Defect Study and Modelling of SnX3-Based Perovskite Solar Cells with SCAPS-1D
Abstract
1. Introduction
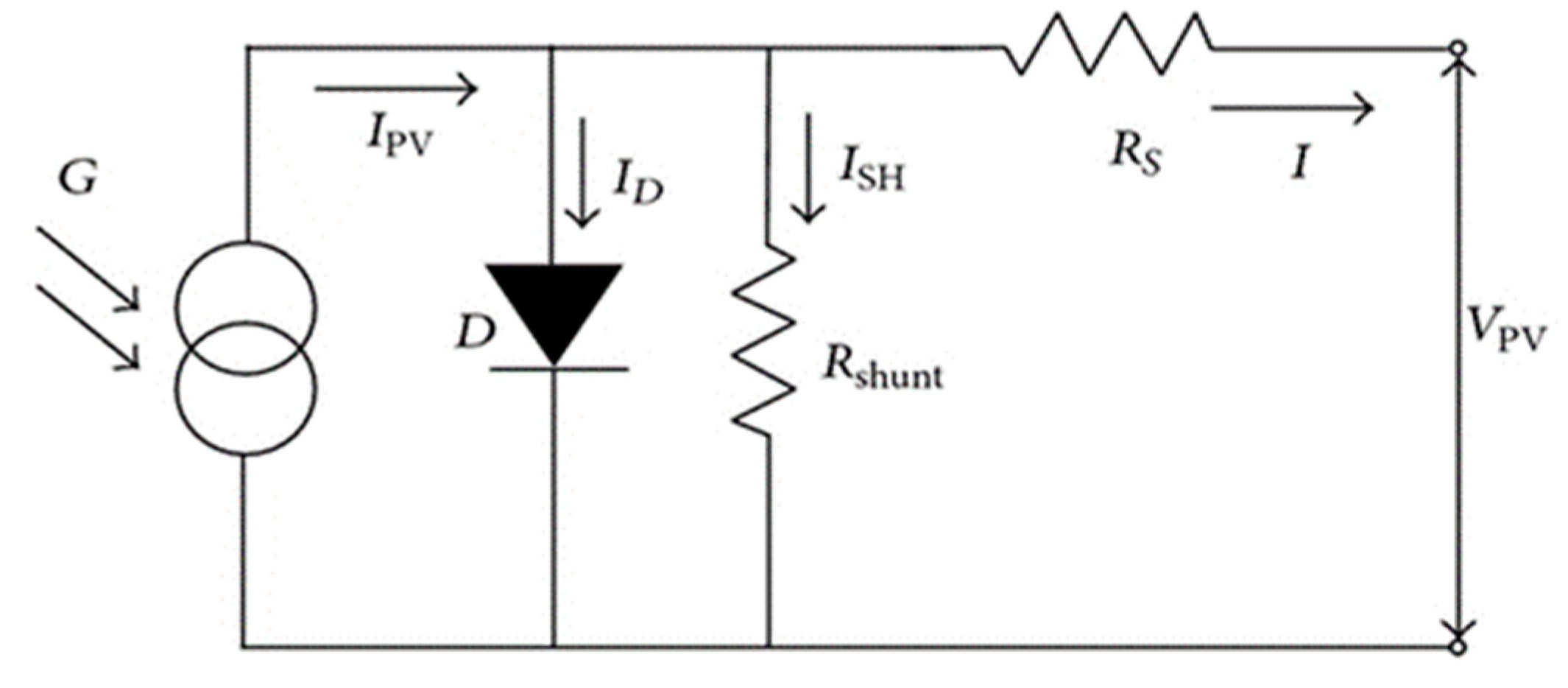
2. Theoretical Concept
3. Device Structure and Simulation Parameters
4. Results and Discussion
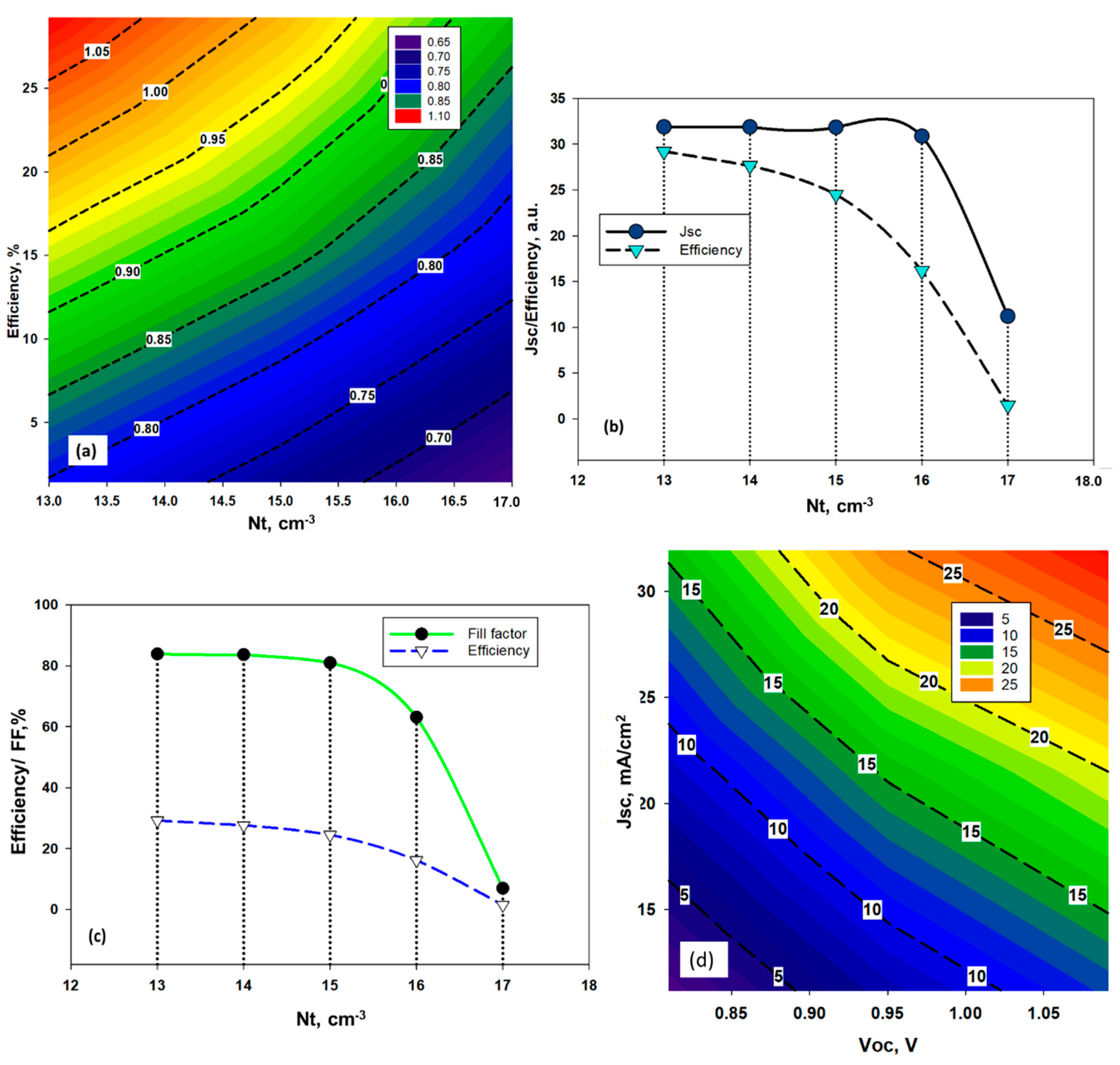
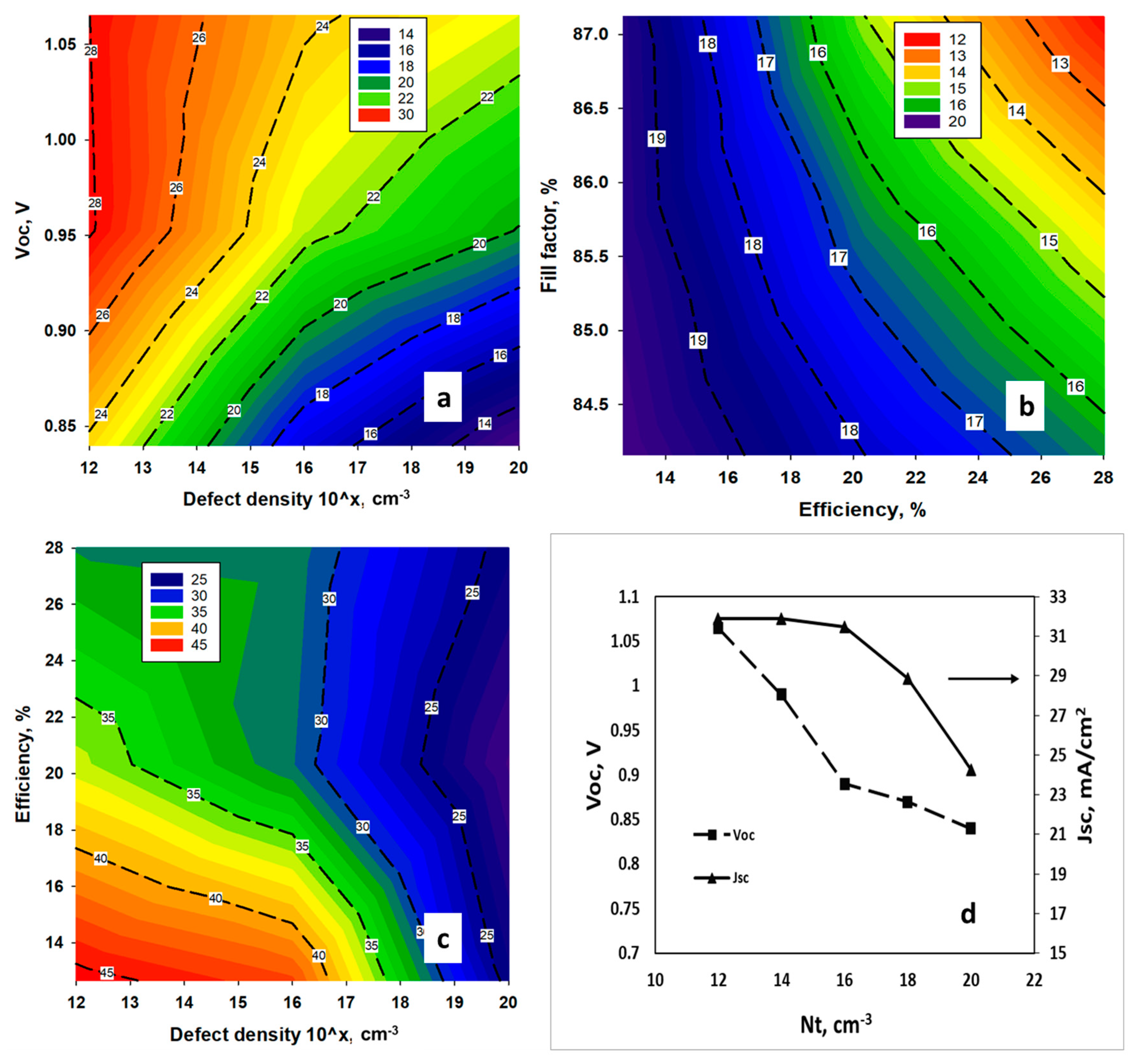
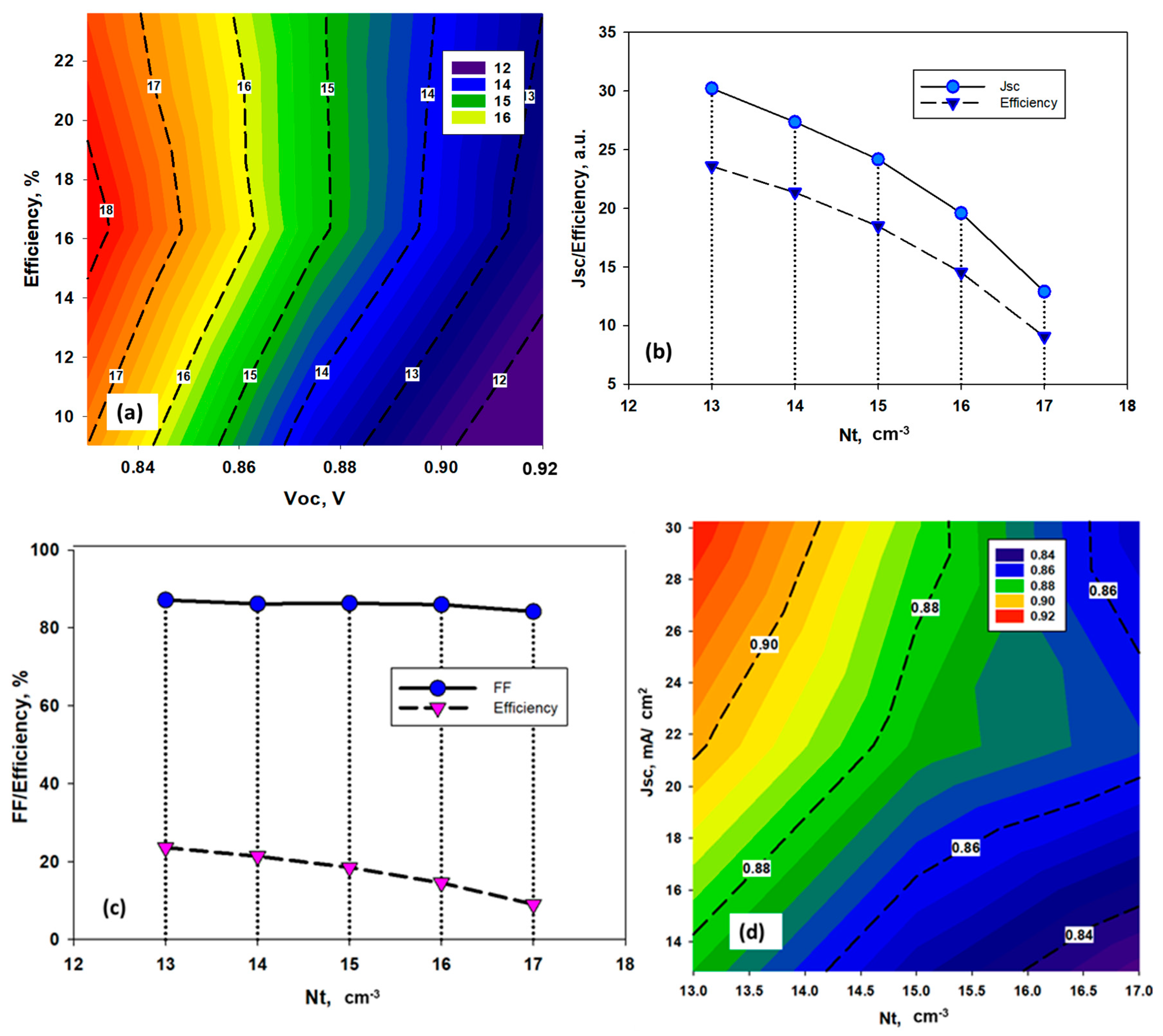
4.1. Effect of Amphoteric Defect Density in the Absorber Layer
4.2. Effect of Interface Defect States
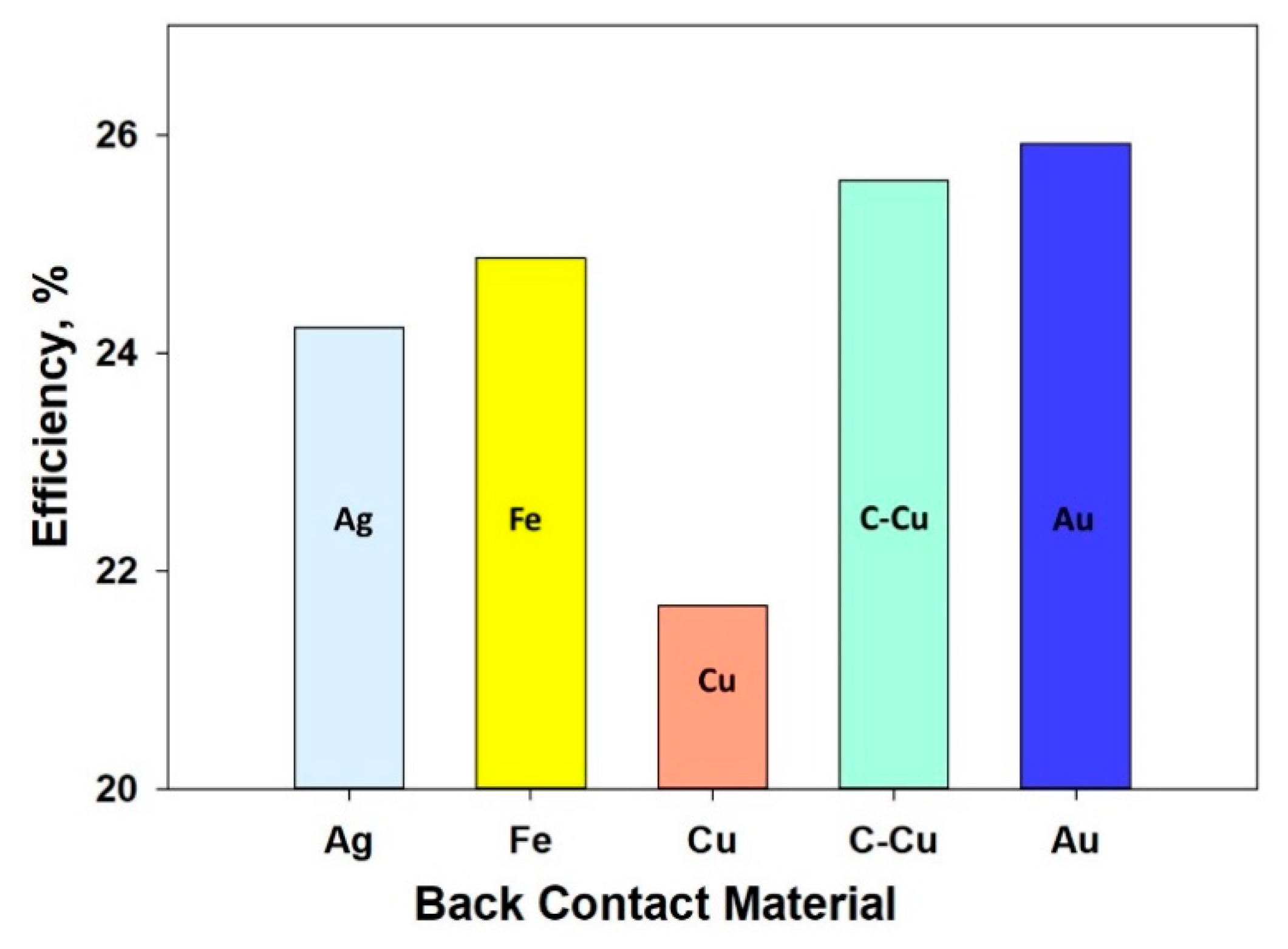
4.3. Metal Work Function
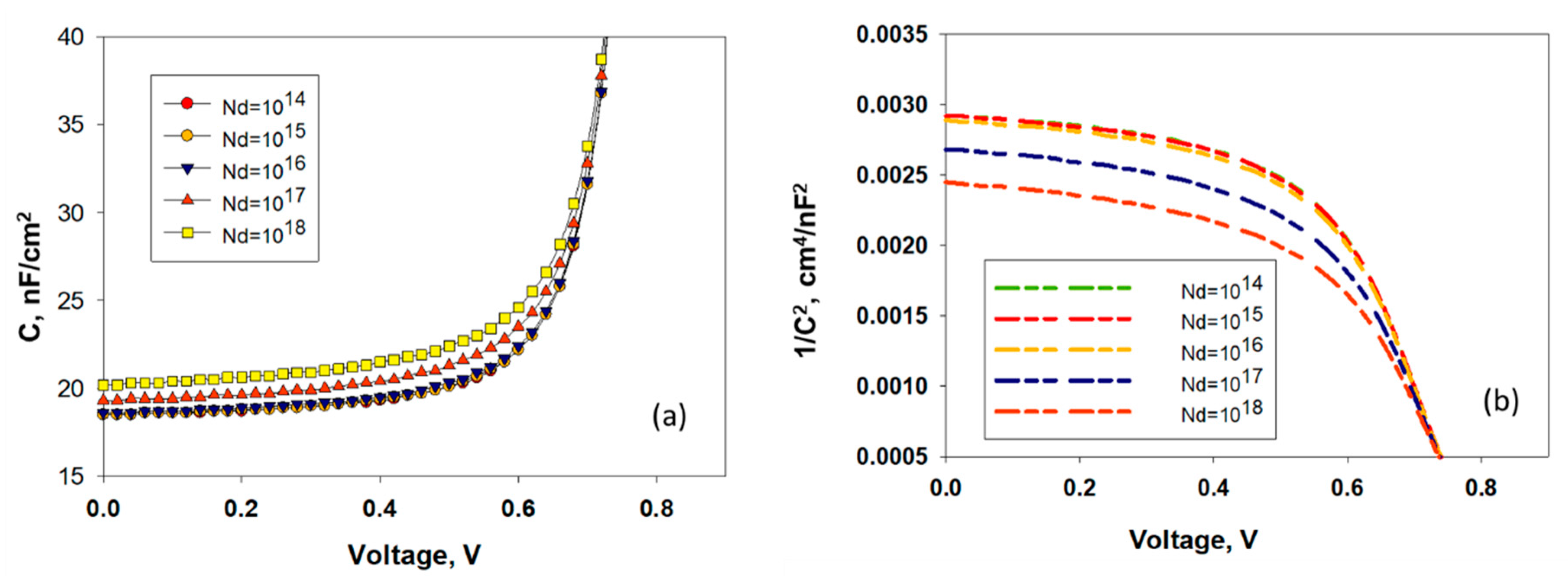
4.4. Effect of Doping Density of the TiO2/MASnBr3 Heterojunction
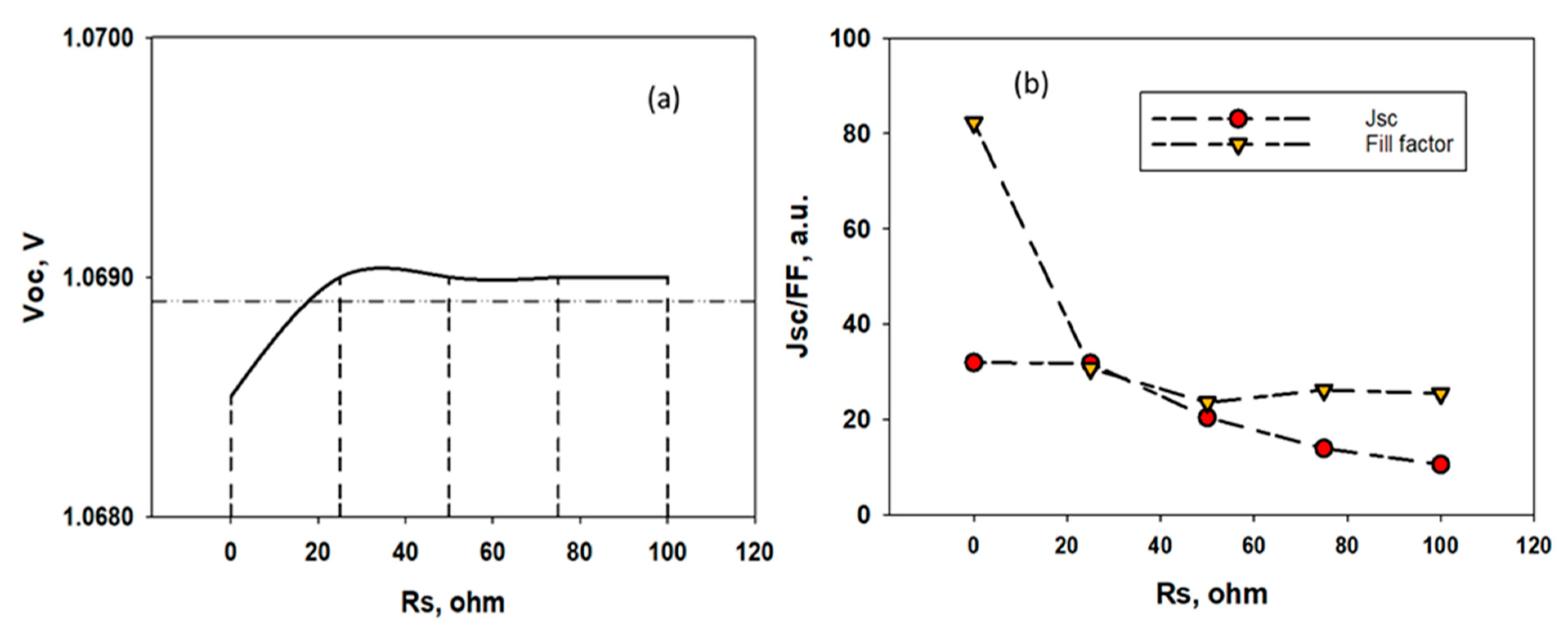
4.5. Optimized Device and Effects of Series Resistances on the Performance of the Solar Cell
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (a) | ||||
| Defect Density, 10x cm−3 | Voc, V | Jsc, mA/cm2 | FF,% | Efficiency,% |
| 13 | 1.09 | 31.89 | 83.87 | 29.2 |
| 14 | 1.04 | 31.88 | 83.64 | 27.64 |
| 15 | 0.95 | 31.85 | 80.98 | 24.5 |
| 16 | 0.83 | 30.87 | 63.1 | 16.17 |
| 17 | 1.85 | 11.2 | 6.98 | 1.46 |
| (b) | ||||
| Defect Density, 10x cm−3 | Voc, V | Jsc, mA/cm2 | FF,% | Efficiency,% |
| 12 | 1.07 | 31.89 | 87.12 | 28.01 |
| 14 | 0.99 | 31.88 | 86.15 | 26.75 |
| 16 | 0.89 | 31.49 | 86.28 | 19.75 |
| 18 | 0.87 | 28.89 | 85.95 | 15.88 |
| 20 | 0.84 | 24.26 | 84.16 | 12.66 |
| (c) | ||||
| Defect Density, 10x cm−3 | Voc, V | Jsc, mA/cm2 | FF,% | Efficiency,% |
| 13 | 0.92 | 30.23 | 87.12 | 23.6 |
| 14 | 0.9 | 27.36 | 86.15 | 21.36 |
| 15 | 0.88 | 24.18 | 86.28 | 18.49 |
| 16 | 0.86 | 19.6 | 85.95 | 14.53 |
| 17 | 0.83 | 12.89 | 84.16 | 9.04 |
Appendix B
| Back Contact Metal | Efficiency,% |
|---|---|
| Ag | 24.23 |
| Fe | 24.87 |
| Cu | 21.68 |
| Cu doped carbon | 25.58 |
| Au | 25.51 |
Appendix C
References
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Shin, S.S.; Yeom, E.J.; Yang, W.S.; Hur, S.; Kim, M.G.; Im, J.; Seo, J.; Noh, J.H.; Seok, S.I. Colloidally prepared La-doped BaSnO3 electrodes for efficient, photostable perovskite solar cells. Science 2017, 356, 167–171. [Google Scholar] [CrossRef]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide–based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic–inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Chen, B.; Yang, M.; Priya, S.; Zhu, K. Origin of J–V hysteresis in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 905–917. [Google Scholar] [CrossRef]
- Im, J.H.; Lee, C.R.; Lee, J.W.; Park, S.W.; Park, N.G. 6.5% efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 2011, 3, 4088–4093. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent annealing of perovskite-induced crystal growth for photovoltaic-device efficiency enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Abate, A.; Saliba, M.; Tress, W.; Jacobsson, T.J.; Grätzel, M.; Hagfeldt, A. The rapid evolution of highly efficient perovskite solar cells. Energy Environ. Sci. 2017, 10, 710–727. [Google Scholar] [CrossRef]
- Saliba, M.; Matsui, T.; Seo, J.Y.; Domanski, K.; Correa-Baena, J.P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A.; et al. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Ono, L.K.; Juarez-Perez, E.J.; Qi, Y. Progress on perovskite materials and solar cells with mixed cations and halide anions. ACS Appl. Mater. Interfaces 2017, 9, 30197–30246. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, Y.; Xiao, S.; Zhang, T.; Meng, X.; G, W.K.N.; Yang, Y.; Chen, H.; Wong, K.S.; Yang, S. Profiling the organic cation-dependent degradation of organolead halide perovskite solar cells. J. Mater. Chem. A 2017, 5, 1103–1111. [Google Scholar]
- Liu, X.; Yang, Z.; Chueh, C.C.; Rajagopal, A.; Williams, S.T.; Sun, Y.; Jen, A.K.Y. Improved efficiency and stability of Pb–Sn binary perovskite solar cells by Cs substitution. J. Mater. Chem. A 2016, 4, 17939. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1–x)I3 Perovskite Solar Cells Covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic-inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Mosconi, E.; Umari, P.; Angelis, F.D. Electronic and optical properties of mixed Sn–Pb organohalide perovskites: A first principles investigation. J. Mater. Chem. A 2015, 3, 9208–9215. [Google Scholar] [CrossRef]
- Im, J.; Stoumpos, C.C.; Jin, H.; Freeman, A.J.; Kanatzidis, M.G. Antagonism between Spin–Orbit Coupling and Steric Effects Causes Anomalous Band Gap Evolution in the Perovskite Photovoltaic Materials CH3NH3Sn1–xPbxI3. J. Phys. Chem. Lett. 2015, 6, 3503–3509. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Chang, R.P.H.; Kanatzidis, M.G. Anomalous Band Gap Behavior in Mixed Sn and Pb Perovskites Enables Broadening of Absorption Spectrum in Solar Cells. J. Am. Chem. Soc. 2014, 136, 8094–8099. [Google Scholar] [CrossRef]
- Zuo, F.; Williams, S.T.; Liang, P.W.; Chueh, C.C.; Liao, C.Y.; Jen, A.K.-Y. Binary-metal perovskites toward high-performance planar-heterojunction hybrid solar cells. Adv. Mater. 2014, 26, 6454–6460. [Google Scholar] [CrossRef]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Serafettin, D.; Ahmet, N.O.; Mustafa, C.; Cebrail, O.; Merve, K. Nanostructured Solar Cells: Recent Progresses in Perovskite Solar Cells; Intech Publishers: London, UK, 2017. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die gesetze der krystallochemie. Naturwissenschaften 1926, 14, 477. [Google Scholar]
- Yi, Z.; Ladi, N.H.; Shai, X.; Li, H.; Shen, Y.; Wang, M. Will organic–inorganic hybrid halide lead perovskites be eliminated from optoelectronic applications? Nanoscale Adv. 2019, 1, 1276. [Google Scholar] [CrossRef]
- Kieslich, G.; Sun, S.; Cheetham, A.K. Solid-state principles applied to organic–inorganic perovskites: New tricks for an old dog. Chem Sci. 2014, 5, 4712. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater 2016, 28, 284. [Google Scholar] [CrossRef]
- Wang, Q.; Phung, N.; Girolamo, D.D.; Vivoc, P.; Abate, A. Enhancement in lifespan of halide perovskite solar cells. Energy Environ. Sci. 2019, 12, 865. [Google Scholar]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater 2015, 5, 1501310. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hörantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Arya, S.; Verma, S.; Gupta, J.; Bandhoria, P.; Bharti, V.; Datt, R.; Gupta, V. Potential substitutes for replacement of lead in perovskite solar cells: A review. Glob. Chall. 2019, 3, 1900050. [Google Scholar] [CrossRef] [PubMed]
- Burgelman, M.; Nollet, P.; Degrave, S. Modelling polycrystalline semiconductor solar cells. Thin Solid Film. 2000, 361, 527–532. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, J.; Wei, J.; Li, Y.; Lv, M.; Yang, S.; Zhang, B.; Yao, J.; Dai, S. Numerical simulation: Toward the design of high-efficiency planar perovskite solar cells. Appl. Phys. Lett. 2014, 104, 253508. [Google Scholar] [CrossRef]
- Lin, P.; Tan, K.; Tan, J.; Wu, L.; Wang, G.; Jin, S.; Lin, Y. Device simulation of solid-state perovskite solar cells. In Proceedings of the 31st European Photovoltaic Solar Energy Conference and Exhibition, Hamburg, Germany, 14–18 September 2015. [Google Scholar]
- Baktash, A.; Amiri, O.; Sasani, A. Improve efficiency of perovskite solar cells by using magnesium doped ZnO and TiO2 compact layers. Superlattices Microstruct. 2016, 93, 128–137. [Google Scholar] [CrossRef]
- Sobayel, K.; Akhtaruzzaman, M.; Rahman, K.S.; Ferdaous, M.T.; Al-Mutairi, Z.A.; Alharbi, H.F.; Alharthi, N.H.; Karim, M.R.; Hasmady, S.; Amin, N. A comprehensive defect study of tungsten disulfide (WS2) as electron transport layer in perovskite solar cells by numerical simulation. Results Phys. 2019, 12, 1097–1103. [Google Scholar] [CrossRef]
- Sobayel, K.; Shahinuzzaman, M.; Amin, N.; Karim, M.R.; Dar, M.A.; Gul, R.; Alghoul, M.A.; Sopian, K.; Hasan, A.K.M.; Akhtaruzzaman, M. Efficiency enhancement of CIGS solar cell by WS2 as window layer through numerical modelling tool. Sol. Energy 2020, 207, 479–485. [Google Scholar] [CrossRef]
- Mahjabin, S.; Haque, M.M.; Sobayel, K.; Jamal, M.S.; Islam, M.A.; Selvanathan, V.; Abdulaziz, A.K.; Alharbi, H.F.; Sopian, K.; Amin, N.; et al. Perceiving of Defect Tolerance in Perovskite Absorber Layer for Efficient Perovskite Solar Cell. IEEE Access 2020, 8, 106346–106353. [Google Scholar] [CrossRef]
- Sobayel, K.; Rahman, K.S.; Karim, M.R.; Aijaz, M.O.; Dar, M.A.; Shar, M.A.; Misran, H.; Amin, N. Numerical modelling on prospective buffer layers for tungsten di-sulfide (WS2) solar cells by scaps-1d. Chalcogenide Lett. 2018, 15, 307–315. [Google Scholar]
- Kang, A.K.; Zandi, M.H.; Gorji, N.E. Simulation analysis of graphene contacted perovskite solar cells using SCAPS-1D. Opt. Quantum Electron. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Frazer, L.; Clark, D.J.; Kim, Y.S.; Rhim, S.H.; Freeman, A.J.; Ketterson, J.B.; Jang, J.I.; Kanatzidis, M.G. Hybrid Germanium Iodide Perovskite Semiconductors: Active Lone Pairs, Structural Distortions, Direct and Indirect Energy Gaps, and Strong Nonlinear Optical Properties. J. Am. Chem. Soc. 2015, 137, 6804. [Google Scholar] [CrossRef]
- Alberi, K.; Scarpulla, M.A. Suppression of compensating native defect formation during semiconductor processing via excess carriers. Sci. Rep. 2016, 6, 27954. [Google Scholar] [CrossRef]
- Flynn, C.; McCullough, S.; Li, L.; Donley, C.; Kanai, Y.; Cahoon, J. Passivation of nickel vacancy defects in nickel oxide solar cells by targeted atomic deposition of boron. J. Phys. Chem. C 2016, 120, 16568–16576. [Google Scholar] [CrossRef]
- Zhu, Z.B.; Liu, Z.; Long, X.; Wei, Z.; Wang, Z.; Zhang, L.; Wang, J.; Yan, F.; Yang, S. High-permance hole-extraction layer of sol–gel-processed NiO nanocrystals for inverted planar per ovskite solar cells. Angew. Chem. 2014, 126, 12779–12783. [Google Scholar] [CrossRef]
- Tang, L.; Chen, X.; Wen, T.; Yang, S.; Zhao, J.; Qiao, H.; Hou, Y.; Yang, G. A solution-processed transparent NiO hole-extraction layer for high-performance inverted perovskite solacells. Chem. Eur. J. 2018, 24, 2845–2849. [Google Scholar] [CrossRef]
- Sun, J.; Lu, J.; Li, B.; Jiang, L.; Chesman, A.; Scully, A.; Gengenbach, T.; Cheng, Y.; Jasieniak, J. Inverted perovskitesolar cells with high fill-factors featuring chemical bath deposited mesoporous NiOhole transporting layers. Nano Energy 2018, 49, 163–171. [Google Scholar] [CrossRef]
- Seo, S.; Park, I.; Kim, M.; Lee, S.; Bae, C.; Jung, H.; Park, N.-G.; Kim, J.; Shin, H. An ultra-thin, un-doped NiO hole transporting layer of highly efficient (16.4%) organic–inorganic hybridperovskite solar cells. Nanoscale 2016, 8, 11403–11412. [Google Scholar] [CrossRef]
- Anwar, F.; Sarwar Satter, S.; Mahbub, R.; Mahmud Ullah S and Afrin, S. Simulation and performance study of nanowire CdS/CdTe solar cell. Int. J. Renew. Energy Res. 2017, 7, 885–893. [Google Scholar]
- Derry, G.N.; Kern M E and Worth, E.H. Recommended values of clean metal surface work functions. J. Vac. Sci. & Technol. A Vac. Surf. Films 2015, 33, 060801. [Google Scholar]
- Thahab, S.M.; Hassan, H.A.; Hassan, Z. Effects of metal work function and operating temperatures on the electrical properties of contacts to n-type GaN. In Proceedings of the 2006 IEEE International Conference on Semiconductor Electronics, kuala Lumpur, Malaysia, 29 November–1 December 2006; pp. 816–819. [Google Scholar]
- Malliaras, G.G.; Salem, J.R.; Brock, P.J.; Scott, C. Electrical characteristics and efficiency of single-layer organic light-emitting diodes. Phys. Rev. B 1998, 58, R13411. [Google Scholar] [CrossRef]
- Sze, S.; Kwok, N. Physics of Semiconductor Devices. In Wiley Inter Science, 3rd ed.; 2007; ISBN 0471143235. Available online: https://www.wiley.com/en-us/Physics+of+Semiconductor+Devices%2C+3rd+Edition-p-9780470068328 (accessed on 6 March 2021).
- Garcia-Belmonte, G.; Munar, A.; Barea, E.; Bisquert, J.; Ugarte, I.; Pacios, R. Charge carrier mobility and lifetime of organic bulk heterojunctions analyzed by impedance spectroscopy. Org. Electron. 2008, 9, 847. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Munar, A.; Sessolo, M.; Soriano, A.; Bolink, H.J. Band unpinning and photovoltaic model for P3HT: PCBM organic bulk heterojunctions under illumination. Chem. Phys. Lett. 2008, 465, 57. [Google Scholar] [CrossRef]
- Nolasco, J.C.; Sanchez-Diaz, A.; Cabre, R.; Ferre-Borrull, J.; Marsal, L.F.; Palomares, E.; Pallares, J. Relation between the barrier interface and the built-in potential in pentacene/C60 solar cell. J. Appl. Phys. Lett. 2010, 97, 013305. [Google Scholar] [CrossRef]
- Ray, D.; Burtone, L.; Leo, K.; Riede, M. Detection of trap charge in small molecular organic bulk heterojunction solar cells. Phys. Rev. B 2010, 82, 125204. [Google Scholar] [CrossRef]
- Boix, P.P.; Garcia-Belmonte, G.; Munecas, U.; Neophytou, M.; Waldauf, C.; Pacios, R. Determination of gap defect states in organic bulk heterojunction solar cells from capacitance measurements. Appl. Phys. Lett. 2009, 95, 233302. [Google Scholar] [CrossRef]
- Mingebach, M.; Deibel, C.; Dyakonov, V. Built-in potential and validity of the Mott-Schottky analysis in organic bulk heterojunction solar cells. Phys. Rev. B 2011, 84, 153201. [Google Scholar] [CrossRef]
- Hosenuzzaman, M.; Rahim, N.A.; Selvaraj, J.; Hasanuzzaman, M.; Malek, A.A.; Nahar, A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation Renew. Sustain. Energy Rev. 2011, 41, 284–297. [Google Scholar] [CrossRef]
- Decock, K.; Khelifi, S.; Burgelman, M. Modelling multivalent defects in thin film solar cells. Thin Solid Films 2011, 519, 7481–7484. [Google Scholar] [CrossRef]
- Zonno, I.; Martinez-Otero, A.; Hebig, J.C.; Kirchartz, T. Understanding mott-Schottky measurements under illumination in organic bulk heterojunction solar cells. Phys. Rev. Appl. 2017, 7, 034018. [Google Scholar] [CrossRef]
- Niemegeers, A.; Burgelman, M. Numerical Modeling of AC-Characteristics of CdTe and CIS Solar Cells. In Proceedings of the Twenty Fifth IEEE Photovoltaic Specialists Conference, Washington, DC, USA, 13–17 May 1996; pp. 901–904. [Google Scholar]
- Kumari, S.; Tripathi, C.; Singh, A.P.; Chauhan, D.; Shrivastav, R.; Dass, S.; Satsangi, V.R. Characterization of Zn-doped hematite thin films for photoelectrochemical splitting of water. Curr. Sci. 2006, 91, 1062–1064. [Google Scholar]
- Abhishiktha, T. Effect of Series Resistance Increase on Fill Factor of PV Cells Extracted from Field Aged Modules of Different Climates; Arizona State University Library, Digital Repository: Tempe, AZ, USA, 2016. [Google Scholar]
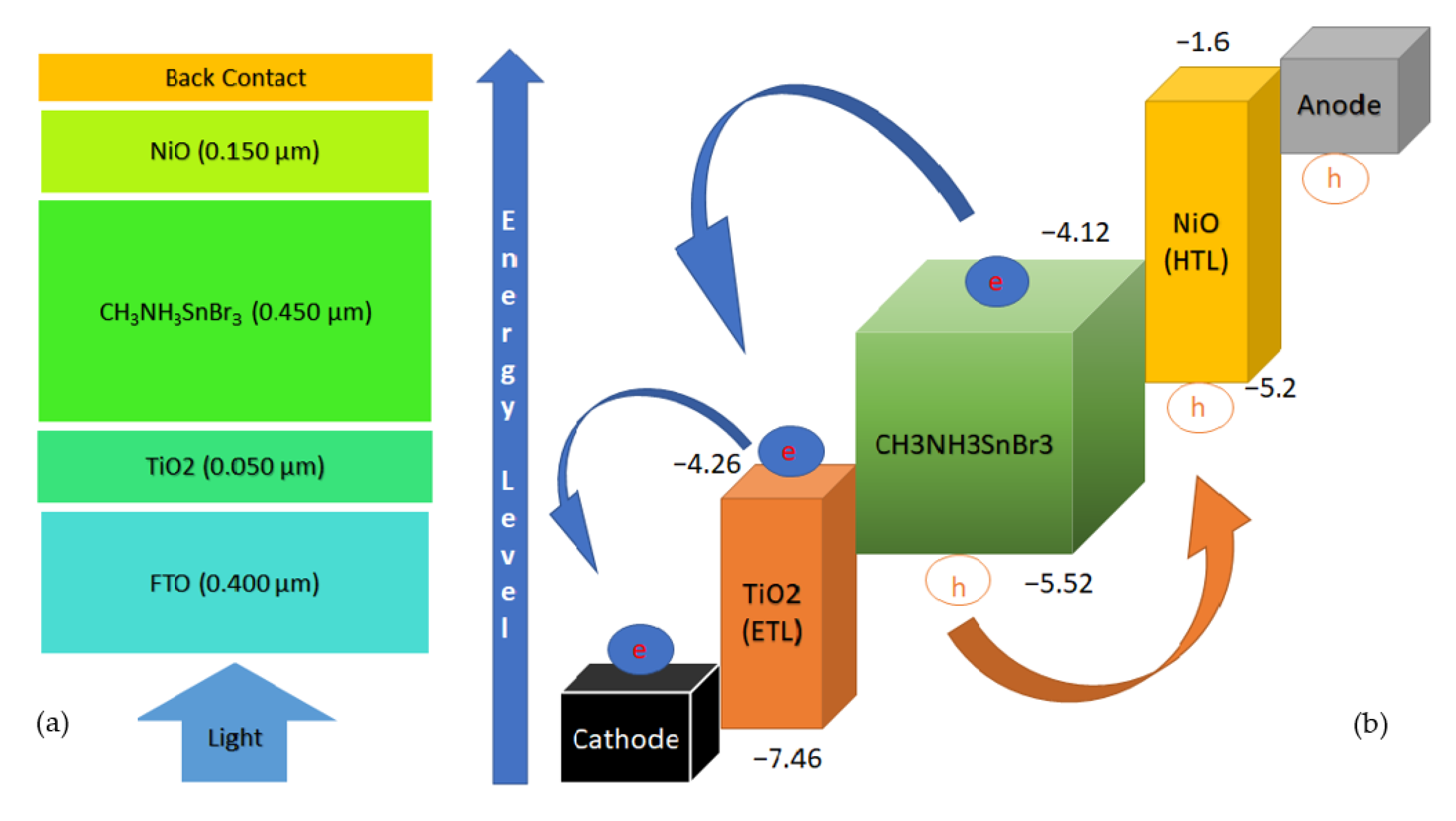

| Parameter | FTO [35,36] | TiO2 [35,37] | CH3NH3SnBr3 [30,37] | NiO [34,37] |
|---|---|---|---|---|
| Thickness (µm) | 0.4 | 0.05 | 0.5 | 0.15 |
| Eg (eV) | 3.5 | 3.26 | 1.3 | 3.6 |
| χ (eV) | 4.0 | 4.2 | 4.17 | 1.8 |
| ε | 9.0 | 10.0 | 10.0 | 11.7 |
| NC (cm−3) | 2.2 × 1018 | 2.2 × 1018 | 2.2 × 1018 | 2.5 × 1020 |
| Nv (cm−3) | 1.8 × 1018 | 1.8 × 1018 | 1.8 × 1018 | 2.5 ×1020 |
| μn (cm2/Vs) | 20 | 20 | 1.6 | 2.8 |
| μp (cm2/Vs) | 10 | 10 | 1.6 | 2.8 |
| ND (cm−3) | 1 × 1019 | 1 × 1017 | 1 × 1013 | 0 |
| NA (cm−3) | 0 | 0 | 1 × 1013 | 3 × 1018 |
| Back Contact Metal | Au | Ag | Fe | Cu | Cu Doped C |
|---|---|---|---|---|---|
| Metal work function (eV) | 5.1 | 4.7 | 4.8 | 4.6 | 5.0 |
| Resistance, Ohm.cm2 | Voc, V | Jsc, mA/cm2 | FF, % | Efficiency, % |
|---|---|---|---|---|
| 0 | 0.814 | 31.88 | 84.89 | 21.66 |
| 1 | 0.814 | 31.88 | 81.21 | 20.72 |
| 2 | 0.814 | 31.88 | 77.67 | 19.8 |
| 3 | 0.814 | 31.87 | 74.03 | 18.88 |
| 4 | 0.814 | 31.80 | 70.43 | 17.86 |
| 5 | 0.814 | 31.78 | 66.88 | 17.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samiul Islam, M.; Sobayel, K.; Al-Kahtani, A.; Islam, M.A.; Muhammad, G.; Amin, N.; Shahiduzzaman, M.; Akhtaruzzaman, M. Defect Study and Modelling of SnX3-Based Perovskite Solar Cells with SCAPS-1D. Nanomaterials 2021, 11, 1218. https://doi.org/10.3390/nano11051218
Samiul Islam M, Sobayel K, Al-Kahtani A, Islam MA, Muhammad G, Amin N, Shahiduzzaman M, Akhtaruzzaman M. Defect Study and Modelling of SnX3-Based Perovskite Solar Cells with SCAPS-1D. Nanomaterials. 2021; 11(5):1218. https://doi.org/10.3390/nano11051218
Chicago/Turabian StyleSamiul Islam, Md., K. Sobayel, Ammar Al-Kahtani, M. A. Islam, Ghulam Muhammad, N. Amin, Md. Shahiduzzaman, and Md. Akhtaruzzaman. 2021. "Defect Study and Modelling of SnX3-Based Perovskite Solar Cells with SCAPS-1D" Nanomaterials 11, no. 5: 1218. https://doi.org/10.3390/nano11051218
APA StyleSamiul Islam, M., Sobayel, K., Al-Kahtani, A., Islam, M. A., Muhammad, G., Amin, N., Shahiduzzaman, M., & Akhtaruzzaman, M. (2021). Defect Study and Modelling of SnX3-Based Perovskite Solar Cells with SCAPS-1D. Nanomaterials, 11(5), 1218. https://doi.org/10.3390/nano11051218










