Insight into the Contributions of Surface Oxygen Vacancies on the Promoted Photocatalytic Property of Nanoceria
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis Process
2.3. Annealing Process
2.4. Subsection Analysis
2.5. Photocatalytic Performance
3. Results and Discussion
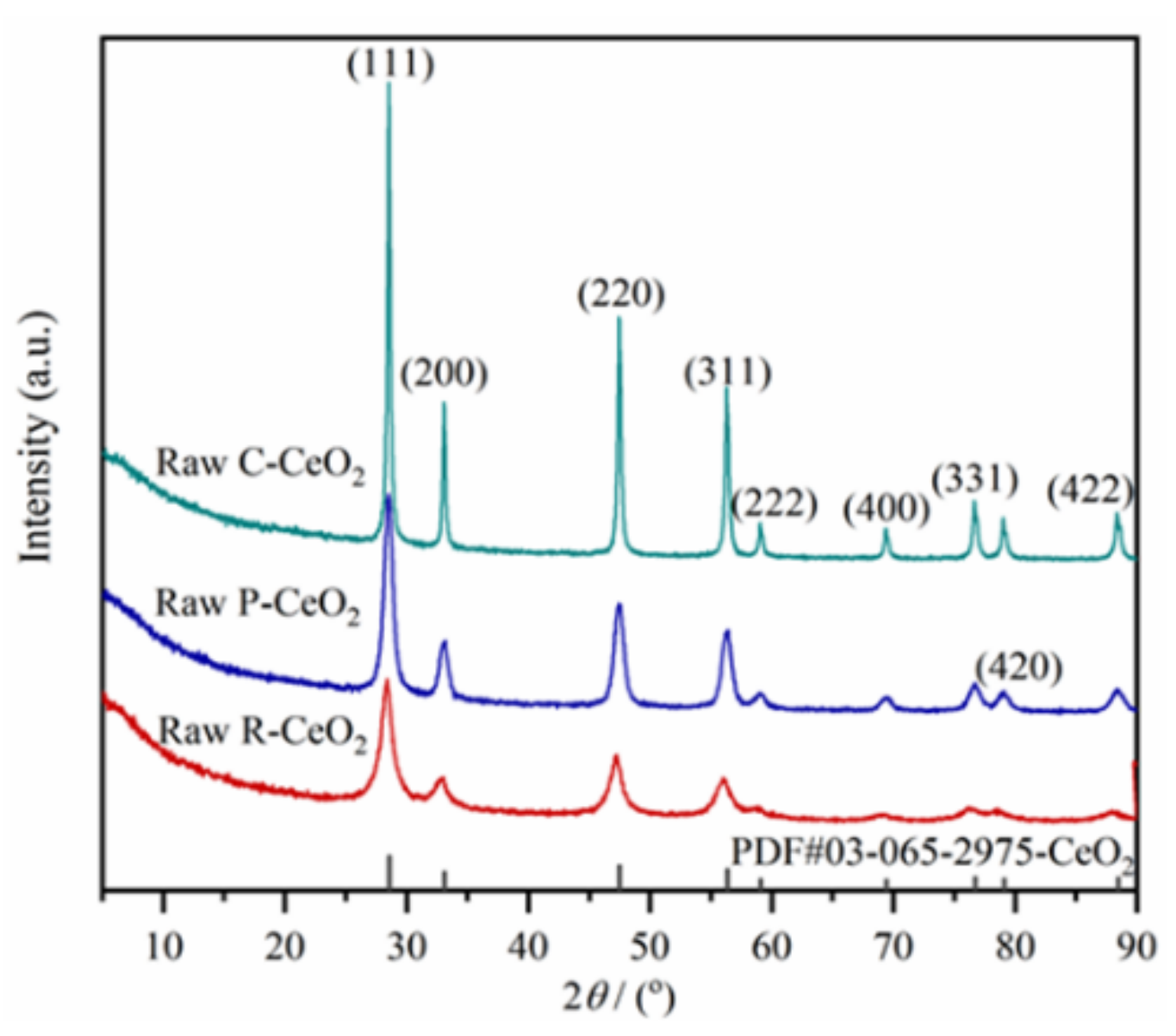
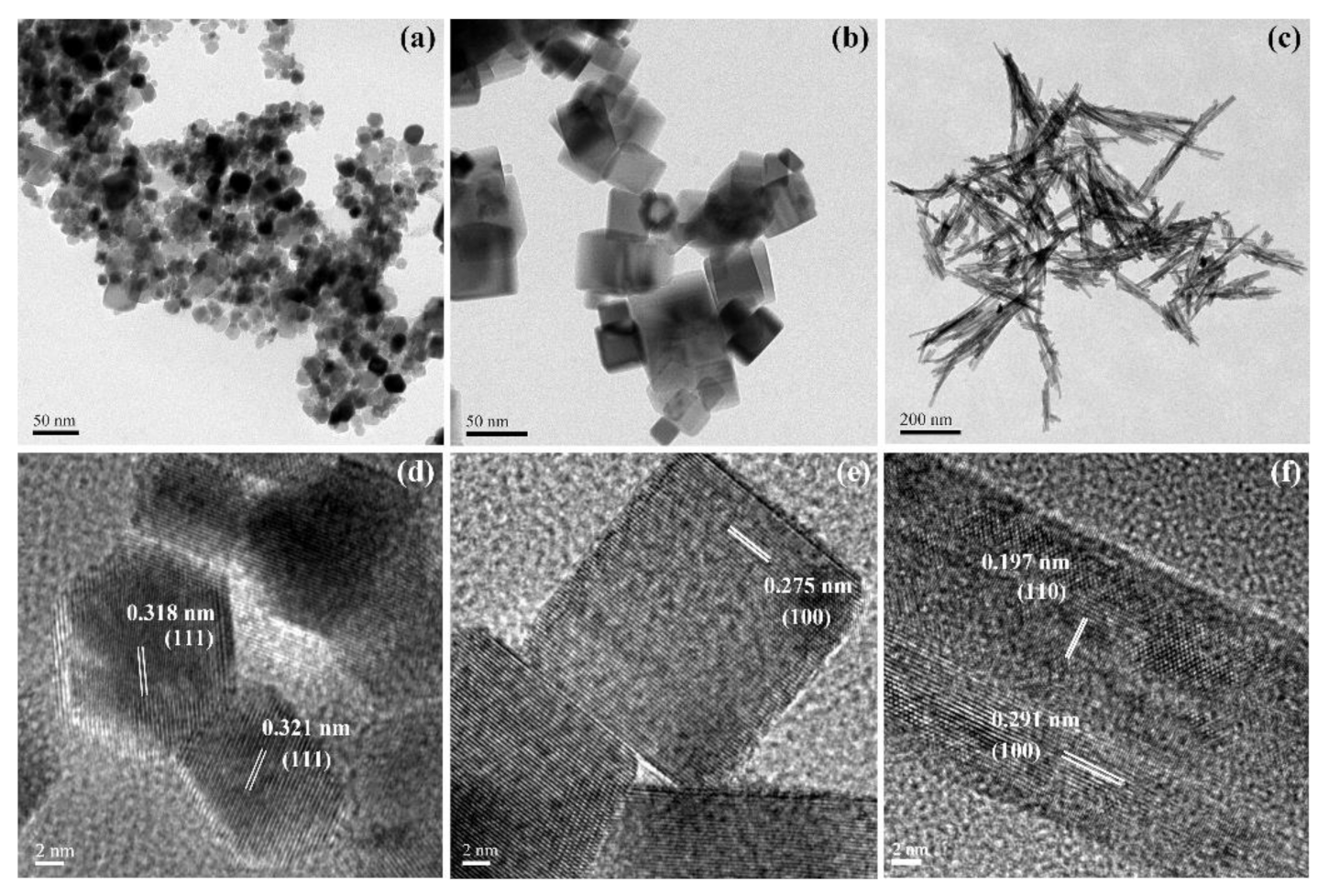
3.1. Phase and Morphology
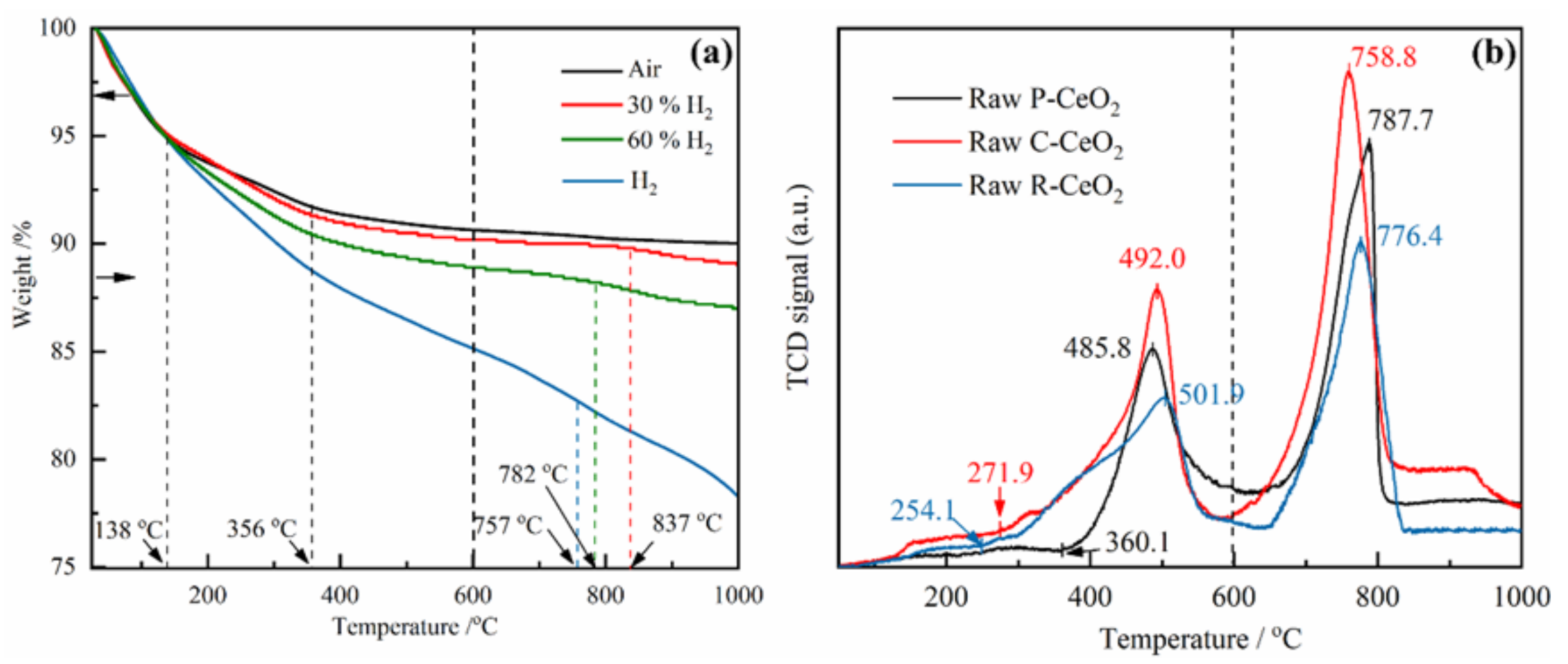
3.2. Reduction of Ceria in H2
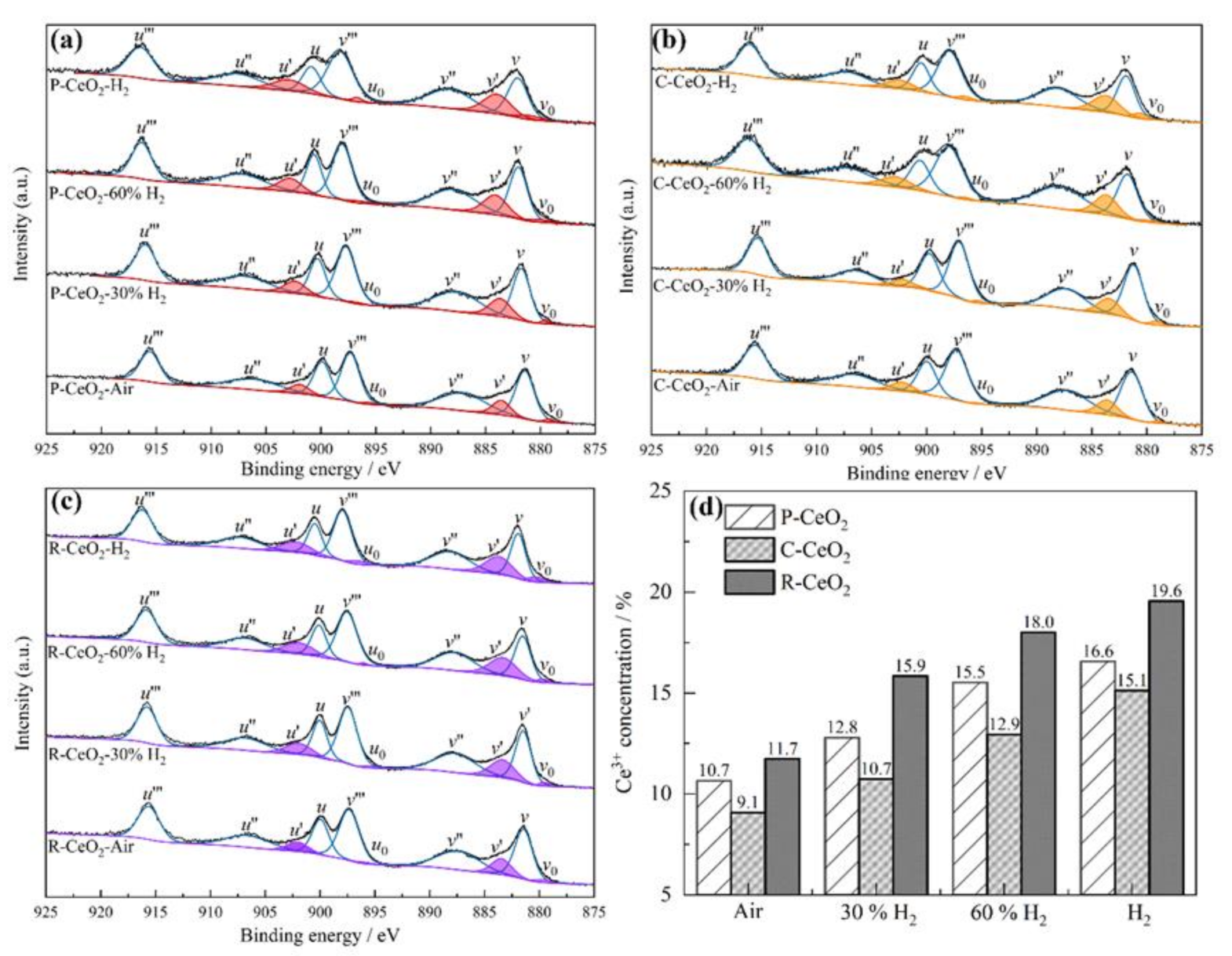
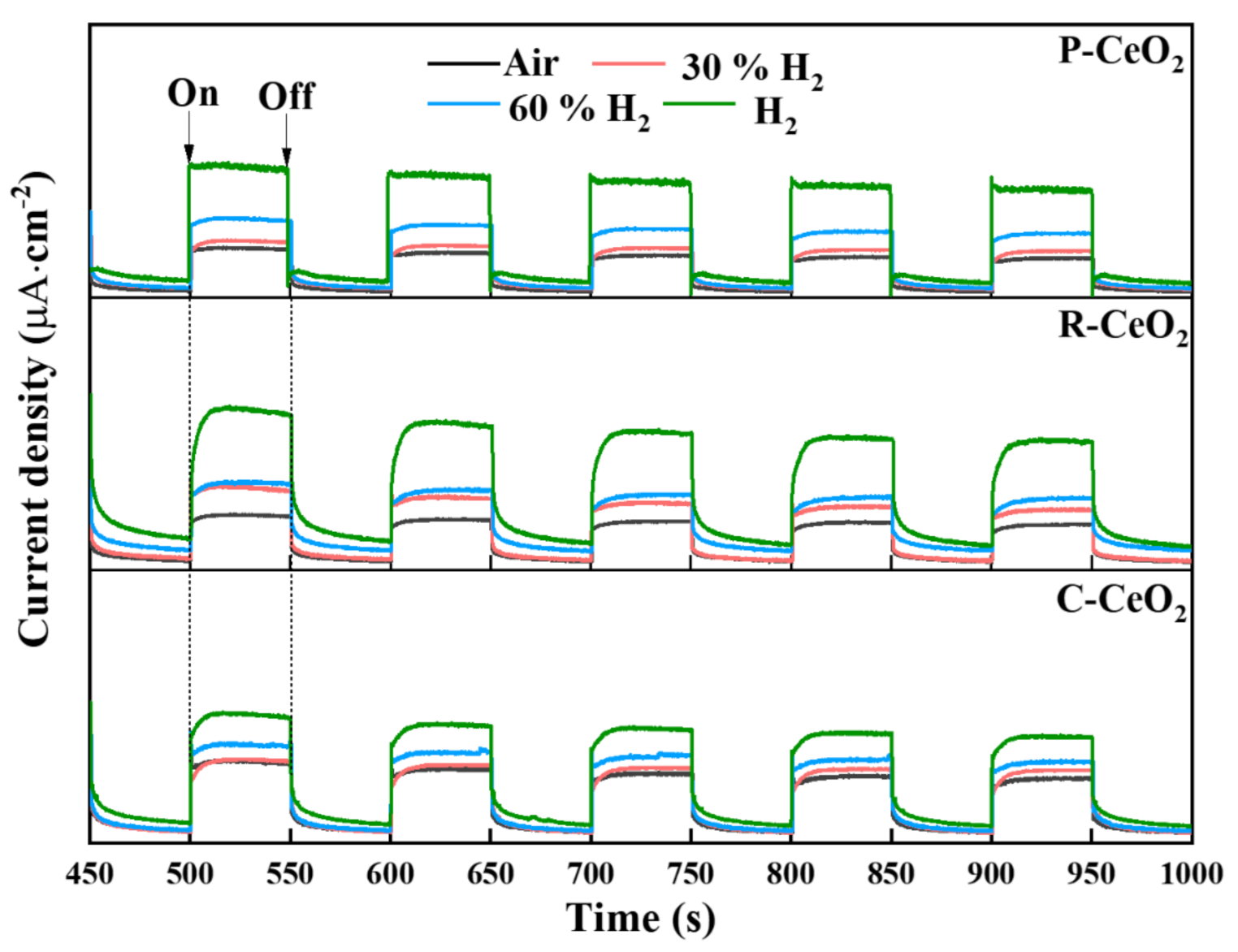
3.3. OVs Characterization
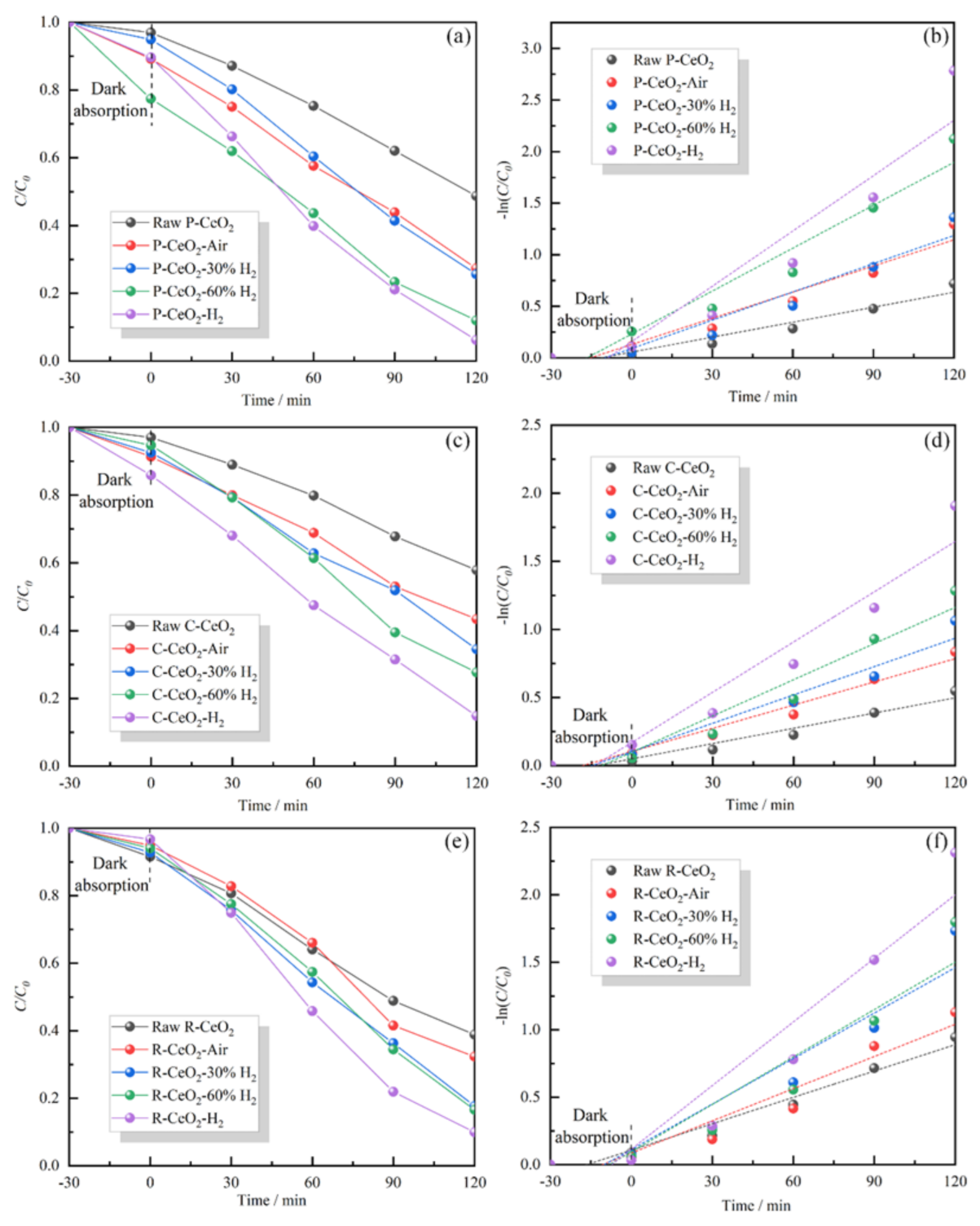
3.4. Photocatalytic Activities
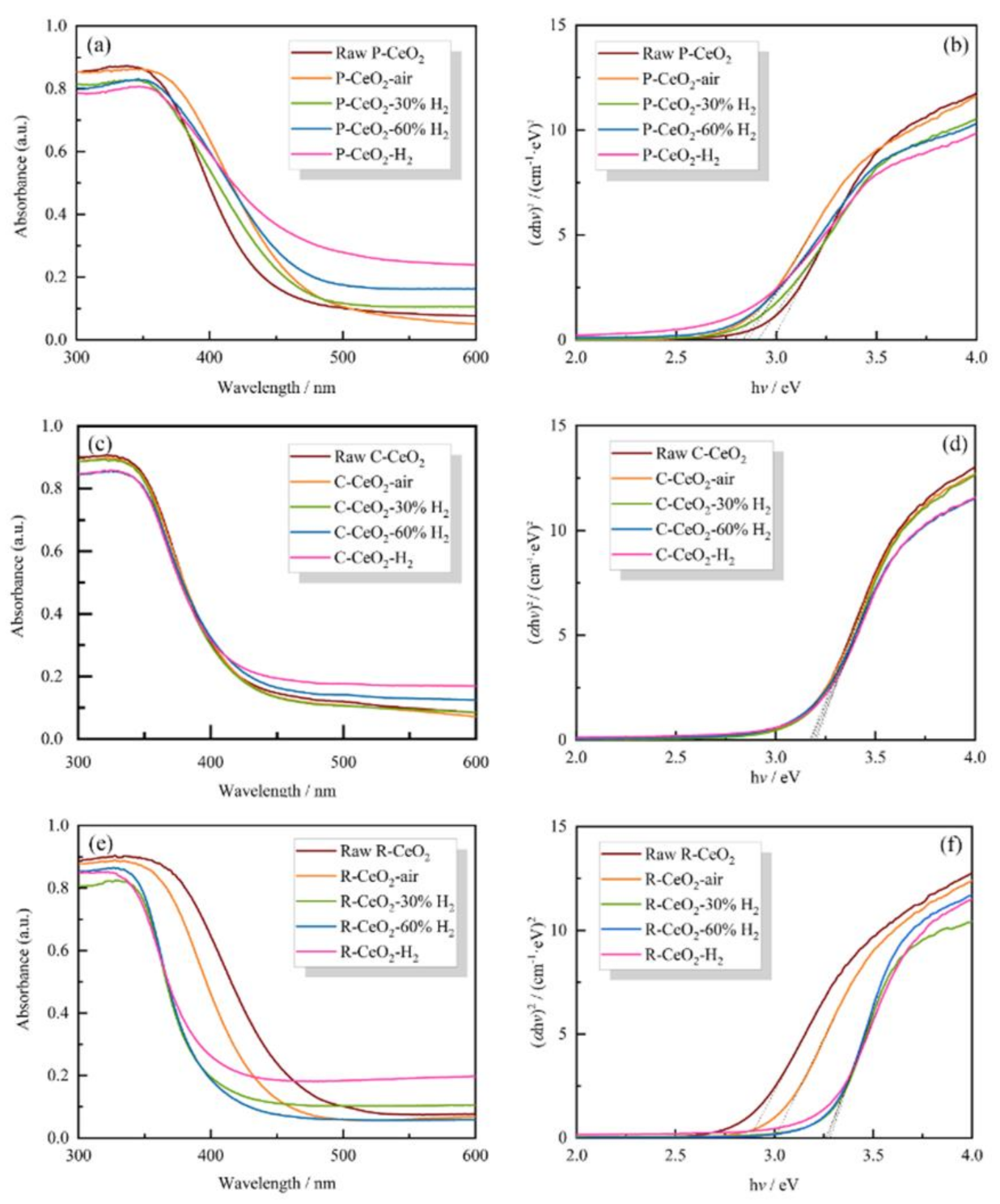
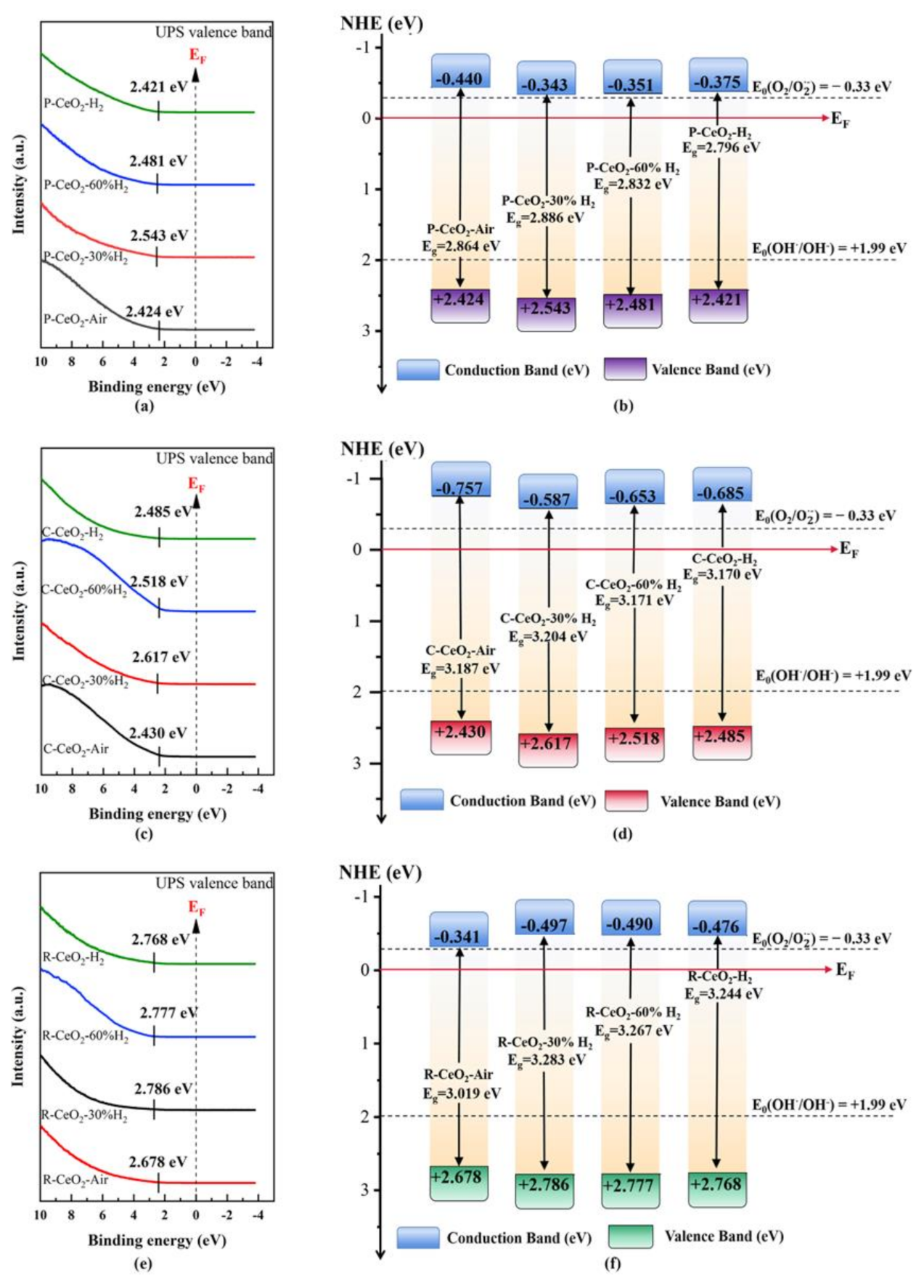
3.5. Band Structure of as Prepared Ceria
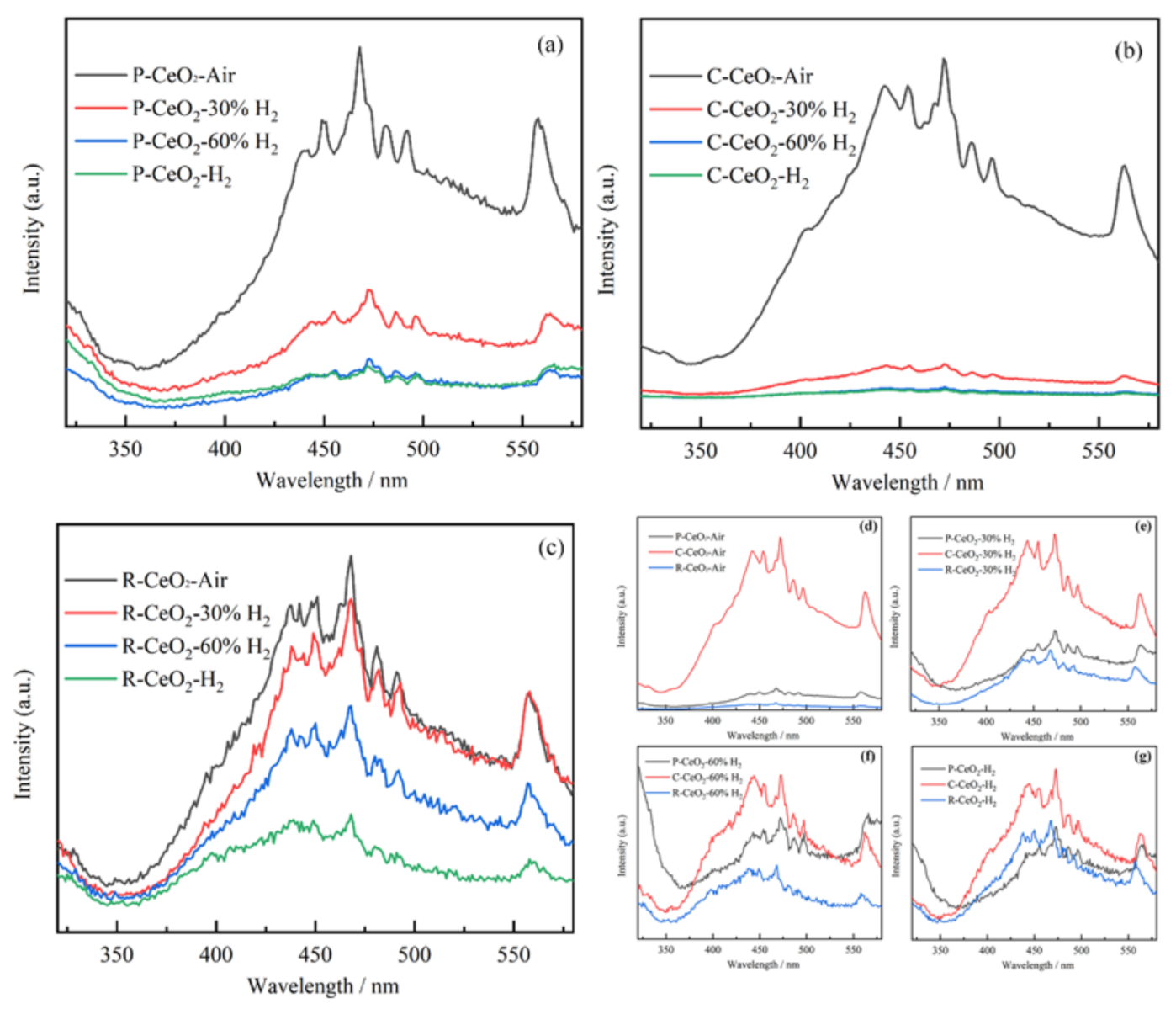
3.6. Separation/Recombination of e−/h+
3.7. Proposed Mechanism for Photocatalytic Enhancement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reed, K.; Cormack, A.; Kulkarni, A.; Mayton, M.; Sayle, D.; Klaessig, F. Exploring the properties and applications of nanoceria: Is there still plenty of room at the bottom? Environ. Sci. Nano 2014, 1, 390–405. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Graziani, M. Use of CeO2-based oxides in the three-way catalysis. Catal. Today 1999, 50, 285–298. [Google Scholar] [CrossRef]
- Jha, A.; Jeong, D.-W.; Jang, W.-J.; Lee, Y.-L.; Roh, H.-S. Hydrogen production from water–gas shift reaction over Ni–Cu–CeO2 oxide catalyst: The effect of preparation methods. Int. J. Hydrog. Energy 2015, 40, 9209–9216. [Google Scholar] [CrossRef]
- Gabrovska, M.; Ivanov, I.; Nikolova, D.; Krstić, J.; Venezia, A.M.; Crişan, D.; Crişan, M.; Tenchev, K.; Idakiev, V.; Tabakova, T. Improved Water–Gas Shift Performance of Au/NiAl LDHs Nanostructured Catalysts via CeO2 Addition. Nanomaterials 2021, 11, 366. [Google Scholar] [CrossRef] [PubMed]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, S.; Sauvin, L.; Castiglioni, R.; Rupp, J.L.; Scheffe, J.R.; Steinfeld, A. Kinetics of CO2 Reduction over Nonstoichiometric Ceria. J. Phys. Chem. C 2015, 119, 16452–16461. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, P.; Suzuki, T.; Anderson, H.U. Nanocrystalline undoped ceria oxygen sensor. Sens. Actuators B Chem. 2003, 95, 73–77. [Google Scholar] [CrossRef]
- Feng, X.; Sayle, D.C.; Wang, Z.L.; Paras, M.S.; Santora, B.; Sutorik, A.C.; Sayle, T.X.; Yang, Y.; Ding, Y.; Wang, X. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 2006, 312, 1504–1508. [Google Scholar] [CrossRef]
- Yang, J.; Xie, N.; Zhang, J.; Fan, W.; Huang, Y.; Tong, Y. Defect Engineering Enhances the Charge Separation of CeO2 Nanorods toward Photocatalytic Methyl Blue Oxidation. Nanomaterials 2020, 10, 2307. [Google Scholar] [CrossRef]
- Mao, X.; Xia, X.; Ning, D.; Li, J.; Chen, C.; Lan, Y.-P. Characterizations and photocatalytic activity of ceria nanoparticles synthesized in KCl–LiCl/KOH–NaOH molten flux from different precursors. J. Nanoparticle Res. 2021, 23, 69. [Google Scholar] [CrossRef]
- Li, M.; Chen, C.; Xu, L.; Jia, Y.; Liu, Y.; Liu, X. Surface defect-rich ceria quantum dots anchored on sulfur-doped carbon nitride nanotubes with enhanced charge separation for solar hydrogen production. J. Energy Chem. 2021, 52, 51–59. [Google Scholar] [CrossRef]
- Huang, Z.-Q.; Li, T.-H.; Yang, B.; Chang, C.-R. Role of surface frustrated Lewis pairs on reduced CeO2 (110) in direct conversion of syngas. Chin. J. Catal. 2020, 41, 1906–1915. [Google Scholar] [CrossRef]
- Wang, M.; Shen, M.; Jin, X.; Tian, J.; Li, M.; Zhou, Y.; Zhang, L.; Li, Y.; Shi, J. Oxygen vacancy generation and stabilization in CeO2−x by Cu introduction with improved CO2 photocatalytic reduction activity. ACS Catal. 2019, 9, 4573–4581. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B Environ. 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Wang, H.; Guan, J.; Li, J.; Li, X.; Ma, C.; Huo, P.; Yan, Y. Fabricated g-C3N4/Ag/m-CeO2 composite photocatalyst for enhanced photoconversion of CO2. Appl. Surf. Sci. 2020, 506, 144931. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, S.H.; Hsiao, C.-H.; Lee, A.T.; Huang, M.H. Mild Synthesis of Size-Tunable CeO2 Octahedra for Band Gap Variation. Chem. Mater. 2020, 32, 2631–2638. [Google Scholar] [CrossRef]
- Xia, X.W.; Lan, Y.P.; Li, J.Q.; Chen, C.Y.; Mao, X.S. Facile synthesis of nanoceria by a molten hydroxide method and its photocatalytic properties. J. Rare Earths 2019, 38, 951–960. [Google Scholar] [CrossRef]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A.I.; Lubomirsky, I.; Haile, S.M.; Rupp, J.L. A review of defect structure and chemistry in ceria and its solid solutions. Chem. Soc. Rev. 2020, 49, 554–592. [Google Scholar] [CrossRef]
- Pinto, F.M.; Suzuki, V.Y.; Silva, R.C.; La Porta, F.A. Oxygen Defects and Surface Chemistry of Reducible Oxides. Front. Mater. 2019, 6, 260. [Google Scholar] [CrossRef]
- Ma, R.; Islam, M.J.; Reddy, D.A.; Kim, T.K. Transformation of CeO2 into a mixed phase CeO2/Ce2O3 nanohybrid by liquid phase pulsed laser ablation for enhanced photocatalytic activity through Z-scheme pattern. Ceram. Int. 2016, 42, 18495–18502. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Ce3+ and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles. Mater. Chem. Phys. 2012, 131, 666–671. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.; Ma, Y.; Qu, Y. Surface engineering on CeO2 nanorods by chemical redox etching and their enhanced catalytic activity for CO oxidation. Nanoscale 2015, 7, 11686–11691. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xia, X.; Li, J.; Chen, C.; Gu, X.; Li, S.; Lan, Y.-P. Self-assembly of structured CeCO3OH and its decomposition in H2 for a novel tactic to obtain CeO2−x with excellent photocatalytic property. J. Alloy. Compd. 2021, 870, 159424. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Kalathil, S.; Nisar, A.; Lee, J.; Cho, M.H. Oxygen vacancy induced band gap narrowing of ZnO nanostructures by an electrochemically active biofilm. Nanoscale 2013, 5, 9238–9246. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Lou, Z.; Chen, Y.; Chen, D.; Tian, S.; Xiong, Y. Tuning the structural properties of CeO2 by Pr and Fe codoping for enhanced visible-light catalytic activity. J. Chem. Technol. Biotechnol. 2019, 94, 1576–1584. [Google Scholar] [CrossRef]
- Yuán, S.; Xu, B.; Zhang, Q.; Liu, S.; Xie, J.; Zhang, M.; Ohno, T. Development of visible light response of CeO2−x with the high content of Ce3+ and its photocatalytic property. ChemCatChem 2018, 10, 1267–1271. [Google Scholar] [CrossRef]
- Padeste, C.; Cant, N.W.; Trimm, D.L. The influence of water on the reduction and reoxidation of ceria. Catal. Lett. 1993, 18, 305–316. [Google Scholar] [CrossRef]
- Binet, C.; Badri, A.; Lavalley, J.-C. A Spectroscopic Characterization of the Reduction of Ceria from Electronic Transitions of Intrinsic Point Defects. J. Phys. Chem. 1994, 98, 6392–6398. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Kalathil, S.; Lee, J.; Cho, M.H. Band gap engineering of CeO2 nanostructure using an electrochemically active biofilm for visible light applications. RSC Adv. 2014, 4, 16782–16791. [Google Scholar] [CrossRef]
- Rao, M.R.; Shripathi, T. Photoelectron spectroscopic study of X-ray induced reduction of CeO2. J. Electron Spectrosc. 1997, 87, 121–126. [Google Scholar]
- Qiu, L.; Liu, F.; Zhao, L.; Ma, Y.; Yao, J. Comparative XPS study of surface reduction for nanocrystalline and microcrystalline ceria powder. Appl. Surf. Sci. 2006, 252, 4931–4935. [Google Scholar] [CrossRef]
- González-Elipe, A.R.; Fernández, A.; Holgado, J.P.; Caballero, A.; Munuera, G. Mixing effects in CeO2/TiO2 and CeO2/SiO2 systems submitted to Ar+ sputtering. J. Vac. Sci. Technol. A 1993, 11, 58–65. [Google Scholar] [CrossRef]
- Sundaram, K.B.; Wahid, P.F.; Melendez, O. Deposition and X-ray photoelectron spectroscopy studies on sputtered cerium dioxide thin films. J. Vac. Sci. Technol. A 1997, 15, 52–56. [Google Scholar] [CrossRef]
- Holgado, J.; Munuera, G.; Espinós, J.; González-Elipe, A. XPS study of oxidation processes of CeOx defective layers. Appl. Surf. Sci. 2000, 158, 164–171. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Peter, Y.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2–TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Lan, Y.P.; Sohn, H.Y. Effect of oxygen vacancies and phases on catalytic properties of hydrogen-treated nanoceria particles. Mater. Res. Express 2018, 5, 035501. [Google Scholar] [CrossRef]
- Lee, Y.; He, G.; Akey, A.J.; Si, R.; Flytzani-Stephanopoulos, M.; Herman, I.P. Raman analysis of mode softening in nanoparticle CeO2−δ and Au-CeO2−δ during CO oxidation. J. Am. Chem. Soc. 2011, 133, 12952–12955. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, A.L.; Ning, J.; Shen, W.J. Electronic and geometric structure of the copper-ceria interface on Cu/CeO2 catalysts. Chin. J. Catal. 2020, 41, 928–937. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, Z.; Gao, G.; Liu, Z.; Wang, Q.; Yan, Y.S. Construction of spindle structured CeO2 modified with rod-like attapulgite as a high-performance photocatalyst for CO2 reduction. Catal. Sci. Technol. 2019, 9, 3788–3799. [Google Scholar] [CrossRef]
- Wang, Z.J.; Gao, Y.Z.; Chabal, Y.J.; Balkus, K.J. Oxidative Dehydrogenation of Cyclohexane and Cyclohexene over Y-doped CeO2 Nanorods. Catal. Lett. 2017, 147, 738–744. [Google Scholar] [CrossRef]
- Xia, X.; Li, J.; Chen, C.; Lan, Y.-P.; Mao, X.; Bai, F. Optimal rare-earth (La, Y and Sm) doping conditions and enhanced mechanism for photocatalytic application of ceria nanorods. Nanotechnology 2021, 32, 195708. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.X.; Sun, L.D.; Zhang, Y.W.; Si, R.; Feng, W.; Zhang, H.P.; Liu, H.C.; Yan, C.H. Shape-Selective Synthesis and Oxygen Storage Behavior of Ceria Nanopolyhedra, Nanorods, and Nanocubes. J. Phys. Chem. B 2005, 109, 24380–24385. [Google Scholar] [CrossRef]
- Murali, A.; Lan, Y.P.; Sohn, H.Y. Effect of oxygen vacancies in non-stoichiometric ceria on its photocatalytic properties. Nano-Struct. Nano Objects 2019, 18, 100257. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Kadkhodaie, A. Synthetic CeO2 Nanoparticle Catalysis of Methylene Blue Photodegradation: Kinetics and Mechanism. Chin. J. Catal. 2010, 31, 1328–1334. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef] [PubMed]
- Djuričić, B.; Pickering, S. Nanostructured cerium oxide: Preparation and properties of weakly-agglomerated powders. J. Eur. Ceram. Soc. 1999, 19, 1925–1934. [Google Scholar] [CrossRef]
- Perrichon, V.; Laachir, A.; Bergeret, G.; Fréty, R.; Tournayan, L.; Touret, O. Reduction of cerias with different textures by hydrogen and their reoxidation by oxygen. J. Chem. Soc. Faraday Trans. 1994, 90, 773. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Esch, F.; Fabris, S.; Zhou, L.; Montini, T.; Africh, C.; Fornasiero, P.; Comelli, G.; Rosei, R. Electron localization determines defect formation on ceria substrates. Science 2005, 309, 752–755. [Google Scholar] [CrossRef]
- Neish, A.C. Preparation of pure cerium salts and the color of cerium oxide. J. Am. Chem. Soc. 1909, 31, 517–523. [Google Scholar] [CrossRef][Green Version]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 1686–1698. [Google Scholar] [CrossRef]
- Le Normand, F.; El Fallah, J.; Hilaire, L.; Légaré, P.; Kotani, A.; Parlebas, J.C. Photoemission on 3d core levels of Cerium: An experimental and theoretical investigation of the reduction of cerium dioxide. Solid State Commun. 1989, 71, 885–889. [Google Scholar] [CrossRef]
- Romeo, M.; Bak, K.; El Fallah, J.; Le Normand, F.; Hilaire, L. XPS Study of the reduction of cerium dioxide. Surf. Interface Anal. 1993, 20, 508–512. [Google Scholar] [CrossRef]
- Skála, T.; Šutara, F.; Škoda, M.; Prince, K.C.; Matolín, V. Palladium interaction with CeO2, Sn–Ce–O and Ga–Ce–O layers. J. Phys. Condens. Matter 2009, 21, 055005. [Google Scholar] [CrossRef]
- Borchert, H.; Frolova, Y.V.; Kaichev, V.V.; Prosvirin, I.P.; Alikina, G.M.; Lukashevich, A.I.; Zaikovskii, V.I.; Moroz, E.M.; Trukhan, S.N.; Ivanov, V.P. Electronic and chemical properties of nanostructured cerium dioxide doped with praseodymium. J. Phys. Chem. B 2005, 109, 5728–5738. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Wang, W.; Zhang, L.; Zheng, Y.; Wang, Z. Insights into the Surface-Defect Dependence of Photoreactivity over CeO2 Nanocrystals with Well-Defined Crystal Facets. ACS Catal. 2015, 5, 4851–4858. [Google Scholar] [CrossRef]
- Du, H.W.; Wang, Y.; Arandiyan, H.; Scott, J.; Wan, T.; Chu, D.W. Correlating morphology and doping effects with the carbon monoxide catalytic activity of Zn doped CeO2 nanocrystals. Catal. Sci. Technol. 2017, 8, 134–138. [Google Scholar] [CrossRef]
- Fan, L.; Wang, K.; Xu, K.; Liang, Z.; Wang, H.; Zhou, S.F.; Zhan, G. Structural Isomerism of Two Ce-BTC for Fabricating Pt/CeO2 Nanorods toward Low-Temperature CO Oxidation. Small 2020, 16, 2003597. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Faisal, M.; Rahman, M.M.; Akhtar, K.; Asiri, A.M.; Khan, A.; Alamry, K.A. Effect of particle size on the photocatalytic activity and sensing properties of CeO2 nanoparticles. Int. J. Electrochem. Sci. 2013, 8, 7284–7297. [Google Scholar]
- Yang, X.; Liu, Y.; Li, J.; Zhang, Y. Effects of calcination temperature on morphology and structure of CeO2 nanofibers and their photocatalytic activity. Mater. Lett. 2019, 241, 76–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, X.; Duan, L.; Shen, H.; Liu, R. Controlling oxygen vacancies and enhanced visible light photocatalysis of CeO2/ZnO nanocomposites. J. Photochem. Photobiol. A Chem. 2020, 392, 112156. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Choi, J.; Kim, T.K. Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of a Z-scheme CeO2–AgI nanocomposite. RSC Adv. 2016, 6, 19341–19350. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chaiwichain, S.; Inceesungvorn, B.; Pingmuang, K.; Phanichphant, S.; Minett, A.I.; Chen, J. BiVO4/CeO2 Nanocomposites with High Visible-Light-Induced Photocatalytic Activity. ACS Appl. Mater. Interfaces 2012, 4, 3718. [Google Scholar] [CrossRef]
- Issarapanacheewin, S.; Wetchakun, K.; Phanichphant, S.; Kangwansupamonkon, W.; Wetchakun, N. A novel CeO2/Bi2WO6 composite with highly enhanced photocatalytic activity. Mater. Lett. 2015, 156, 28–31. [Google Scholar] [CrossRef]
- Bakkiyaraj, R.; Balakrishnan, M.; Bharath, G.; Ponpandian, N. Facile synthesis, structural characterization, photocatalytic and antimicrobial activities of Zr doped CeO2 nanoparticles. J. Alloy. Compd. 2017, 724, 555–564. [Google Scholar] [CrossRef]
- Syed, K.Y.A.; Balamurugan, A.; Devarajan, V.P.; Subramanian, R. Hydrothermal Synthesis of Gadolinium (Gd) Doped Cerium Oxide (CeO2) Nanoparticles: Characterization and Antibacterial Activity. Orient. J. Chem. 2017, 33, 2405–2411. [Google Scholar]
- Younis, A.; Chu, D.W.; Kaneti, Y.V.; Li, S. Tuning the surface oxygen concentration of {111} surrounded ceria nanocrystals for enhanced photocatalytic activities. Nanoscale 2016, 8, 378–387. [Google Scholar] [CrossRef]
- Magdalane, C.M.; Kaviyarasu, K.; Priyadharsini, G.M.A.; Bashir, A.K.H.; Maaza, M. Improved photocatalytic decomposition of aqueous Rhodamine-B by solar light illuminated hierarchical yttria nanosphere decorated ceria nanorods. J. Mater. Res. Technol. 2019, 8, 2898–2909. [Google Scholar] [CrossRef]
- Kesarla, M.K.; Fuentez-Torres, M.O.; Alcudia-Ramos, M.A.; Ortiz-Chi, F.; Espinosa-González, C.G.; Aleman, M.; Torres-Torres, J.G.; Godavarthi, S. Synthesis of g-C3N4/N-doped CeO2 composite for photo catalytic degradation of an herbicide. J. Mater. Res. Technol. 2019, 8, 1628–1635. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Qian, J.; Miao, N.; Yao, C.; Chen, Z. Synthesis and characterization of CeO2/TiO2 nanotube arrays and enhanced photocatalytic oxidative desulfurization performance. J. Alloy. Compd. 2016, 661, 363–371. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Duan, X.; Hu, G.; Liu, Q.C.; Ren, S.; Li, J.L.; Kong, M. Roles of photo-generated holes and oxygen vacancies in enhancing photocatalytic performance over CeO2 prepared by molten salt method. Adv. Powder Technol. 2020, 31, 4072–4081. [Google Scholar] [CrossRef]
- Ikegami, L.T. Oxide ionic conductivity and microstructures of Sm- or La-doped CeO2-based systems. Solid State Ion. 2002, 154–155, 461–466. [Google Scholar]
- Mao, X.; Xia, X.; Li, J.; Chen, C.; Zhang, J.; Ning, D.; Lan, Y.-P. Homogenously Rare-Earth-Ion-Doped Nanoceria Synthesis in KOH-NaOH Molten Flux: Characterization and Photocatalytic Property. J. Mater. Eng. Perform. 2021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Xia, X.; Li, J.; Mao, X.; Chen, C.; Ning, D.; Chu, Z.; Zhang, J.; Liu, F. Insight into the Contributions of Surface Oxygen Vacancies on the Promoted Photocatalytic Property of Nanoceria. Nanomaterials 2021, 11, 1168. https://doi.org/10.3390/nano11051168
Lan Y, Xia X, Li J, Mao X, Chen C, Ning D, Chu Z, Zhang J, Liu F. Insight into the Contributions of Surface Oxygen Vacancies on the Promoted Photocatalytic Property of Nanoceria. Nanomaterials. 2021; 11(5):1168. https://doi.org/10.3390/nano11051168
Chicago/Turabian StyleLan, Yuanpei, Xuewen Xia, Junqi Li, Xisong Mao, Chaoyi Chen, Deyang Ning, Zhiyao Chu, Junshan Zhang, and Fengyuan Liu. 2021. "Insight into the Contributions of Surface Oxygen Vacancies on the Promoted Photocatalytic Property of Nanoceria" Nanomaterials 11, no. 5: 1168. https://doi.org/10.3390/nano11051168
APA StyleLan, Y., Xia, X., Li, J., Mao, X., Chen, C., Ning, D., Chu, Z., Zhang, J., & Liu, F. (2021). Insight into the Contributions of Surface Oxygen Vacancies on the Promoted Photocatalytic Property of Nanoceria. Nanomaterials, 11(5), 1168. https://doi.org/10.3390/nano11051168





