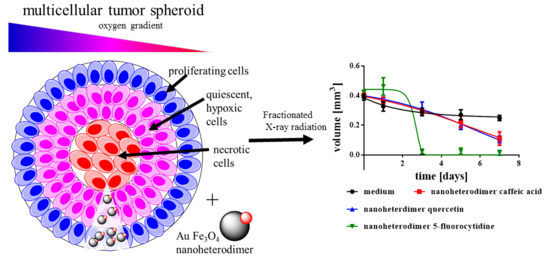
Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Synthesis of the Au-Fe3O4 Nanoheterodimers
2.4. Ligand Exchange Procedure
2.5. Multicellular Tumor Spheroids (MCTS)
2.6. Preparation for Transmission Electron Microscopy (TEM) Images
2.7. Determination of Glucose Consumption and Lactate Secretion
2.7.1. Lactate Assay
2.7.2. Estimation of Sugar by Folin and Wu Method
2.8. Fractionating of the MCTS
2.9. Cell Viability
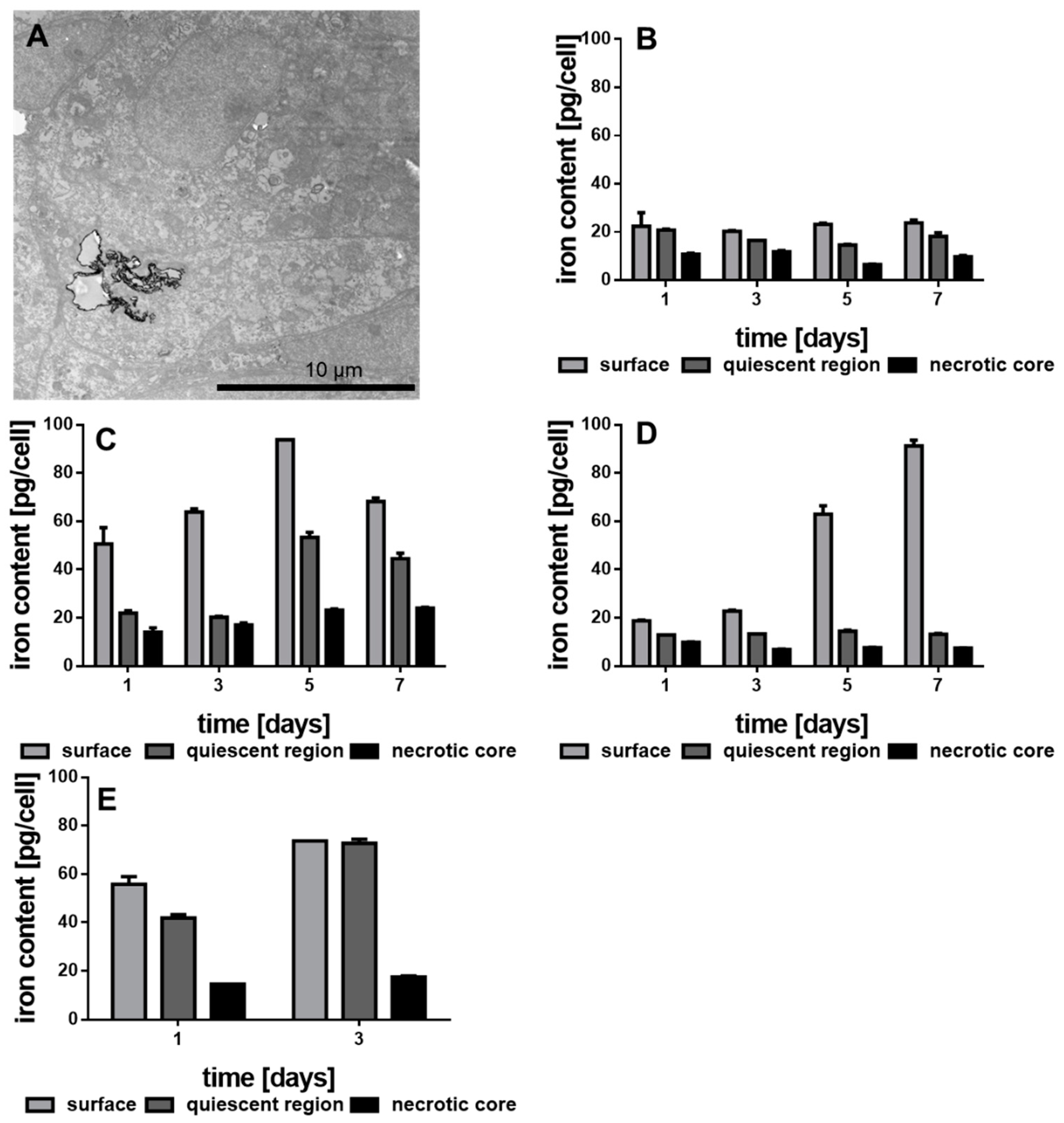
2.10. Intracellular Iron Content by Prussian Blue Reaction
2.11. Intracellular Reactive Oxygen Species (ROS) Concentration
3. Results and Discussion
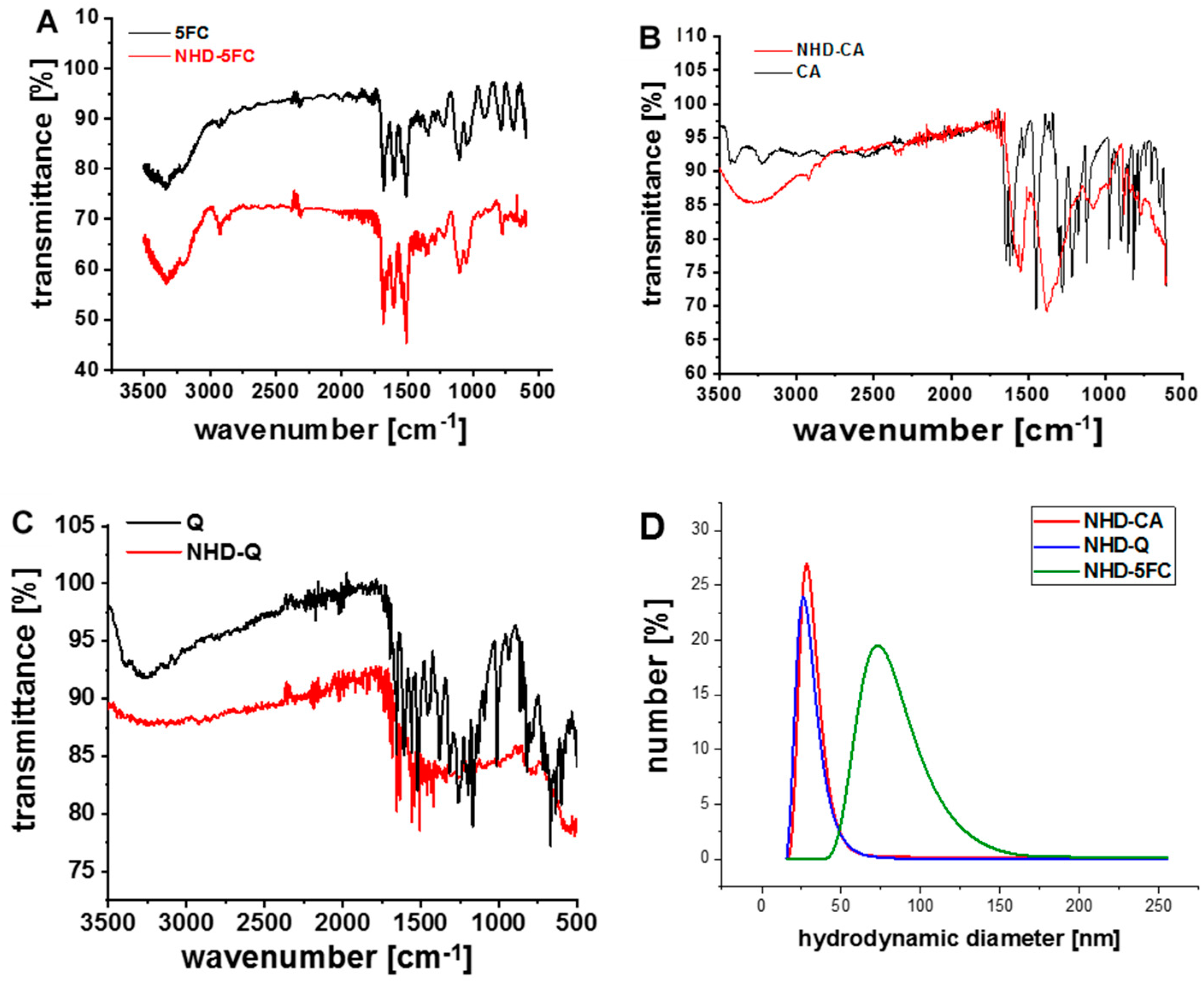
3.1. Characterization of the Functionalized Au-Fe3O4 Nanoheterodimers (NHDs)
3.2. Growth of the MCTS without Irradiation
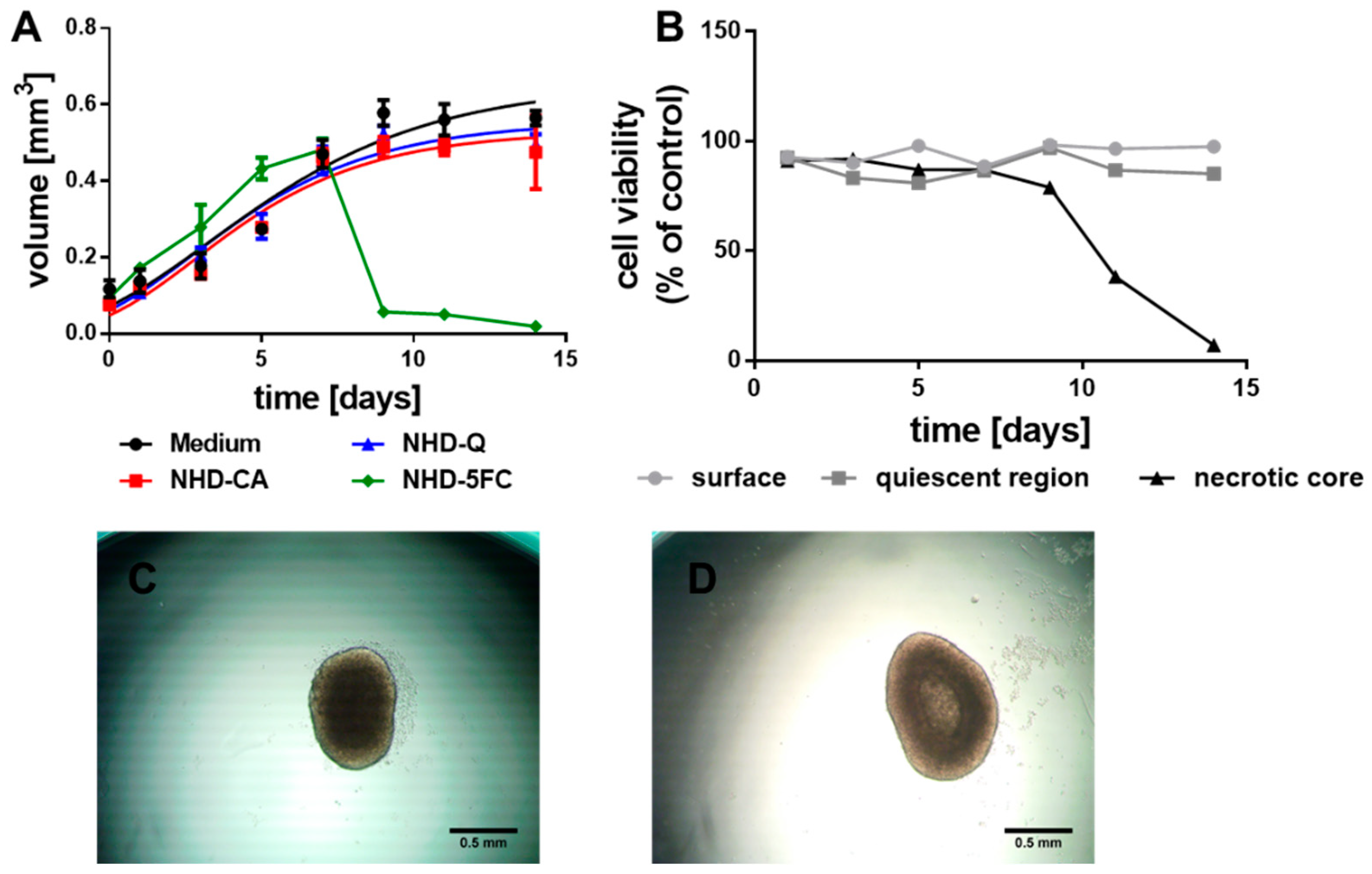
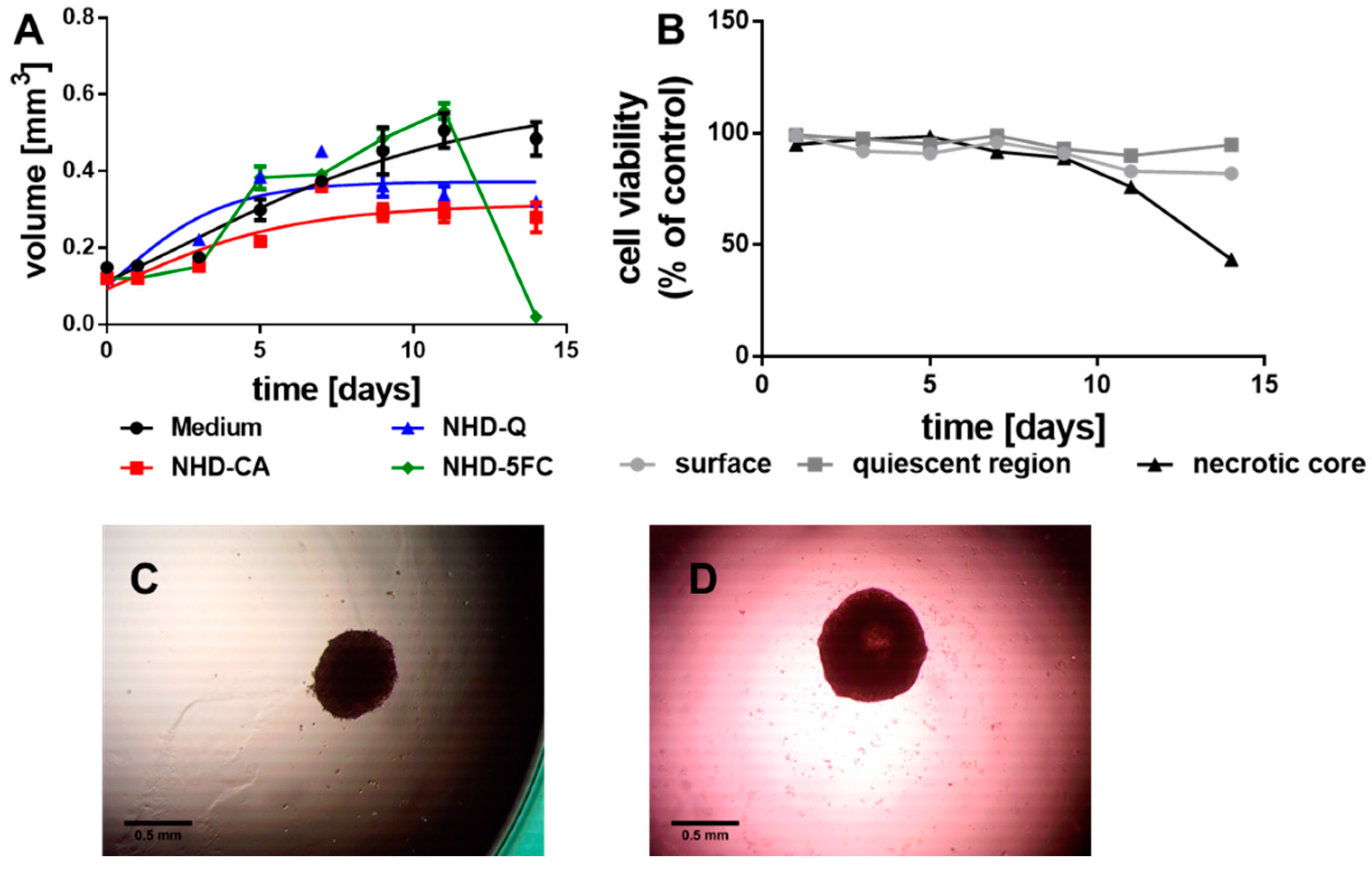
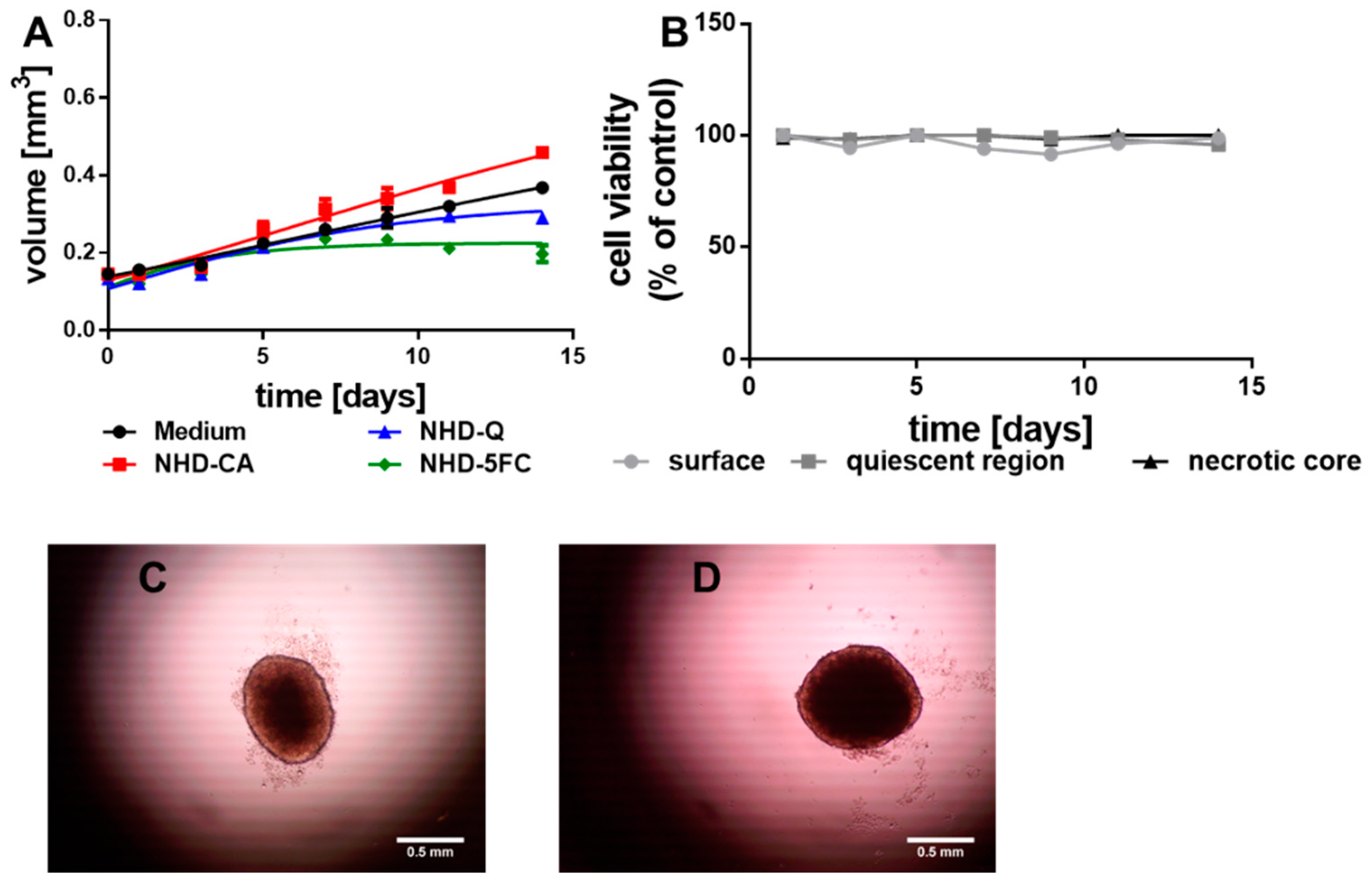
3.2.1. MCF-7 MCTS
3.2.2. MDA-MB-231 MCTS
3.2.3. MCF-10A Multicellular Spheroids (MCS)
3.3. Metabolism of the Spheroids
3.4. Internalization of the Functionalized Au-Fe3O4 NHDs
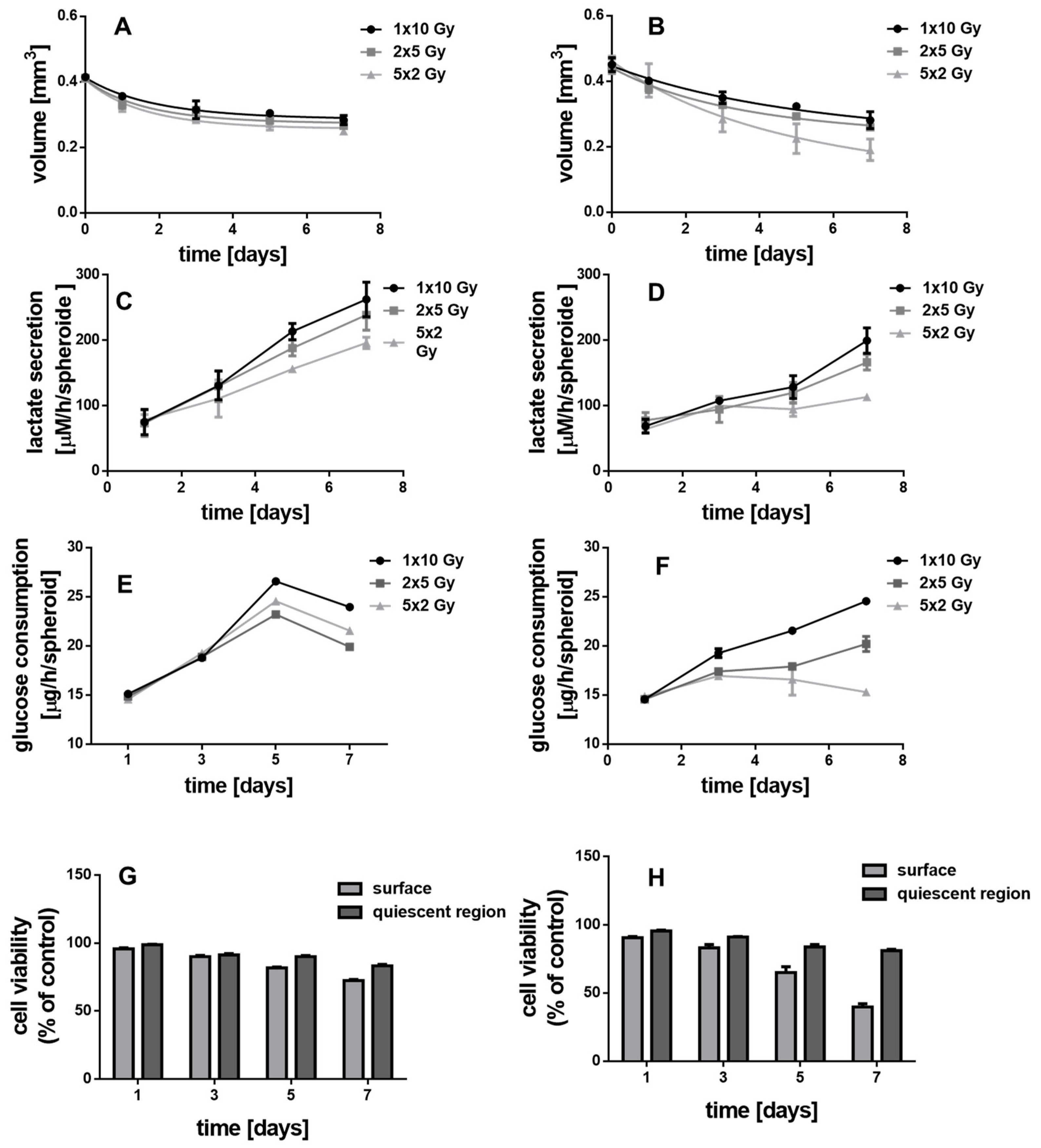
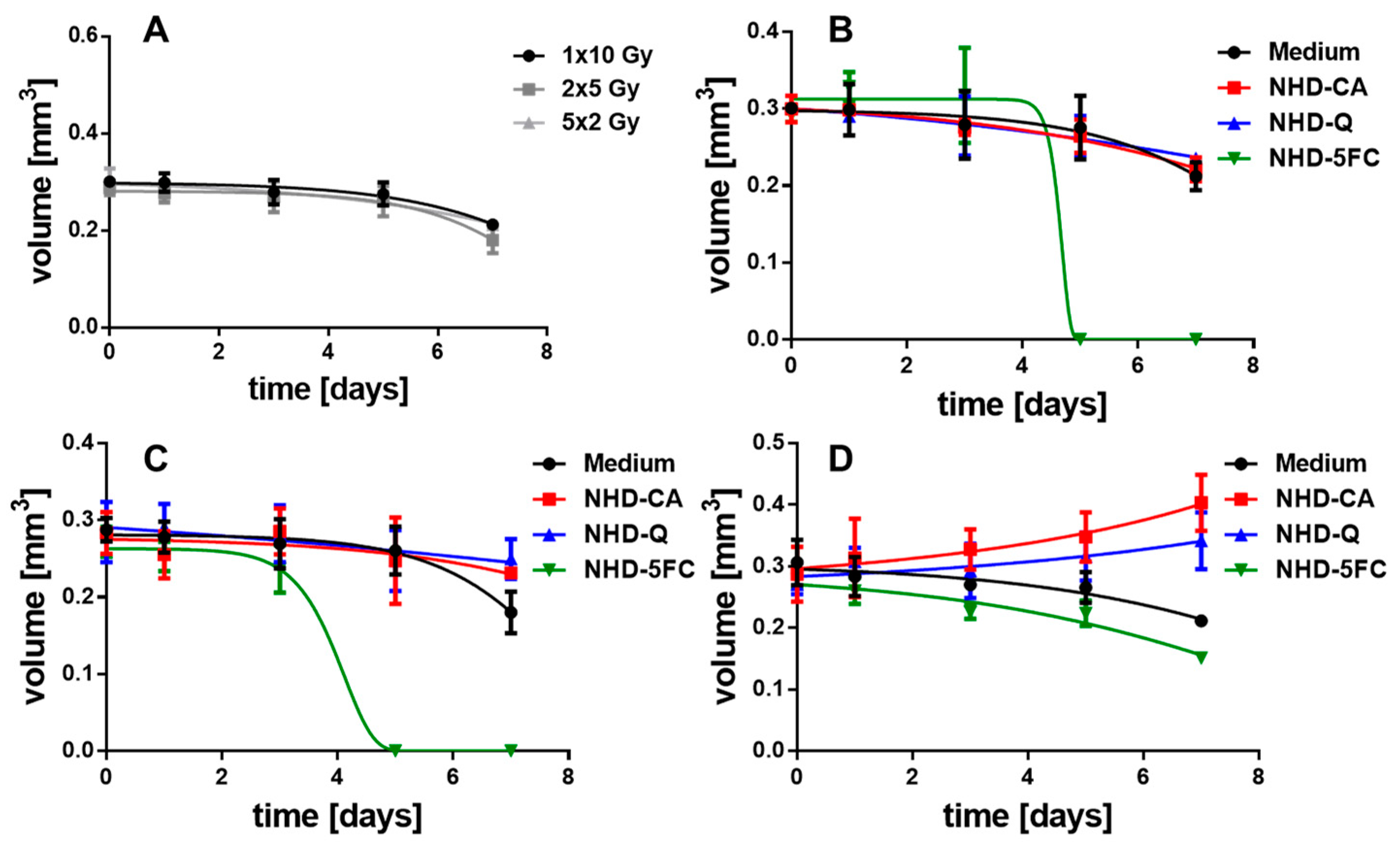
3.5. Irradiated MCTS without Au-Fe3O4 NHDs
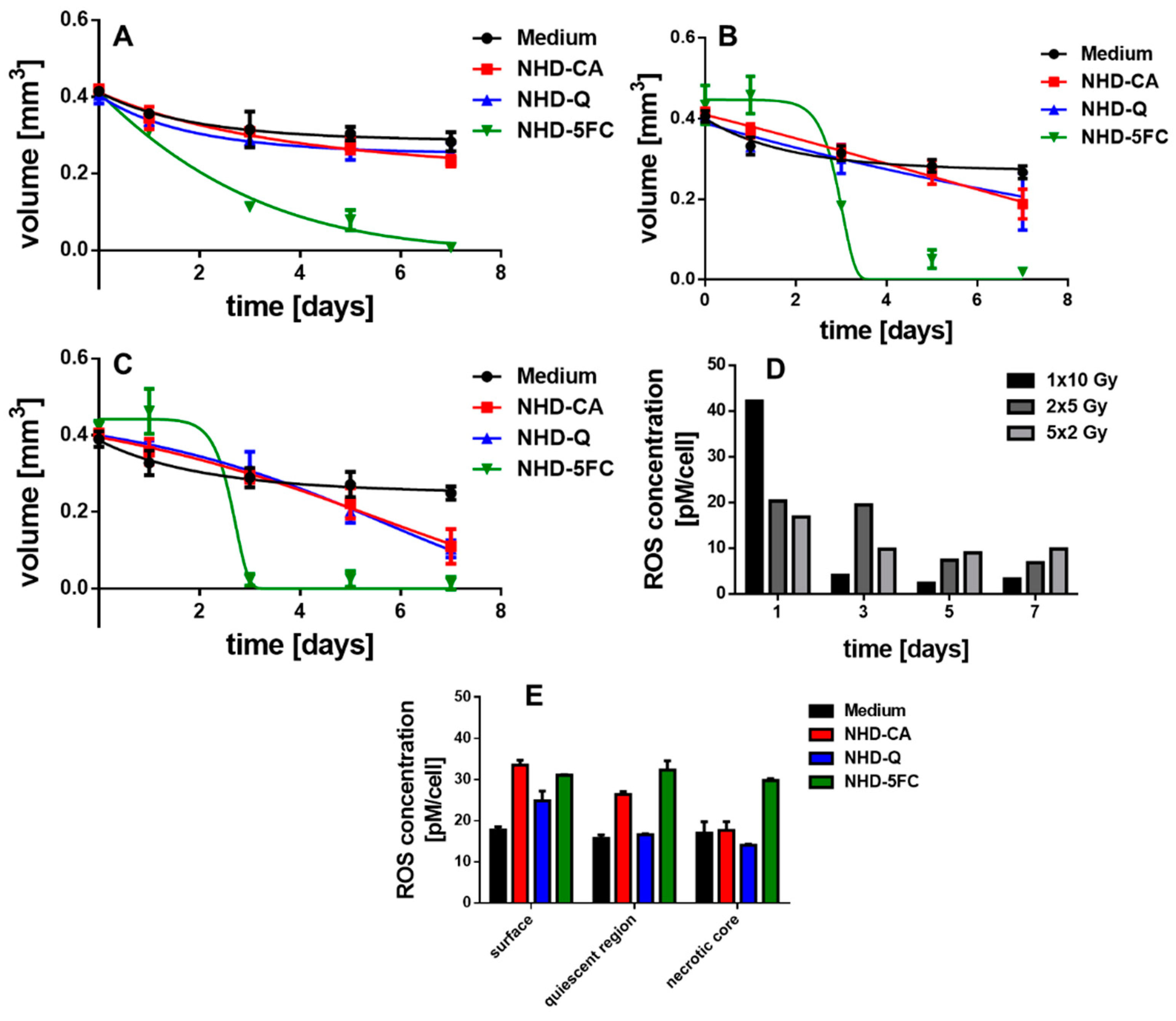
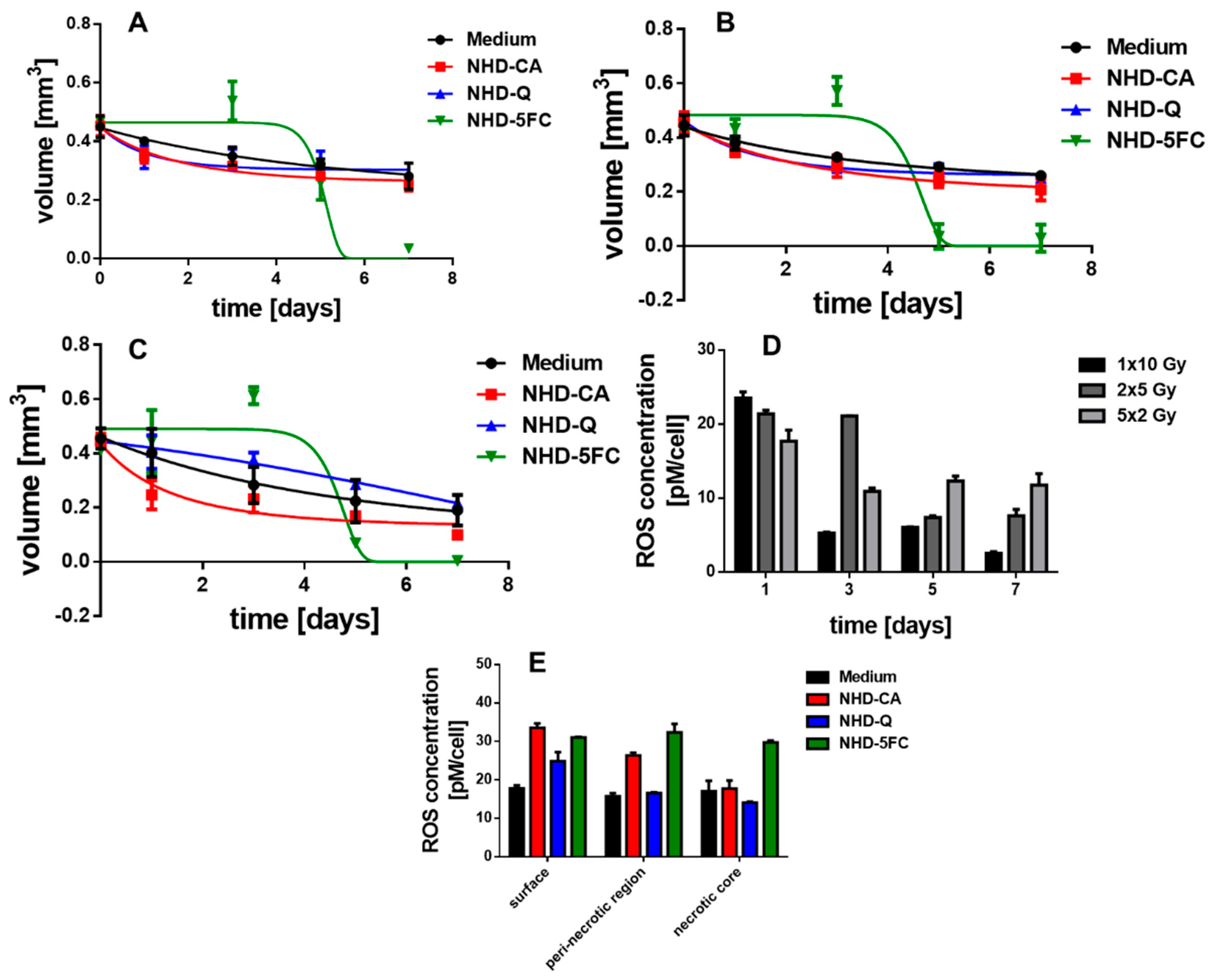
3.6. Irradiated MCTS Incubated with Au-Fe3O4 NHDs
3.7. Irradiated MCF-10 A MCS Incubated with Au-Fe3O4 NHDs
3.8. ROS Generation in Irradiated MCTS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koulis, T.A.; Phan, T.; Olivotto, I.A. Hypofractionated Whole Breast Radiotherapy: Current Perspectives. Breast Cancer Targets Ther. 2015, 7, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Pignol, J.-P.; Levine, M.N.; Julia, J.A.; MacKenzie, R.; Parpia, S.; Shelley, W.; Grimard, L.; Bowen, J.; Lukka, H.; et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. N. Engl. J. Med. 2010, 362, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.J.; Sibson, N.R.; Kiltie, A.E. Treatment of Breast and Prostate Cancer by Hypofractionated Radiotherapy: Potential Risks and Benefits. Clin. Oncol. 2015, 27, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Herskind, C.; Ma, L.; Liu, Q.; Zhang, B.; Schneider, F.; Veldwijk, M.R.; Wenz, F. Biology of High Single Doses of IORT: RBE, 5 R´s, and other Biological Aspects. Radiat. Oncol. 2017, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Graham, P.; Hao, J.; Ni, J.; Deng, J.; Bucci, J.; Malouf, D.; Gillatt, D.; Li, Y. Cancer Stem Cells and Signaling Pathways in Radioresistance. Oncotarget 2015, 10, 11002–11017. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Lai, C.; Kuten, A.; Belkacemi, Y.; On behalf of AROME. “The Infinite Maze” of Breast Cancer, Signaling Pathways and Radioresistance. Breast 2013, 22, 411–418. [Google Scholar] [CrossRef]
- Lee, J.; Lilly, G.D.; Doty, C.; Podsiadlo, P.; Kotov, N.A. In Vitro Toxicity Testing of Nanoparticles in 3D Cell Culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Grabowska, A.M. In Vitro Tissue Microarrays for Quick and Efficient Spheroid Characterization. SLAS Discov. 2018, 23, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D Tumor Spheroid Models for in vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6, srep19103. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Yeap, S.K.; Ho, C.L.; Rahim, R.A.; Alitheen, N.B. Development of Multicellular Tumor Spheroid (MCTS) Culture from Breast Cancer Cell and a High Throughput Screening Method Using MTT Assay. PLoS ONE 2012, 7, e44640. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Eetezadi, S.; Allen, C. Multicellular Tumor Spheroid for Evaluation of Cytotoxicity and Tumor Growth Inhibitory Effects of Nanomedicines In Vitro: A Comparison of Docetaxel-Loaded Block Copolymer Micelles and Taxotere®. PLoS ONE 2013, 8, e62630. [Google Scholar] [CrossRef]
- Dubessy, C.; Merlin, J.-L.; Marchal, C.; Guillemin, F. Spheroids in Radiobiology and Photodynamic Therapy. Crit. Rev. Oncol. Hemat. 2000, 36, 179–192. [Google Scholar] [CrossRef]
- Schwachhöfer, J.H.M. Multicellular Tumor Spheroids in Radiotherapy Research. Anticancer Res. 1990, 10, 963–970. [Google Scholar]
- Olive, P.G.; Durand, R.E. Drug and Radiation Resistance in Spheroids: Cell Contact and Kinetics. Cancer Metastasis Rev. 1994, 13, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.E.; Sutherland, R.M. Effects of Intracellular Contact on Repair of Radiation Damage. Exp. Cell Res. 1972, 71, 75–80. [Google Scholar] [CrossRef]
- Al-Rahmadan, A.; Mortensen, A.; Carlsson, J.; Nestor, M.V. Analysis of Radiation Effects in Two Irradiated Tumor Spheroid Models. Oncol. Lett. 2018, 15, 3008–3016. [Google Scholar] [CrossRef] [PubMed]
- Khaitan, D.; Chadna, S.; Arya, M.B.; Dwarakanath, B.S. Establishment and Characterization of Multicellular Spheroids from a Human Glioma Cell Line; Implications for Tumor Therapy. J. Transl. Med. 2006, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.T.; Rainaldi, G.; Indovina, P.L. Multicellular Tumor Spheroids in Radiation Biology. Int. J. Radiat. Biol. 1999, 75, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Harreiss, C.; Menter, C.; Hümmer, J.; Distel, L.V.R.; Meyer, K.; Hock, R.; Kryschi, C. NOBF4-Funtionalized Au-Fe3O4 Nanoheterodimers for Radiation Therapy: Synergy Effect due to Simultaneous Reactive Oxygen and Nitrogen Species Formation. ACS Appl. Mater. Interfaces 2018, 10, 17071–17080. [Google Scholar] [CrossRef]
- Klein, S.; Smuda, M.; Harreiß, C.; Menter, C.; Distel, L.V.R.; Kryschi, C. Bifunctional Au-Fe3O4 Nanoheterodimers Acting as X-ray Protector in Healthy Cells and as X-ray Enhancer in Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 39613–39623. [Google Scholar] [CrossRef]
- Klein, S.; Stiegler, L.M.S.; Harreiss, C.; Distel, L.V.R.; Neuhuber, W.; Spieker, E.; Hirsch, A.; Kryschi, C. Understanding the Role of Surface Charge in Cellular Uptake and X-ray-Induced ROS Enhancing of Au-Fe3O4 Nanoheterodimers. ACS Appl. Bio Mater. 2018, 1, 2002–2011. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef]
- Mesbashi, A. A Review on Gold Nanoparticles Radiosensitization Effect in Radiation Therapy of Cancer. Rep. Pract. Oncol. Radiother. 2010, 15, 176–180. [Google Scholar] [CrossRef]
- Ngwa, W.; Kumar, R.; Sridhar, S.; Korideck, H.; Zygmanski, P.; Cormack, R.A.; Berbeco, R.; Mahrigiorgos, G.M. Targeted Radiotherapy with Gold Nanoparticles: Current Status and Future Perspectives. Nanomedicine 2014, 9, 1063–1082. [Google Scholar] [CrossRef]
- Haume, K.; Rosa, S.; Grellet, S.; Simałek, M.A.; Butterworth, K.T.; Solov’yov, A.V.; Prise, K.M.; Golding, J.; Mason, N.J. Goldnanoparticles for cancer radiotherapy: A review. Cancer Nanotechnol. 2016, 7, 8. [Google Scholar] [CrossRef]
- Klein, S.; Sommer, A.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. Superparamagnetic Iron Oxide Nanoparticles as Radiosensitizer via Enhanced Reactive Oxygen Species Formation. Biochem. Biophys. Res. Commun. 2012, 61, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Sommer, A.; Distel, L.V.R.; Hazemann, J.-L.; Kröner, W.; Neuhuber, W.; Müller, P.; Proux, O.; Kryschi, C. Superparamagnetic Iron Oxide Nanoparticles as Novel X-ray Enhancer for Low-Dose Radiation Therapy. J. Phys. Chem. B 2014, 118, 6159–6166. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Kizaloğlu, M.; Portilla, L.; Park, H.; Rejek, T.; Hümmer, J.; Meyer, K.; Hock, R.; Distel, L.V.R.; Halik, M.; et al. Enhanced in Vitro Biocompatibility and Water Dispersibility of Magnetite and Cobalt Ferrite Nanoparticles Employed as ROS Formation Enhancer in Radiation Cancer Therapy. Small 2018, 14, 1704111. [Google Scholar] [CrossRef]
- Miwa, M.; Ura, M.; Nishida, M.; Sawada, N.; Ishikawa, T.; Mori, K.; Shimma, N.; Umeda, I.; Ishitsuka, H. Design of a Novel Oral Fluoropyrimidine Carbamate, Capecitabine, which Generates 5-Fluorouracil Selectively in Tumors by Enzymes Concentrated in Human Liver and Cancer Tissue. Eur. J. Cancer 1998, 34, 1274–1281. [Google Scholar] [CrossRef]
- Bonadonna, G.; Valagussa, P.; Moliterni, A.; Zambetti, M.; Brambilla, C. Adjuvant Cyclophosphamide, Methotrexate, and Fluorouracil in Node-Positive Breast Cancer—The Results of 20 Years of Follow-up. N. Engl. J. Med. 1995, 332, 901–906. [Google Scholar] [CrossRef]
- Major, P.P.; Egan, E.; Herrick, D.; Kufe, D.W. 5-Fluorouracil Incorporation in DNA of Human Breast Carcinoma Cells. Cancer Res. 1982, 42, 3005–3009. [Google Scholar] [PubMed]
- Kaysen, J.; Spriggs, D.; Kufe, D. Incorporation of 5-Fluorodeoxycytidine and its Metabolites into Nucleic Acids of Human MCF-7 Breast Carcinoma Cells. Cancer Res. 1986, 46, 4534–4538. [Google Scholar] [PubMed]
- Lee, S.Y.; Jeong, E.-K.; Jeon, H.M.; Kim, C.H.; Kang, H.S. Implication of Necrosis-Linked p53 Aggregation in Acquired Apoptotic Resistance to 5-FU in MCF-7 Multicellular Tumor Spheroids. Oncol. Rep. 2010, 24, 73–79. [Google Scholar] [PubMed]
- Garg, A.K.; Buchholz, T.A.; Aggarwal, B.B. Chemosensitization and Radiosensitization of Tumors by Plant Polyphenols. Antioxid. Redox Sign. 2005, 11, 1630–1647. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and Apoptosis-Inducing Effects of Quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into its therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Chang, W.-C.; Hsieh, C.-H.; Hsiao, M.-W.; Lin, W.-C.; Hung, Y.-C.; Ye, J.-C. Caffeic Acid Induces Apoptosis in Human Cervical Cancer Cells Through the Mitochondrial Pathway. Taiwan J. Obstet. Gynecol. 2010, 49, 419–424. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Benzekry, S.; Lamont, C.; Beheshti, A.; Tracz, A.; Ebos, J.M.L.; Hlatky, L.; Hahnfeldt, P. Classical Mathematical Models for Description and Prediction of Experimental Tumor Growth. PLoS Comput. Biol. 2014, 10, e1003800. [Google Scholar] [CrossRef]
- Enderling, H.; Chaplain, M.A.J. Mathematical Modeling of Tumor Growth and Treatment. Curr. Pharm. Des. 2014, 20, 4934–4940. [Google Scholar] [CrossRef] [PubMed]
- Boutry, S.; Forge, D.; Burtea, C.; Mahieu, I.; Murariu, O.; Laurent, S.; Vander Elst, L.; Muller, R.N. How to quantify iron in an aqueous or biological matrix: A technical note. Contrast Media Mol. Imaging 2009, 4, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Raynal, I.; Prigent, P.; Peyramaure, S.; Najid, A.; Rebuzzi, C.; Corot, C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles; mechanisms and comparison of ferumoxides and ferumoxtran-10. Investig. Radiol. 2004, 39, 56–63. [Google Scholar] [CrossRef]
- Comsa, S.; Cimpean, A.M.; Raica, M. The story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar]
- Madunić, I.V.; Madunić, J.; Antunović, M.; Paradžik, M.; Garaj-Vrhovac, V.; Breljak, D.; Marijanović, I.; Gajski, G. Apigenin, a Dietary Flavonoid, Induces Apoptosis, DNA Damage, and Oxidative Stress in Human Breast Cancer MCF-7 and MDA MB-231 Cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 2018, 391, 537–550. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple Negative Breast Cancer Cell Lines: One Tool in the Search for Better Treatment of Triple Negative Breast Cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Kamphorst, J.J.; Markert, E.K.; Schug, Z.T.; Tardito, S.; Gottlieb, E. Cancer Metabolism at a Glance. J. Cell Sci. 2016, 129, 3367–3373. [Google Scholar] [CrossRef]
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The Roles of Glucose Metabolic Reprogramming in Chemo- and Radio-Resistance. J. Exp. Clin. Cancer Res. 2019, 38, 218. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.R.; Brautigan, D.L.; Wu, H.; Yarmohammadi, H.; Kubicka, E.; Serbulea, V.; Leitinger, N.; Liu, W.; Haaga, J.R. Cinnamic Acid Derivatives Enhance Efficacy of Transarterial Embolization in a Rat Model of Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2017, 40, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial Transport of Chlorogenic acid, Caffeic Acid, and Their Colonic Metabolites in Intestinal Caco-2 Cell Monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.H.; Alfieri, A.A.; Young, C.W. Quercetin, an Inhibitor of Lactate Transport and a Hyperthermic Sensitizer of HeLa Cells. Cancer Res. 1984, 44, 102–106. [Google Scholar] [PubMed]
- Laudański, P.; Swiatecka, J.; Kovalchuk, O.; Wołczyński, S. Expression of GLUT1 gene in breast cancer cell lines MCF-7 and MDA-MB-231. Ginekol. Pol. 2003, 4, 782–785. [Google Scholar]
- Sattler, U.G.A.; Meyer, S.S.; Quennet, V.; Hoerner, C.; Knoerzer, H.; Fabian, C.; Yaromina, A.; Zips, D.; Walenta, S.; Baumann, M.; et al. Glycolytic Metabolism and Tumor Response to Fractionated Irradiation. Radiother. Oncol. 2010, 94, 102–109. [Google Scholar] [CrossRef]
- Alhallak, K.; Jenkins, S.V.; Lee, D.E.; Greene, N.P.; Quinn, K.P.; Griffin, R.J.; Dings, R.P.M.; Rajaram, N. Optical Imaging of Radiation-Induced Metabolic Changes in Radiation-Sensitive and Resistant Cancer Cells. J. Biomed. Opt. 2017, 22. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, S.; Distel, L.V.R.; Neuhuber, W.; Kryschi, C. Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids. Nanomaterials 2021, 11, 1167. https://doi.org/10.3390/nano11051167
Klein S, Distel LVR, Neuhuber W, Kryschi C. Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids. Nanomaterials. 2021; 11(5):1167. https://doi.org/10.3390/nano11051167
Chicago/Turabian StyleKlein, Stefanie, Luitpold V. R. Distel, Winfried Neuhuber, and Carola Kryschi. 2021. "Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids" Nanomaterials 11, no. 5: 1167. https://doi.org/10.3390/nano11051167
APA StyleKlein, S., Distel, L. V. R., Neuhuber, W., & Kryschi, C. (2021). Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids. Nanomaterials, 11(5), 1167. https://doi.org/10.3390/nano11051167