Feasibility Synthesis and Characterization of Gadolinia Doped Ceria Coatings Obtained by Cathodic Arc Evaporation
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Source 2021, 492, 229630. [Google Scholar] [CrossRef]
- Badwal, S.; Foger, K. Solid oxide electrolyte fuel cell review. Ceram. Int. 1996, 22, 257. [Google Scholar] [CrossRef]
- Ivers-Tiffée, E.; Weber, A.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805. [Google Scholar] [CrossRef]
- Chaubey, N.; Wani, B.N.; Bharadwaj, S.R.; Chattopadhyaya, M.C. Physicochemical properties of rare earth doped ceria Ce0.9Ln0.1O1.95 (Ln = Nd, Sm, Gd) as an electrolyte material for IT-SOFC/SOEC. Solid State Sci. 2013, 20, 135–141. [Google Scholar] [CrossRef]
- Yahiro, H.; Eguchi, K.; Arai, H. Electrical properties and reducibilities of ceria-rare earth oxide systems and their application to solid oxide fuel cell. Solid State Ion. 1989, 36, 71. [Google Scholar] [CrossRef]
- Anirban, S.K.; Dutta, A. Revisiting ionic conductivity of rare earth doped ceria: Dependency on different factors. Int. J. Hydrogen 2020, 45, 25139. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984. [Google Scholar] [CrossRef]
- Ishihara, T.; Matsuda, H.; Takida, Y. Effects of rare earth cations doped for La site on the oxide ionic conductivity of LaGaO3-based perovskite type oxide. Solid State Ion. 1995, 79, 147. [Google Scholar] [CrossRef]
- Zhang, G.B.; Smyth, D.M. Defects and transport of the brownmillerite oxides with high oxygen ion conductivity—Ba2In2O5. Solid State Ion. 1995, 82, 161. [Google Scholar] [CrossRef]
- Joubert, O.; Ganne, M.; Vannier, R.N.; Mairesse, G. Solid phase synthesis and characterization of new BIMEVOX series: Bi4V2−xMxO11 x (M = CrIII, FeIII). Solid State Ion. 1996, 83, 199. [Google Scholar] [CrossRef]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retous, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856. [Google Scholar] [CrossRef] [PubMed]
- Briois, P.; Lapostolle, F.; Billard, A. Investigations of Apatite-Structure Coatings Deposited by Reactive Magnetron Sputtering Dedicated to IT-SOFC. Plasma Process. Polym. 2007, 4, S99–S103. [Google Scholar] [CrossRef]
- Ma, C.Y.; Briois, P.; Bohlmark, J.; Lapostolle, F.; Billard, A. La9.33Si6O26 electrolyte thin films for IT-SOFC application deposited by a HIPIMS/DC hybrid magnetron sputtering process. Ionics 2008, 14, 471–476. [Google Scholar] [CrossRef]
- Huang, W.; Shuk, P.; Greenblatt, M. Properties of sol-gel prepared Ce1−xSmxO2−x2 solid electrolytes. Solid State Ion. 1997, 100, 23. [Google Scholar] [CrossRef]
- Yahiro, H.; Eguchi, Y.; Eguchi, K.; Arai, H. Oxygen ion conductivity of the ceria-samarium oxide system with fluorite structure. J. Appl. Electrochem. 1988, 18, 527. [Google Scholar] [CrossRef]
- Arabaci, A. Effect of Sm and Gd dopants on tsructurals characteristics and ionic conductivity of ceria. Ceram. Int. 2015, 41, 5836. [Google Scholar] [CrossRef]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 2000, 129, 95. [Google Scholar] [CrossRef]
- Butler, V.; Catlow, C.; Fender, B.; Harding, J. Dopant ion radius and ionic conductivity in cerium dioxide. Solid State Ion. 1983, 8, 109. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.; Jiang, S.; Khor, K. Low-temperature SOFC with thin film GDC electrolyte prepared in situ by solid-state reaction. Solid State Ion. 2004, 170, 9. [Google Scholar] [CrossRef]
- Fang, Q.; Zhang, J.Y. Preparation of Ce1−xGdxO2−0.5x thin films by UV assisted sol–gel method. Surf. Coat. Technol. 2002, 100, 151–152. [Google Scholar] [CrossRef]
- Christie, G.M.; van Berkel, F.P.F. Microstructure-ionic conductivity relationships in ceria-gadolinia electrolytes. Solid State Ion. 1996, 83, 17. [Google Scholar] [CrossRef]
- Arabacı, A.; Öksüzömer, M.F. Preparation and characterization of 10 mol% Gd doped CeO2 (GDC) electrolyte for SOFC applications. Ceram. Int. 2012, 38, 6509. [Google Scholar] [CrossRef]
- Gourba, E.; Ringuede, A.; Cassir, M.; Billard, A.; Päivãsaari, J.; Niinisto, J.; Putkonen, M.; Niinisto, L. Characterisation of thin films of ceria-based electrolytes for Intermediate Temperature—Solid oxide fuel cells (IT-SOFC). Ionics 2003, 9, 15. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, S.; Kim, W.; Yoon, H. Fabrication and characterization GDC electrolyte thin films by e-beam technique for IT-SOFC. Curr. Appl. Phys. 2011, 11, S163–S168. [Google Scholar] [CrossRef]
- Briois, P.; Billard, A. A comparison of electrical properties of sputter-deposited electrolyte coatings dedicated to intermediate temperature solid oxide fuel cells. Surf. Coat. Technol. 2006, 201, 1328. [Google Scholar] [CrossRef]
- Mickan, M.; Coddet, P.; Vulliet, J.; Caillard, A.; Sauvage, T.; Thomann, A.-L. Optimized magnetron sputtering process for the deposition of gadolinia doped ceria layers with controlled structural properties. Surf. Coat. Technol. 2020, 398, 126095. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, W.; Hertz, J. Structure and ionic conductivity of nanoscale gadolinia-doped ceria thin films. Solid State Ion. 2013, 249–250, 139–143. [Google Scholar] [CrossRef]
- Chapusot, V.; Pierson, J.-F.; Lapostolle, F.; Billard, A. Arc-evaporated nanocomposite zirconium-based boronitride coatings. Mater. Chem. Phys. 2009, 114, 780–784. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, X. Effect of O2 pressure on the preferred orientation of TiO2 films prepared by filtered arc deposition. Thin Solid Films 1998, 326, 171. [Google Scholar] [CrossRef]
- Koller, C.; Dalbauer, V.; Schmelz, A.; Raab, R.; Polcik, P.; Ramm, J.; Mayrhofer, P. Structure, mechanical properties, and thermal stability of arc evaporated (Al1-xCrx)2O3 coatings. Surf. Coat. Technol. 2018, 342, 37. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31. [Google Scholar] [CrossRef] [Green Version]
- Gourba, E.; Briois, P.; Ringuedé, A.; Cassir, M.; Billard, A. Electrical properties of gadolinia-doped ceria thin films deposited by sputtering in view of SOFC application. J. Solid State Electrochem. 2004, 8, 633. [Google Scholar] [CrossRef]
- Anders, A. Approaches to rid cathodic arc plasmas of macro- and nanoparticles: A review. Surf. Coat. Technol. 1999, 120–121, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Boxman, R.; Goldsmith, S. Macroparticle contamination in cathodic arc coatings: Generation, transport and control. Surf. Coat. Technol. 1992, 52, 39–50. [Google Scholar] [CrossRef]
- Rakhovskii, V.I. Experimental Study of the Dynamics of Cathode Spots Development. IEEE Trans. Plasma Sci. 1976, 4, 81–102. [Google Scholar] [CrossRef]
- Billard, A.; Perry, F.; Frantz, C. Stable and unstable conditions of the sputtering mode by modulating at low frequency the current of a magnetron discharge. Surf. Coat. Technol. 1997, 94–95, 345. [Google Scholar] [CrossRef]
- Pierson, J.-F.; Billard, A.; Belmonte, T.; Michel, H.; Frantz, C. Influence of oxygen flow rate on the structural and mechanical properties of reactively magnetron sputter-deposited Zr–B–O coatings. Thin Solid Films 1999, 347, 78. [Google Scholar] [CrossRef]
- Suzuki, T.; Kosacki, I.; Anderson, H.U. Microstructure—Electrical conductivity relationships in nanocrystalline ceria thin films. Solid State Ion. 2002, 151, 111. [Google Scholar] [CrossRef]
- Bauerle, J. Study of solid electrolyte polarization by a complex admittance method. J. Phys. Chem. Solids 1969, 30, 2657. [Google Scholar] [CrossRef]
- Tschöpe, A.; Sommer, E.; Birringer, R. Grain Size Dependent Electrical Conductivity of Polycrystalline Cerium Oxide. Solid State Ion. 2001, 139, 255. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Núñez, P.; Frade, J.; Abrantes, J. Conductivity of CGO and CSO ceramics obtained from freeze-dried precursors. Electrochim. Acta 2003, 48, 1551. [Google Scholar] [CrossRef]
- Balazs, G.B.; Glass, R.S. Ac impedance studies of rare earth oxide doped ceria. Solid State Ion. 1995, 76, 155. [Google Scholar] [CrossRef]
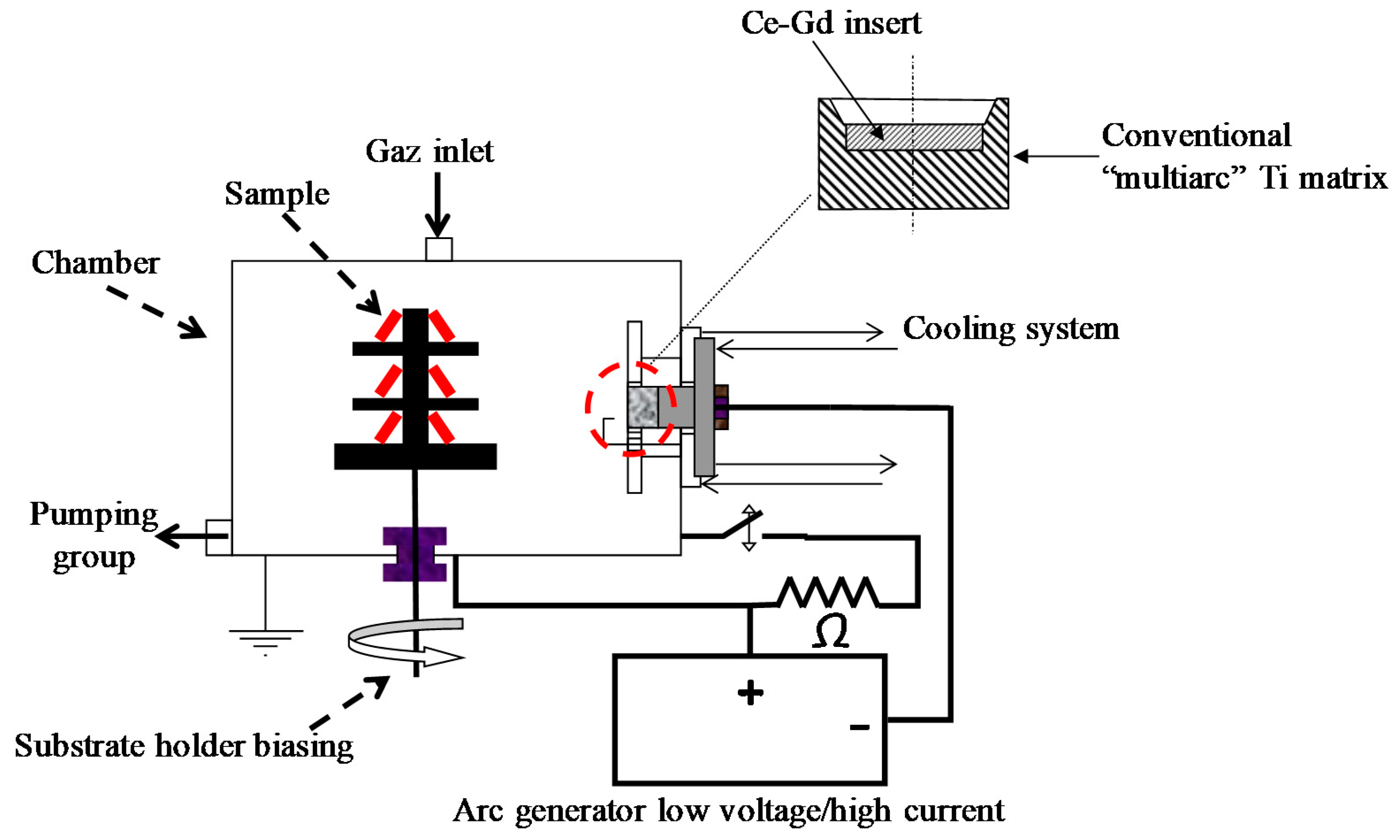
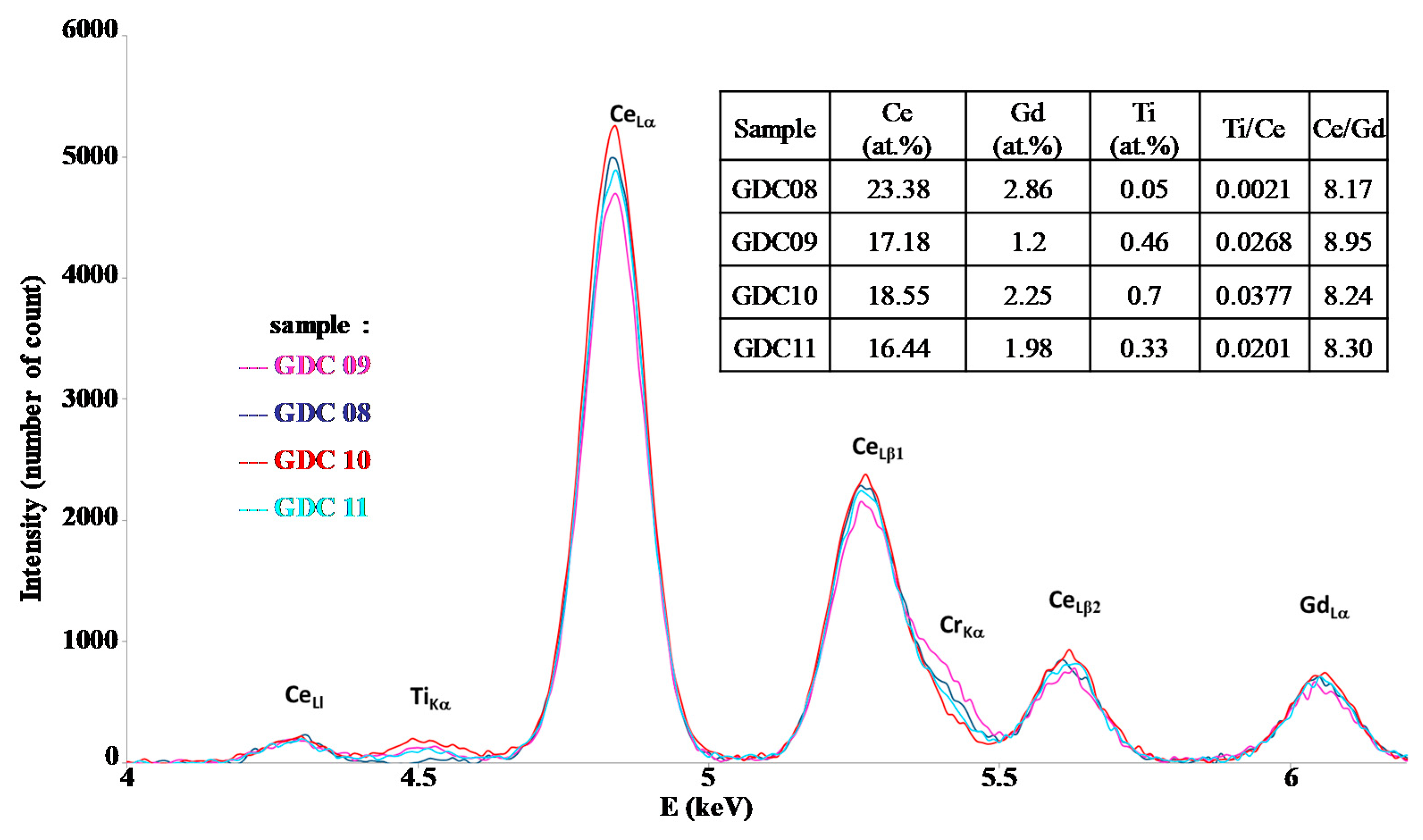



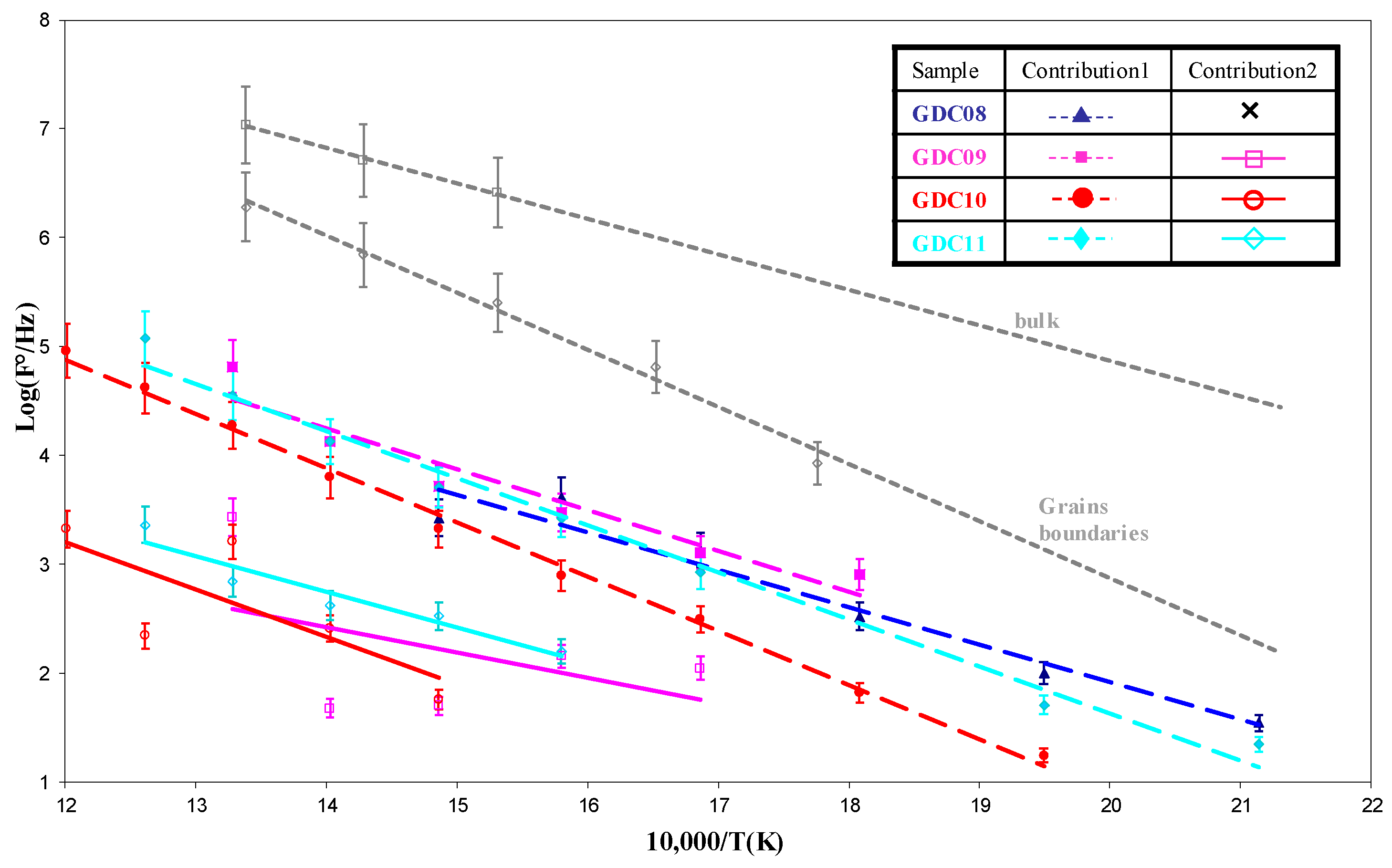
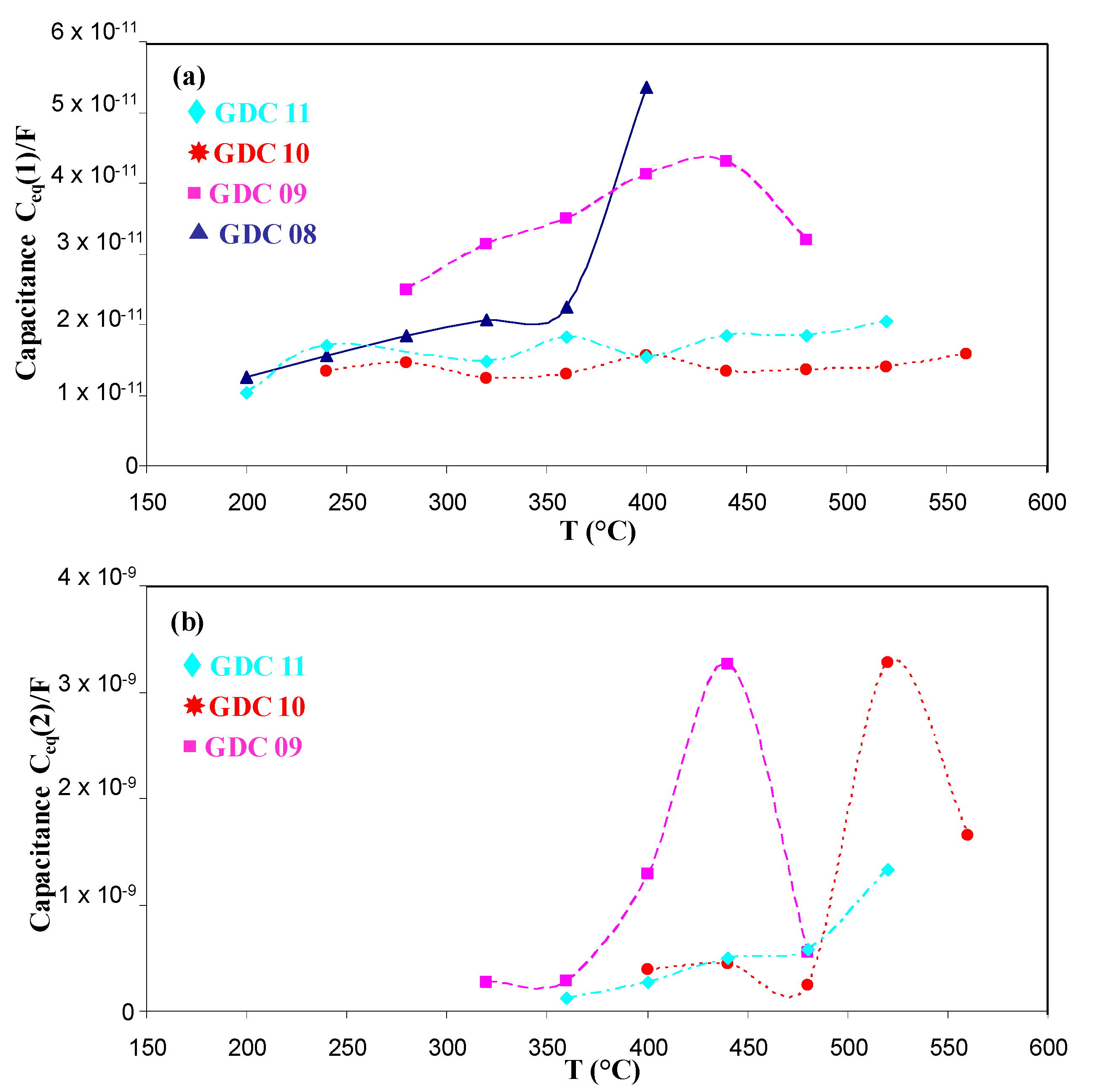
| Sample | Cleaning by Bias | Bias (V/mA) | Magnet | DAr (Sccm) | DO2 (Sccm) | Pressure (Pa) | Intensity (A) | Voltage (V) |
|---|---|---|---|---|---|---|---|---|
| GDC 08 | Yes | −50/500 | 2 | 20 | 40 | 0.6 | 60 | 38.5 |
| GDC 09 | −50/400 | 0 | 40 | 0.6 | 40 | |||
| GDC 10 | −30/700 | 1 | 30 | 0.4 | 18 | |||
| GDC 11 | −50/600 | 1 | 40 | 0.6 | 40 |
| Sample | Bragg Angle (°) | Lattice Parameter (nm) | Grain Size (nm) | Thickness (nm) |
|---|---|---|---|---|
| GDC08 | 16.62 | 0.542 | 13.1 | 700 |
| GDC09 | 16.10 | 0.559 | 5.7 | 500 |
| GDC10 | 16.57 | 0.543 | 13.7 | 740 |
| GDC11 | 16.17 | 0.556 | 10.4 | 550 |
| Previous work [33] | 16.65 | 0.541 | 38.1 | 1000 |
| GDC08 | Contribution 1 (Bulk) | |||||
| T(°C) | R (Ω) | CPE (F) | n | |||
| 200 | 3.64 × 108 | 1.65 × 10−11 | 0.95 | |||
| 240 | 1.03 × 108 | 2.89 × 10−11 | 0.9041 | |||
| 280 | 2.63 × 107 | 5.13 × 10−11 | 0.8657 | |||
| 320 | 5.72 × 106 | 8.03 × 10−11 | 0.85 | |||
| 360 | 1.73 × 106 | 1.03 × 10−10 | 0.85 | |||
| 400 | 1.12 × 106 | 3.89 × 10−10 | 0.796 | |||
| GDC09 | Contribution 1 (Bulk) | Contribution 2 (Grain Boundaries) | ||||
| T(°C) | R (Ω) | CPE (F) | n | R (Ω) | CPE (F) | n |
| 280 | 8.06 × 106 | 5.33 × 10−11 | 0.91 | Not detectable | ||
| 320 | 3.99 × 106 | 7.05 × 10−11 | 0.91 | 5.40 × 106 | 8.66 × 10−10 | 0.82 |
| 360 | 1.55 × 106 | 8.45 × 10−11 | 0.91 | 4.00 × 106 | 1.43 × 10−9 | 0.76 |
| 400 | 7.38 × 105 | 1.59 × 10−10 | 0.87 | 2.48 × 106 | 1.08 × 10−8 | 0.63 |
| 440 | 2.78 × 105 | 2.96 × 10−10 | 0.83 | 1.03 × 106 | 4.48 × 10−8 | 0.54 |
| 480 | 7.59 × 104 | 3.20 × 10−11 | 1 | 1.07 × 105 | 3.49 × 10−9 | 0.81 |
| GDC10 | Contribution 1 (Bulk) | Contribution 2 (Grain Boundaries) | ||||
| T(°C) | R (Ω) | CPE (F) | n | R (Ω) | CPE (F) | n |
| 240 | 6.80 × 108 | 1.86 × 10−11 | 0.93 | Not detectable | ||
| 280 | 1.68 × 108 | 2.65 × 10−11 | 0.9 | |||
| 320 | 4.17 × 107 | 1.79 × 10−11 | 0.95 | |||
| 360 | 1.57 × 107 | 2.55 × 10−11 | 0.92 | |||
| 400 | 4.84 × 106 | 3.34 × 10−11 | 0.92 | 7.05 × 106 | 1.62 × 10−9 | 0.76 |
| 440 | 1.90 × 106 | 1.65 × 10−11 | 0.98 | 1.40 × 106 | 1.35 × 10−9 | 0.85 |
| 480 | 6.21 × 105 | 1.36 × 10−11 | 1 | 4.19 × 105 | 3.12 × 10−10 | 0.97 |
| 520 | 2.74 × 105 | 1.40 × 10−11 | 1 | 2.20 × 105 | 1.86 × 10−8 | 0.76 |
| 560 | 1.10 × 105 | 1.58 × 10−11 | 1 | 4.60 × 104 | 5.16 × 10−9 | 0.88 |
| GDC11 | Contribution 1 (Bulk) | Contribution 2 (Grain Boundaries) | ||||
| T(°C) | R (Ω) | CPE (F) | n | R (Ω) | CPE (F) | n |
| 200 | 6.99 × 108 | 1.13 × 10−11 | 0.98 | Not detectable | ||
| 240 | 1.86 × 108 | 2.68 × 10−11 | 0.92 | |||
| 320 | 1.30 × 107 | 2.63 × 10−11 | 0.9321 | |||
| 360 | 3.34 × 106 | 3.24 × 10−11 | 0.94 | 8.08 × 106 | 3.87 × 10−10 | 0.835 |
| 400 | 2.03 × 106 | 2.32 × 10−11 | 0.96 | 1.78 × 106 | 2.68 × 10−10 | 1 |
| 440 | 6.54 × 105 | 3.23 × 10−11 | 0.95 | 7.64 × 105 | 2.40 × 10−9 | 0.8 |
| 480 | 2.48 × 105 | 1.83 × 10−11 | 1 | 4.00 × 105 | 3.07 × 10−9 | 0.8 |
| 520 | 6.59 × 104 | 2.04 × 10−11 | 1 | 5.27 × 104 | 8.97 × 10−9 | 0.8 |
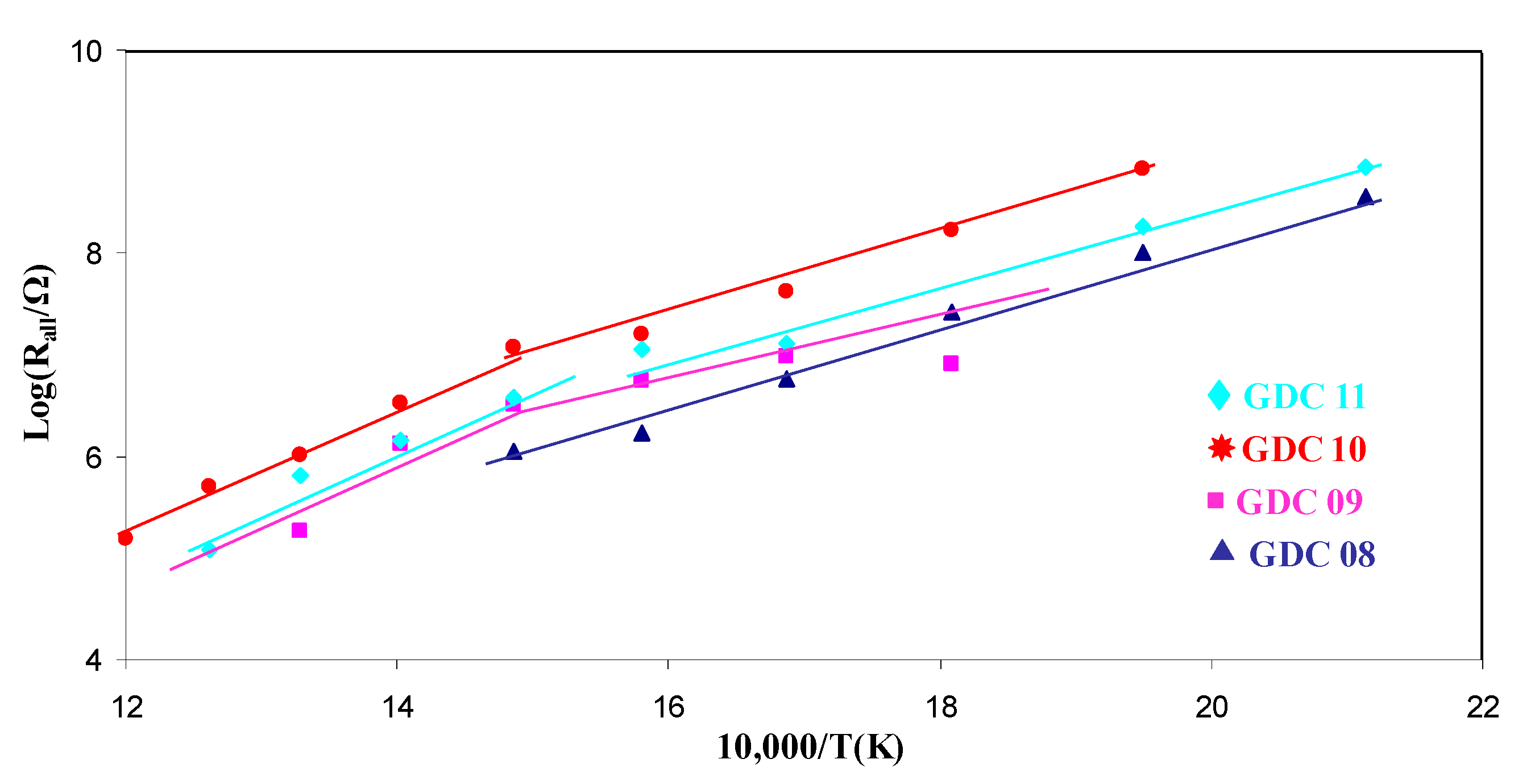
| Sample | Bulk (eV) | Grain Boundaries (eV) | Apparent (eV) |
|---|---|---|---|
| GDC 08 | 0.84 | - | 0.84 |
| GDC 09 | 0.81 | 0.84 | 0.62 |
| GDC 10 | 1.0 | 1.44 | 0.92 |
| GDC 11 | 0.91 | 1.24 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briois, P.; Aubry, E.; Ringuedé, A.; Cassir, M.; Billard, A. Feasibility Synthesis and Characterization of Gadolinia Doped Ceria Coatings Obtained by Cathodic Arc Evaporation. Nanomaterials 2021, 11, 1211. https://doi.org/10.3390/nano11051211
Briois P, Aubry E, Ringuedé A, Cassir M, Billard A. Feasibility Synthesis and Characterization of Gadolinia Doped Ceria Coatings Obtained by Cathodic Arc Evaporation. Nanomaterials. 2021; 11(5):1211. https://doi.org/10.3390/nano11051211
Chicago/Turabian StyleBriois, Pascal, Eric Aubry, Armelle Ringuedé, Michel Cassir, and Alain Billard. 2021. "Feasibility Synthesis and Characterization of Gadolinia Doped Ceria Coatings Obtained by Cathodic Arc Evaporation" Nanomaterials 11, no. 5: 1211. https://doi.org/10.3390/nano11051211
APA StyleBriois, P., Aubry, E., Ringuedé, A., Cassir, M., & Billard, A. (2021). Feasibility Synthesis and Characterization of Gadolinia Doped Ceria Coatings Obtained by Cathodic Arc Evaporation. Nanomaterials, 11(5), 1211. https://doi.org/10.3390/nano11051211






