D. In-situ degradation of soil-sorbed 17β-estradiol using carboxymethyl cellulose stabilized manganese oxide nanoparticles: Column studies. Environ. Pollut. 2017, 223, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Ning, Q.; Yin, Z.; Liu, Y.; Tan, X.; Zeng, G.; Jiang, L.; Liu, S.; Tian, S.; Liu, N.; Wang, X. Fabrication of Stabilized Fe–Mn Binary Oxide Nanoparticles: Effective Adsorption of 17β-Estradiol and Influencing Factors. Int. J. Environ. Res. Public Health 2018, 15, 2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Duan, X.; Wang, M.; Su, X. A label-free fluorescent sensor based on silicon quantum dots-MnO2 nanosheets for the detection of α-glucosidase and its inhibitor. Analyst 2019, 144, 7398–7405. [Google Scholar] [CrossRef]
- Lee, P.C.; Li, N.S.; Hsu, Y.P.; Peng, C.; Yang, H.W. Direct glucose detection in whole blood by colorimetric assay based on glucose oxidase-conjugated graphene oxide/MnO. Analyst 2019, 144, 3038–3044. [Google Scholar] [CrossRef]
- Liu, J.; Meng, L.; Fei, Z.; Dyson, P.J.; Jing, X.; Liu, X. MnO2 nanosheets as an artificial enzyme to mimic oxidase for rapid and sensitive detection of glutathione. Biosens. Bioelectron. 2017, 90, 69–74. [Google Scholar] [CrossRef]
- Hao, L.; Xue, L.; Huang, F.; Cai, G.; Qi, W.; Zhang, M.; Han, Q.; Wang, Z.; Lin, J. A Microfluidic Biosensor Based on Magnetic Nanoparticle Separation, Quantum Dots Labeling and MnO. Micromachines 2020, 11, 281. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Tian, X.M.; Yang, C.; Liu, P.; Luo, N.Q.; Liang, Y.; Li, H.B.; Chen, D.H.; Wang, C.X.; Li, L.; et al. Ultrahigh relaxivity and safe probes of manganese oxide nanoparticles for in vivo imaging. Sci. Rep. 2013, 3, 3424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Ji, Y.; Wang, M.; Miao, F.; Ma, H.; Shen, H.; Jia, N. Water-soluble and biocompatible MnO@PVP nanoparticles for MR imaging in vitro and in vivo. J. Biomed. Nanotechnol. 2013, 9, 976–984. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, W.; Li, H.; Xu, X.; Cao, X.; Zhu, S.; Liang, J.; Chen, X. In Vivo Dual-Modality Fluorescence and Magnetic Resonance Imaging-Guided Lymph Node Mapping with Good Biocompatibility Manganese Oxide Nanoparticles. Molecules 2017, 22, 2208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, G.H.; Kim, S.M.; Lee, D.G.; Lee, W.J.; Lee, J.H.; Lee, I.S. Fe(3)O(4)/MnO hybrid nanocrystals as a dual contrast agent for both T(1)- and T(2)-weighted liver MRI. Biomaterials 2013, 34, 2069–2076. [Google Scholar] [CrossRef]
- Tang, H.; Chen, D.; Li, C.; Zheng, C.; Wu, X.; Zhang, Y.; Song, Q.; Fei, W. Dual GSH-exhausting sorafenib loaded manganese-silica nanodrugs for inducing the ferroptosis of hepatocellular carcinoma cells. Int. J. Pharm. 2019, 572, 118782. [Google Scholar] [CrossRef]
- Fan, H.; Yan, G.; Zhao, Z.; Hu, X.; Zhang, W.; Liu, H.; Fu, X.; Fu, T.; Zhang, X.B.; Tan, W. A Smart Photosensitizer-Manganese Dioxide Nanosystem for Enhanced Photodynamic Therapy by Reducing Glutathione Levels in Cancer Cells. Angew. Chem. Int. Ed. Engl. 2016, 55, 5477–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, D.; Wang, L.; Tian, L.; Zhao, S.; Zhang, X.; Li, H. Synergistic photothermal/photodynamic suppression of prostatic carcinoma by targeted biodegradable MnO. Drug Deliv. 2019, 26, 661–672. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Yin, Y.; Zan, W.; Sun, X.; Yang, Q. Hybrid manganese dioxide-bovine serum albumin nanostructure incorporated with doxorubicin and IR780 for enhanced breast cancer chemo-photothermal therapy. Drug Deliv. 2019, 26, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, W.; Kumar, A.; Rong, X.; Yang, W.; Chen, H.; Xie, J.; Wang, Y. Acridine Orange Encapsulated Mesoporous Manganese Dioxide Nanoparticles to Enhance Radiotherapy. Bioconjug. Chem. 2020, 31, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Hyoju, R.; Wang, P.; Shi, L.; Li, C.; Li, M.; Wang, X. Hydrogen-Peroxide-Responsive Protein Biomimetic Nanoparticles for Photothermal-Photodynamic Combination Therapy of Melanoma. Lasers Surg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, H.; Hu, Y.; Bai, L.; Xue, J. MnO nanoparticles with potential application in magnetic resonance imaging and drug delivery for myocardial infarction. Int. J. Nanomed. 2018, 13, 6177–6188. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Xiao, W.; Song, X.; Wang, W.; Dong, X. Recent Advances in Tumor Microenvironment Hydrogen Peroxide-Responsive Materials for Cancer Photodynamic Therapy. Nano-Micro Lett. 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Raja, I.S.; Kang, M.S.; Kim, K.S.; Jung, Y.J.; Han, D.W. Two-Dimensional Theranostic Nanomaterials in Cancer Treatment: State of the Art and Perspectives. Cancers 2020, 12, 1657. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, W.; Zhong, H.; Luo, T.; Niu, M.; Xu, K.; Tian, J. Tumor Vessel Targeted Self-Assemble Nanoparticles for Amplification and Prediction of the Embolization Effect in Hepatocellular Carcinoma. ACS Nano 2020, 14, 14907–14918. [Google Scholar] [CrossRef]
- Bi, H.; Dai, Y.; Yang, P.; Xu, J.; Yang, D.; Gai, S.; He, F.; An, G.; Zhong, C.; Lin, J. Glutathione and H2O2 consumption promoted photodynamic and chemotherapy based on biodegradable MnO2–Pt@Au25 nanosheets. Chem. Eng. J. 2019. [Google Scholar] [CrossRef]
- Urandur, S.; Banala, V.T.; Shukla, R.P.; Gautam, S.; Marwaha, D.; Rai, N.; Sharma, M.; Sharma, S.; Ramarao, P.; Mishra, P.R. Theranostic lyotropic liquid crystalline nanostructures for selective breast cancer imaging and therapy. Acta Biomater. 2020, 113, 522–540. [Google Scholar] [CrossRef]
- He, T.; Xu, H.; Zhang, Y.; Yi, S.; Cui, R.; Xing, S.; Wei, C.; Lin, J.; Huang, P. Glucose Oxidase-Instructed Traceable Self-Oxygenation/Hyperthermia Dually Enhanced Cancer Starvation Therapy. Theranostics 2020, 10, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Morello, M.; Zatta, P.; Zambenedetti, P.; Martorana, A.; D’Angelo, V.; Melchiorri, G.; Bernardi, G.; Sancesario, G. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: An immunohistochemical study. Brain Res. Bull. 2007, 74, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Betancourt, J.; Anaya-Martínez, V.; Gutierrez-Valdez, A.L.; Ordoñez-Librado, J.L.; Montiel-Flores, E.; Espinosa-Villanueva, J.; Reynoso-Erazo, L.; Avila-Costa, M.R. Manganese mixture inhalation is a reliable Parkinson disease model in rats. Neurotoxicology 2012, 33, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Meyer, S.; Weber, T.; Böker, C.; Marschall, T.; Mangerich, A.; Beneke, S.; Bürkle, A.; Schwerdtle, T. Molecular mechanisms of Mn induced neurotoxicity: RONS generation, genotoxicity, and DNA-damage response. Mol. Nutr. Food Res. 2013, 57, 1255–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verina, T.; Schneider, J.S.; Guilarte, T.R. Manganese exposure induces α-synuclein aggregation in the frontal cortex of non-human primates. Toxicol. Lett. 2013, 217, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dlamini, W.W.; Nelson, G.; Nielsen, S.S.; Racette, B.A. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am. J. Ind. Med. 2020, 63, 36–43. [Google Scholar] [CrossRef]
- Greene, A.; Hashemi, J.; Kang, Y. Development of MnO. Nanotechnology 2021, 32, 025713. [Google Scholar] [CrossRef] [PubMed]
- Bennewitz, M.F.; Lobo, T.L.; Nkansah, M.K.; Ulas, G.; Brudvig, G.W.; Shapiro, E.M. Biocompatible and pH-sensitive PLGA encapsulated MnO nanocrystals for molecular and cellular MRI. ACS Nano 2011, 5, 3438–3446. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Chen, D.Y.; Dodd, S.J.; Bouraoud, N.; Koretsky, A.P.; Krishnan, K.M. The use of silica coated MnO nanoparticles to control MRI relaxivity in response to specific physiological changes. Biomaterials 2012, 33, 3560–3567. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.M.; Javorina, A.K.; Schrand, A.M.; Duhart, H.M.; Ali, S.F.; Schlager, J.J. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol. Sci. 2006, 92, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Rahman, M.F.; Duhart, H.M.; Newport, G.D.; Patterson, T.A.; Murdock, R.C.; Hussain, S.M.; Schlager, J.J.; Ali, S.F. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 2009, 30, 926–933. [Google Scholar] [CrossRef]
- Afeseh Ngwa, H.; Kanthasamy, A.; Gu, Y.; Fang, N.; Anantharam, V.; Kanthasamy, A.G. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 2011, 256, 227–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alarifi, S.; Ali, D.; Alkahtani, S. Oxidative Stress-Induced DNA Damage by Manganese Dioxide Nanoparticles in Human Neuronal Cells. Biomed. Res. Int. 2017, 2017, 5478790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wu, G.; Zhang, Z.; Ma, X.; Liu, L. Neurotoxicity of Mn. Ecotoxicol. Environ. Saf. 2020, 188, 109909. [Google Scholar] [CrossRef] [PubMed]
- Frick, R.; Müller-Edenborn, B.; Schlicker, A.; Rothen-Rutishauser, B.; Raemy, D.O.; Günther, D.; Hattendorf, B.; Stark, W.; Beck-Schimmer, B. Comparison of manganese oxide nanoparticles and manganese sulfate with regard to oxidative stress, uptake and apoptosis in alveolar epithelial cells. Toxicol. Lett. 2011, 205, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Alhadlaq, H.A.; Akhtar, M.J.; Ahamed, M. Different cytotoxic and apoptotic responses of MCF-7 and HT1080 cells to MnO. Toxicology 2019, 411, 71–80. [Google Scholar] [CrossRef]
- Jiang, X.; Gray, P.; Patel, M.; Zheng, J.; Yin, J.J. Crossover between anti- and pro-oxidant activities of different manganese oxide nanoparticles and their biological implications. J. Mater. Chem. B 2020, 8, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Tootoonchi, M.H.; Hashempour, M.; Blackwelder, P.L.; Fraker, C.A. Manganese oxide particles as cytoprotective, oxygen generating agents. Acta Biomater. 2017, 59, 327–337. [Google Scholar] [CrossRef]
- Oszlánczi, G.; Vezér, T.; Sárközi, L.; Horváth, E.; Kónya, Z.; Papp, A. Functional neurotoxicity of Mn-containing nanoparticles in rats. Ecotoxicol. Environ. Saf. 2010, 73, 2004–2009. [Google Scholar] [CrossRef]
- Lundborg, M.; Eklund, A.; Lind, B.; Camner, P. Dissolution of metals by human and rabbit alveolar macrophages. Br. J. Ind. Med. 1985, 42, 642–645. [Google Scholar] [CrossRef] [Green Version]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J.; et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Babadi, V.Y.; Tanwir, F. Manganese dioxide nanoparticle induces Parkinson like neurobehavioral abnormalities in rats. Bratisl. Lek. Listy 2018, 119, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Kumari, M.; Kumari, S.I.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity assessment of manganese oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral exposure. J. Appl. Toxicol. 2013, 33, 1165–1179. [Google Scholar] [CrossRef]
- Katsnelson, B.A.; Minigaliyeva, I.A.; Panov, V.G.; Privalova, L.I.; Varaksin, A.N.; Gurvich, V.B.; Sutunkova, M.P.; Shur, V.Y.; Shishkina, E.V.; Valamina, I.E.; et al. Some patterns of metallic nanoparticles’ combined subchronic toxicity as exemplified by a combination of nickel and manganese oxide nanoparticles. Food Chem. Toxicol. 2015, 86, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Ehlerding, E.B.; Shi, S.; Graves, S.A.; Goel, S.; Engle, J.W.; Liang, J.; Cai, W. Intrinsically Zirconium-89-Labeled Manganese Oxide Nanoparticles for. J. Biomed. Nanotechnol. 2018, 14, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, S.; Lin, H.; Zhao, M.; Yao, H.; Zhang, L.; Peng, W.; Chen, Y. Theranostic 2D ultrathin MnO. Biomaterials 2018, 155, 54–63. [Google Scholar] [CrossRef]

| In Vitro Studies | |||||||
| Mn Nanoform (Size; Shape by TEM; Surface Modification) | Cell System | Concentration Tested; Stability | Exposure Time | Cellular Internalization | Toxicity Endpoints | Results | References |
| Elemental Mn (40 nm; irregularly shaped cubes) | PC-12/rat pheochromocytoma | 1–100 μg Mn/mL stability not measured | 24 h | Effective internalization (the CytoViva 150 URI system) | Cellular morphology, cytotoxicity (MTT), ROS level; depletion of DA, DOPAC, and HVA | Moderate cytotoxicity; induction of concentration-dependent DA, DOPAC, and HVA depletion; >10-fold increase in ROS | Hussain et al. 2006 [49] |
| Elemental Mn (40 nm; e irregularly shaped spheres) | PC-12/rat pheochromocytoma | 10 μg Mn/mL Average diameter in water: 5030 nm; in culture medium: 2390 nm. | 24 h | Not measured | Expression of genes associated with dopaminergic system and redox status | Significant dopaminergic neurotoxicity; no effect on redox status related genes, suppression of Th and Park2, up-regulation of Snca (genes involved in DA metabolism and PD pathogenesis) | Wang et al. 2009 [50] |
| Elemental Mn (20 nm; irregular shape) | N27/rat dopaminergic neural cell line | 25–400 μg Mn/mL Stability not measured | 3, 6, 9 h | Effective internalization after 6 h of incubation (TEM) | Cytotoxicity (Sytox green), mitochondrial superoxide production, H2O2 induction, autophagy | Cell viability decrease, oxidative stress induction, proapoptotic protein kinase Cδ (PKCδ) cleavage, autophagy induction | Afeseh Ngwa et al. 2011 [51] |
| MnO2 (40 nm; round shape) | SH-SY5Y/human neuroblastoma | 10, 30, 60 μg MnO2/mL Average diameter 299.60 nm in water | 24, 48 h | Not measured | Cell morphology, cytotoxicity (MTT, NRU), MMP, ROS level, oxidative stress (LPO, GSH, SOD, CAT level), PS translocation, chromosome condensation, caspase-3 state, genotoxicity | Cell viability decrease, ROS induction, oxidative stress markers increase, apoptosis (caspase-3 activation, PS translocation, fragmentation of chromosomes) | Alarifi et al. 2017 [52] |
| Mn3O4 (25 nm; spheres) | PC-12/rat pheochromocytoma | 5–20 μg Mn3O4/mL Average diameter 123 nm in water and 115 nm in culture medium | 24 h | Not measured | Cytotoxicity (CCK-8, LDH release), intracellular ROS level, oxidative stress (SOD, MDA, and GSH), apoptosis, cytosolic Ca2+ concentration, MMP, apoptosis-related proteins level, DA and DA-related proteins level | Cell viability decrease, ROS induction, oxidative stress markers increase, up-regulation of Bax and suppression of Bcl-2 expression, caspase-3 and caspase-9 cleavage, cytosolic Ca2+ concentration increase, DA level decrease, decreased expression of DOPA decarboxylase | Chen et al. 2020 [53] |
| Mn3O4 (30 nm; spheres) | CCL-149/rat lung epithelium | 5, 10, 20 μg Mn3O4/mL Average diameter 100 nm | 24 h | Effective internalization (TEM, ICP-MS) | ROS level, GSH level, caspase-3 activity, apoptosis, LDH release | ROS induction, apoptosis, red-ox status disturbances, | Frick et al. 2011 [54] |
| MnO2 (20–120 nm; nanoflakes) | MCF-7/human breast cancer; HT1080/human fibrosarcoma | 5–200 μg MnO2/mL Average diameter 350 nm in culture medium | 24 h | Effective internalization (ICP-MS) | Cytotoxicity (MTT, NRU, LDH release), ROS level, oxidative stress markers (GSH, TBARS, SOD, CAT), apoptosis, MMP, cell cycle | Cell viability decrease and oxidative stress induction (more significant for HT1080), apoptosis, cell cycle disturbances, up-regulation of pro-apoptotic genes, and down-regulation of anti-apoptotic genes | Alhadlaq et al. 2018 [55] |
| MnO2, Mn2O3, and Mn3O4 (50 nm; spherical shape) | Caco-2/human colorectal adenocarcinoma | 25 μM NP/mL Average diameter MnO2—232.1 nm Mn2O3—435.7 nm, Mn3O4—273 nm in water Stability not measured | 24 h | Effective internalization (TEM) | Cytotoxicity (Alamar blue) | Cell viability decrease; incubation wit NPs protected from H2O2 cytotoxicity (effect decreased with growing concentrations of NPs), ROS-scavenging activity highest for MnO2 | Jiang et al. (2020) [56] |
| MnO2, Mn3O4 (different structures) | bTC3/Murine insulinoma | 3.126–200 μg NP/mL Stability not measured | 48 h | Effective internalization after 3.5 h (TEM) | Cytotoxicity (bioluminescence, MTS) | Low cytotoxicity (significant for high concentrations of hausmannite Mn3O4); incubation wit NPs protected from H2O2 cytotoxicity (effect decreased from 50 μg NP/mL with growing concentrations of NPs) | Tootoonchi et al. 2017 [57] |
| Mn3O4 (9 nm) | Cytotoxicity: HEK 293/Human embryonic kidney cells apoptosis: NP69/Human nasopharyngeal epithelial cells CNE-2/human nasopharyngeal carcinoma cells | 10, 100, 150 Mn3O4 Stability not measured | 24, 48 h | Effective internalization (TEM) | Cytotoxicity (MTT), apoptosis | Cell viability unaltered, no apoptosis | Xiao et al. 2013 [23] |
| Mn3O4 (10 nm; spherical shape; coated with PEG and Cy7.5) | PC-3 (human prostate adenocarcinoma), A549 (human lung carcinoma), HepG2 (human hepatocellular carcinoma) | 200–1000 μg NP/mL Stability not measured | 24 h | Effective internalization (confocal microscopy) | Cytotoxicity (CCK-8) | Cell viability unaltered | Zhan et al. 2017 [25] |
| MnO (60 nm; coated with PEG and Cy5.5) | NRVM (neonatal rat ventricular myocytes), CF (cardiac fibroblasts), and H9c2 (rat myoblast) | 0–100 μM NP Stability not measured | 48 h | Not measured | Cytotoxicity (MTT) | Cell viability unaltered | Zheng et al. 2018 [33] |
| MnO (20 nm; coated with PEG) | SPCA-1 (human lung carcinoma) | 0–100 μg NP/mL Average diameter 30 nm in PBS | 24 h | Not measured | Cytotoxicity (MTT) | Cell viability unaltered | Hu et al. 2013 [24] |
| MnO2 (3 nm thick; nanosheets; conjugated with Au nanoclusters and DSP) | L929 (fibroblast cells) | 0–200 μg NP/mL Stability not measured | 24 h | Effective internalization (confocal microscopy) | Cytotoxicity (MTT) | Cell viability unaltered | Bi et al. 2019 [38] |
| MnO (7.3 nm;loaded into LCN wit BA) | Cytotoxicity: HEK 293 (human embryonic kidney cells) Internalization: MDA-MB-231, 4T1 (human breast cancer cells) | 5, 10 μg Mn/mL | 48 h | Effective internalization after 12 h (confocal microscopy, flow cytometry) | Cytotoxicity (MTT) | Cell viability unaltered | Urandur et al. 2020 [39] |
| MnO2 (2 nm thick; nanosheets; loaded with GOx) | A375 (human melanoma) | 0–1 nM NP/mL Stability not measured | 24 h | Not measured | Cytotoxicity (MTT, CAM + PI staining) | Cell viability unaltered | He et al. 2020 [40] |
| In Vivo Studies | |||||||
| Mn Nanoform (Size; Shape) | Animals | Dose Tested/Route | Exposure Time | Bio-Accumulation | Toxicity Endpoints | Results | References |
| MnO2 (23 nm) | Male Wistar rats | Daily doses of 2.63 and 5.26 mg Mn/kg; intratracheal instillation | 3, 6, and 9 weeks | Increased Mn level in blood and brain | Open field behaviour changes, electrophysiology, body and organ weights | Behavioural changes: increased immobility, decreased rearing, electrophysiological brain activity pattern altered, no weight gain from the 6th week on | Oszlánczi et al. [58] |
| MnO2 (30–60 nm) | Male Wistar rats | Daily doses of 50 and 100 μg MnO2/kg; intraperitoneal injection | 15 days | Not measured | Behaviour changes, Sucrose preference, Catecholamine concentration, ROS and LPO level, histopathological analysis of tissues | Depressive-like behaviours (increased immobility, anhedonia), oxidative stress induction and catecholamine level decrease in hippocampus tissue, necrotic, and apoptotic cells in brain tissue | Sadeghi et al. 2018 [62] |
| MnO2 (42.63 nm) | Male and female Wistar rats | Daily dose of 30, 300, 1000 mg MnO2/kg; oral gavage | 28 days | Increased Mn level in blood, liver, heart, kidneys, spleen | DNA damage (comet assay, micronucleus test, chromosomal aberration assay), blood biochemistry changes, fractionation of brain for ATPases, histopathological analysis of tissues | DNA damage, increased MN frequency and chromosome aberration for doses of 300 and 1000 mg/kg, discrepancies in brain tissue enzyme activity and blood biochemical parameters, tissue damage for the highest dose | Singh et al. 2013 [63] |
| Mn3O4 (~18 nm; spherical shape) | Female rats | 2.5 and 1.25 mg Mn3O4/kg; 3 times a week, 18 doses in total; intraperitoneal injection | 6 weeks | Increased Mn level in brain and kidneys | Behaviour changes, urine analysis, blood biochemistry and hematology changes, histopathological analysis of tissues | Discrepancies in some blood and urine parameters | Katsnelson et al. 2015 [64] |
| Mn3O4 (10 nm; spherical shape; coated with PEG and Cy7.5) | Male BALB/c mice | 20 mg/kg Mn3O4; intravenously | 14 days | NPs present in liver and kidneys; rapid biodegradation and clearance | Blood biochemistry, histopathological analysis of tissues | No tissue damage, no discrepancies in biochemical markers for liver and kidney functions | Zhan et al. 2018 [65] |
| MnO2 (2 nm thick nanosheets; coated with soy phospholipid) | Female Kunming mice | 5, 10, 20 mg MnO2/kg; intravenously | 30 days | Not measured | Body weight changes, histopathological analysis of tissues | No body weight discrepancies, no tissue damage | Liu et al. 2018 [66] |
| MnO (60 nm; coated with PEG and Cy5.5) | C57BL/6J mice | 7 mg Mn/kg (4 and 24 h) 35 mg Mn/kg (28 h) | 4 h, 24 h, 28 days | NPs present after 4, but not after 24 h; rapid clearance | Behaviour changes, histopathological analysis of tissues | No behaviour changes, no tissue damage | Zheng et al. 2018 [33] |
| MnO (7.3 nm; loaded into LCN wit BA) | BALB/b mice | 40 mg/kg Mn; intravenously | 21 days | Cleared from blood after 8 h (MnO + BA NPs) or >48 h(LCN + MnO + BA) | Histopathological analysis of tissues | No tissue damage | Urandur et al. 2020 [39] |
| MnO2 (2 nm thick; nanosheets; loaded with GOx) | Nude mice | 5 mg/kg; intratumorally | 30 days | Not measured | Body weight changes, histopathological analysis of tissues | No body weight discrepancies, no tissue damage | He et al. 2020 [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobańska, Z.; Roszak, J.; Kowalczyk, K.; Stępnik, M. Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models. Nanomaterials 2021, 11, 1084. https://doi.org/10.3390/nano11051084
Sobańska Z, Roszak J, Kowalczyk K, Stępnik M. Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models. Nanomaterials. 2021; 11(5):1084. https://doi.org/10.3390/nano11051084
Chicago/Turabian StyleSobańska, Zuzanna, Joanna Roszak, Kornelia Kowalczyk, and Maciej Stępnik. 2021. "Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models" Nanomaterials 11, no. 5: 1084. https://doi.org/10.3390/nano11051084
APA StyleSobańska, Z., Roszak, J., Kowalczyk, K., & Stępnik, M. (2021). Applications and Biological Activity of Nanoparticles of Manganese and Manganese Oxides in In Vitro and In Vivo Models. Nanomaterials, 11(5), 1084. https://doi.org/10.3390/nano11051084






