Enhanced Light Absorption by Facile Patterning of Nano-Grating on Mesoporous TiO2 Photoelectrode for Cesium Lead Halide Perovskite Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used and Nanopatterned PDMS Mold Preparation
2.2. Device Fabrication
2.3. Characterization and Analysis
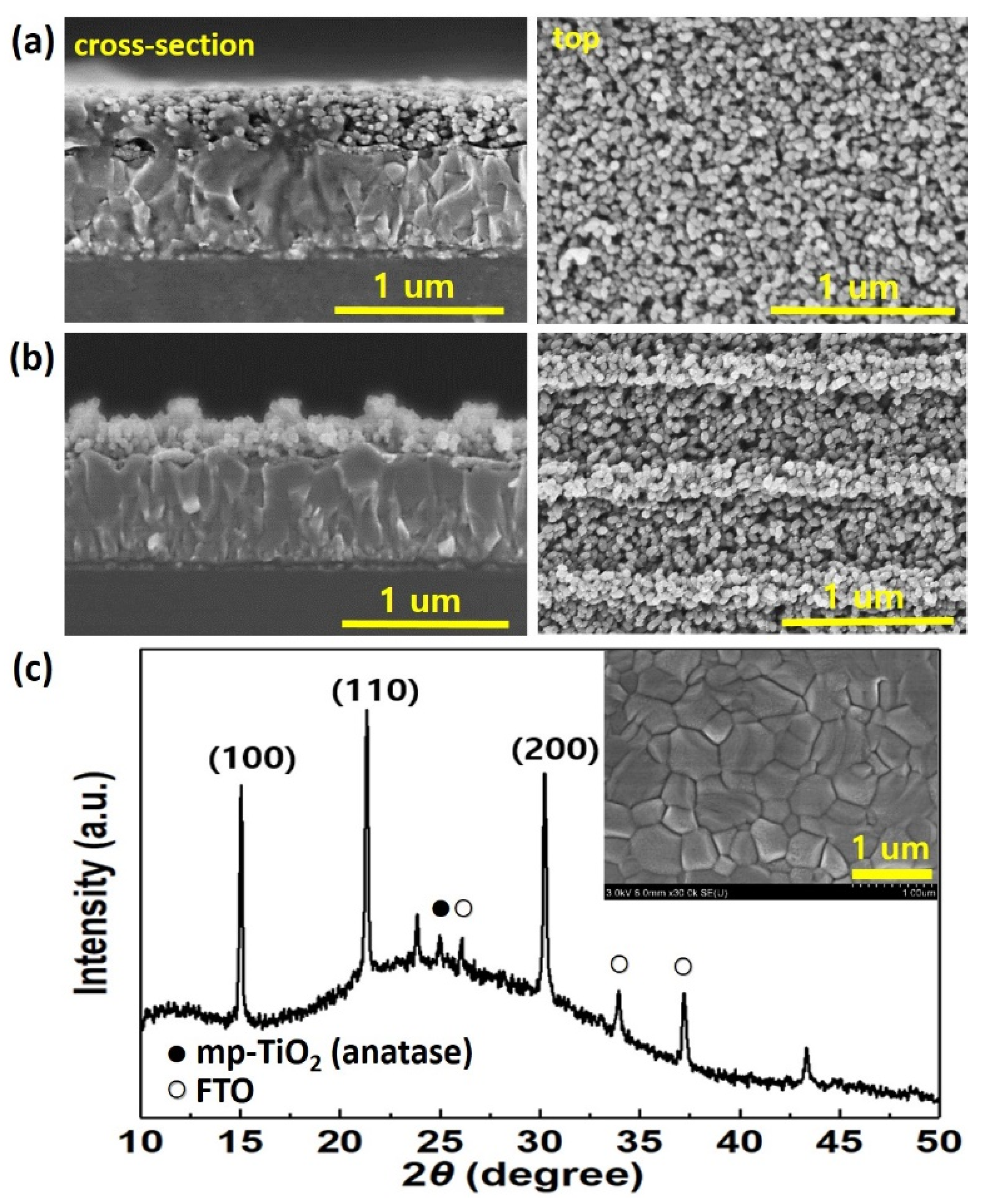
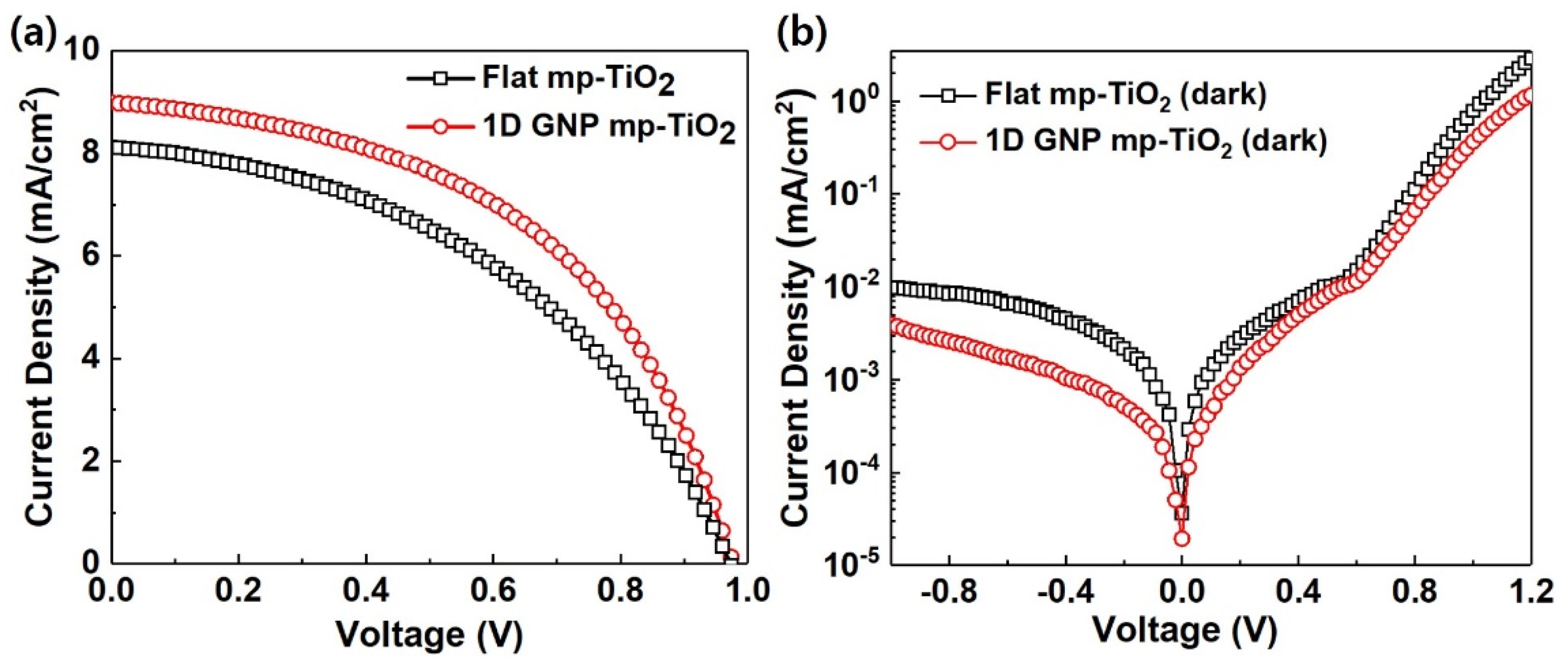
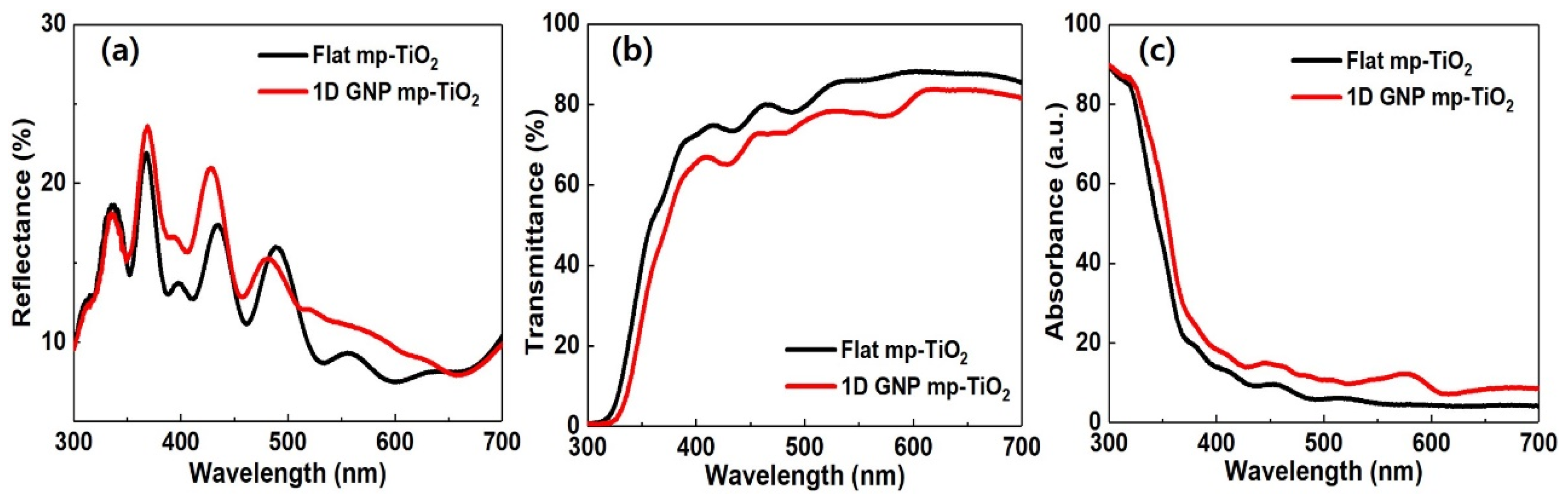
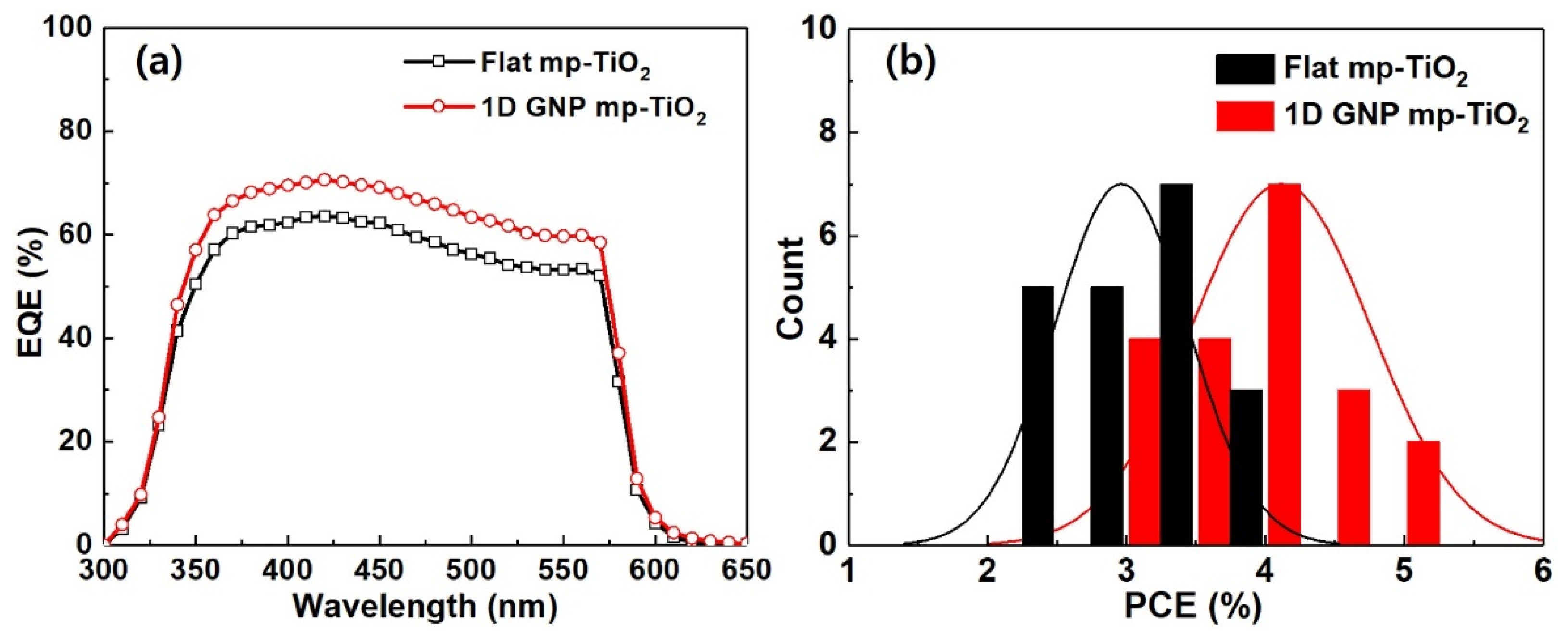
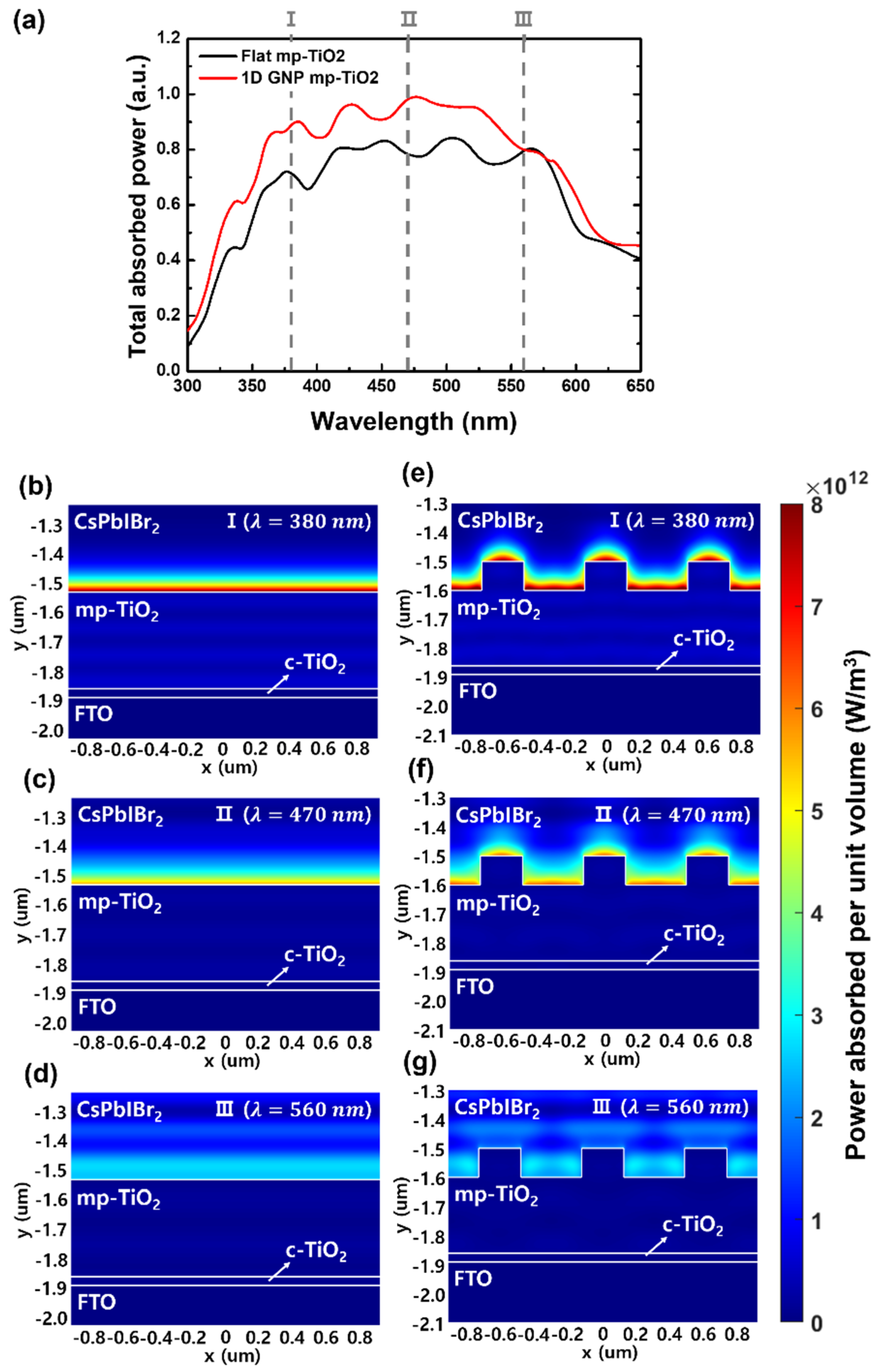
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, N.K. Research direction toward scalable, stable, and high efficiency perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903106. [Google Scholar] [CrossRef]
- Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 4 January 2021).
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 17042. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.K.; Xue, J.; Yang, Y. A Review of Perovskites Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
- Chan, S.H.; Wu, M.C.; Lee, K.M.; Chen, W.C.; Lin, T.H.; Su, W.F. Enhancing perovskite solar cell performance and stability by doping barium in methylammonium lead halide. J. Mater. Chem. A 2017, 5, 18044–18052. [Google Scholar] [CrossRef]
- Chen, J.; Park, N.G. Materials and methods for interface engineering toward stable and efficient perovskite solar cells. ACS Energy Lett. 2020, 5, 2742–2786. [Google Scholar] [CrossRef]
- Cheacharoen, R.; Rolston, N.; Harwood, D.; Bush, K.A.; Dauskardt, R.H.; McGehee, M.D. Design and understanding of encapsulated perovskite solar cells to withstand temperature cycling. Energy Environ. Sci. 2018, 11, 144–150. [Google Scholar] [CrossRef]
- Duan, J.; Xu, H.; Sha, W.E.I.; Zhao, Y.; Wang, Y.; Yang, X.; Tang, Q. Inorganic perovskite solar cells: An emerging member of the photovoltaic community. J. Mater. Chem. A 2019, 7, 21036–21068. [Google Scholar] [CrossRef]
- Ouedraogo, N.A.N.; Chen, Y.; Xiao, Y.Y.; Meng, Q.; Han, C.B.; Yan, H.; Zhang, Y. Stability of all inorganic perovskite solar cells. Nano Energy 2020, 67, 104249. [Google Scholar] [CrossRef]
- Xiang, W.; Tress, W. Review on recent progress of all-inorganic metal halide perovskites and solar cells. Adv. Mater. 2019, 31, 1902851. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Hu, T.; Zhao, Y.; He, B.; Tang, Q. Carbon-electrode-tailored all-Inorganic perovskite solar cells to harvest solar and water-vapor energy. Angew. Chem. Int. Ed. 2018, 57, 5746–5749. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Kan, M.; Zhao, Y. Bifunctional stabilization of all-inorganic alpha-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 2018, 140, 12345–12348. [Google Scholar] [CrossRef]
- Straus, D.B.; Guo, S.; Abeykoon, A.M.M.; Cava, R.J. Understanding the instability of the halide perovskite CsPbI3 through temperature-dependent structural analysis. Adv. Mater. 2020, 32, 2001069. [Google Scholar] [CrossRef]
- Liang, J.; Wang, C.; Wang, Y.; Xu, Z.; Lu, Z.; Ma, Y.; Zhu, H.; Hu, Y.; Xiao, C.; Yi, X.; et al. All-inorganic perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 15829–15832. [Google Scholar] [CrossRef]
- Mariotti, S.; Hutter, O.S.; Phillips, L.J.; Yates, P.J.; Kundu, B.; Durose, K. Stability and performance of CsPbI2Br thin films and solar cell devices. ACS Appl. Mater. Interfaces 2018, 10, 3750–3760. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhang, X.; Liu, C.; Feng, T.; Chen, Z.; Zhang, W.; Zheng, W.; Zhang, H.; Yang, B. Inorganic CsPbI2Br perovskite solar cells: The progress and perspective. Sol. RRL 2019, 3, 1800239. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yin, X.; Liu, J.; Wen, S.; Wu, Y.; Que, W. Inorganic CsPbIBr2-based perovskite solar cells: Fabrication technique modification and efficiency improvement. Sol. RRL 2019, 3, 1900135. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Chen, J.; Fan, J.; Mai, Y.; Schropp, R.E.I. Ultra-thin MoOx as cathode buffer layer for the improvement of all-inorganic CsPbIBr2 perovskite solar cells. Nano Energy 2017, 41, 75. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Q.; Chen, D.; Zhang, Z.; Lin, Z.; Chang, J.; Zhang, J.; Zhang, C.; Hao, Y. Intermolecular exchange boosts efficiency of air-stable, carbon-based all-inorganic planar CsPbIBr2 perovskite solar cells to over 9%. Adv. Energy Mater. 2018, 8, 1802080. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, X.; Liu, J.; Que, W. Highly efficient CsPbIBr2 perovskite solar cells with efficiency over 9.8% fabricated using a preheating-assisted spin-coating method. J. Mater. Chem. A 2019, 7, 19008–19016. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Wang, X.; Liu, S. Interface-modification-induced gradient energy band for highly efficient CsPbIBr2 perovskite solar cells. Adv. Energy Mater. 2019, 9, 1803785. [Google Scholar] [CrossRef]
- Liang, J.; Liu, Z.; Qiu, L.; Hawash, Z.; Meng, L.; Wu, Z.; Jiang, Y.; Ono, L.K.; Qi, Y. Enhancing optical, electronic, crystalline, and morphological properties of cesium lead halide by Mn substitution for high-stability all-inorganic perovskite solar cells with carbon electrodes. Adv. Energy Mater. 2018, 8, 1800504. [Google Scholar] [CrossRef]
- Cui, J.; Chen, C.; Han, J.; Cao, K.; Zhang, W.; Shen, Y.; Wang, M. Surface plasmon resonance effect in inverted perovskite solar cells. Adv. Sci. 2016, 3, 1500312. [Google Scholar] [CrossRef]
- Tavakoli, M.M.; Tsui, K.H.; Zhang, Q.; He, J.; Yao, Y.; Li, D.; Fan, Z. Highly efficient flexible perovskite solar cells with antireflection and self-cleaning nanostructures. ACS Nano 2015, 9, 10287. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Deng, X.; Zhang, C.; Yu, M.; Zhou, X.; Wang, Z.; Chen, Z.; Huang, S. Enhancing photovoltaic performance of perovskite solar cells with silica nanosphere antireflection coatings. Sol. Energy 2018, 169, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, P.; Zhou, X.; Li, C.; Li, H.; Hu, X.; Li, F.; Liu, X.; Li, M.; Song, Y. Diffraction-grated perovskite induced highly efficient solar cells through nanophotonic light trapping. Adv. Energy Mater. 2018, 8, 1702960. [Google Scholar] [CrossRef]
- Deng, K.; Liu, Z.; Wang, M.; Li, L. Nanoimprinted grating-embedded perovskite solar cells with improved light management. Adv. Funct. Mater. 2019, 29, 1900830. [Google Scholar] [CrossRef]
- Subhani, W.S.; Wang, K.; Du, M.; Liu, S.F. Goldschmidt-rule-deviated perovskite CsPbIBr2 by barium substitution for efficient solar cells. Nano Energy 2019, 61, 165–172. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Gao, L.; Cao, J.; Han, Q.; Yu, F.; Kamata, Y.; Zhang, C.; Fan, M.; Wei, G.; et al. Excellent moisture stability and efficiency of inverted all-inorganic CsPbIBr2 perovskite solar cells through molecule interface engineering. ACS Appl. Mater. Interfaces 2020, 12, 13931–13940. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Qin, C.; Zhang, T.; Gu, Y.; Chen, H.; Zheng, H.; Li, S. Solution-processed inorganic perovskite flexible photodetectors with high performance. Nanoscale Res. Lett. 2019, 14, 284. [Google Scholar] [CrossRef]
- Bai, Y.; Mora-Seró, I.; Angelis, F.D.; Bisquert, J.; Wang, W. Titanium dioxide nanomaterials for photovoltaic applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef] [PubMed]
- Shahiduzzaman, M.; Visal, S.; Kuniyoshi, M.; Kaneko, T.; Umezu, S.; Katsumata, T.; Iwamori, S.; Kakihana, M.; Taima, T.; Isomura, M.; et al. Low-temperature-processed brookite-based TiO2 heterophase junction enhances performance of planar perovskite solar cells. Nano Lett. 2019, 19, 598–604. [Google Scholar] [CrossRef]
- Wooh, S.; Yoon, H.; Jung, J.H.; Lee, Y.G.; Koh, J.H.; Lee, B.; Kang, Y.S.; Char, K. Efficient light harvesting with micropatterned 3D pyramidal photoanodes in dye-sensitized solar cells. Adv. Mater. 2013, 25, 3111–3116. [Google Scholar] [CrossRef]
- Kim, J.; Koh, J.K.; Kim, B.; Kim, J.H.; Kim, E. Nanopatterning of mesoporous inorganic oxide films for efficient light harvesting of dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 6864–6869. [Google Scholar] [CrossRef]
- Mahpeykar, S.M.; Xiong, Q.; Wang, X. Resonance-induced absorption enhancement in colloidal quantum dot solar cells using nanostructured electrodes. Opt. Express 2014, 22, A1576. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Nam, S.K.; Jung, K.; Moon, J.H. 2D photonic crystal nanodisk array as electron transport layer for highly efficient perovskite solar cells. Nano Energy 2019, 56, 365–372. [Google Scholar] [CrossRef]
- Jiang, Y.; Almansouri, I.; Huang, S.; Young, T.; Li, Y.; Peng, Y.; Hou, Q.; Spiccia, L.; Bach, U.; Cheng, Y.B.; et al. Optical analysis of perovskite/silicon tandem solar cells. J. Mater. Chem. C 2016, 4, 5679–5689. [Google Scholar] [CrossRef]
- Jang, S.; Yoon, J.; Ha, K.; Kim, M.C.; Kim, D.H.; Kim, S.M.; Kang, S.M.; Park, S.J.; Jung, H.S.; Choi, M. Facile fabrication of three-dimensional TiO2 structures for highly efficient perovskite solar cells. Nano Energy 2016, 22, 499–506. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.-H.; Yu, Y.-H.; Sun, H.-B. Laser interference fabrication of large-area functional periodic structure surface. Front. Mech. Eng. 2018, 13, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Haque, S.; Martins, A.; Fortunato, E.; Martins, R.; Mendes, M.J.; Schuster, C.S. Light trapping in solar cells: Simple design rules to maximize absorption. Optica 2020, 7, 1377–1384. [Google Scholar] [CrossRef]


| Device | JSC (mA/cm2) | VOC (V) | FF (%) | PCE (%) | RS (Ωcm2) | RSH (Ωcm2) |
|---|---|---|---|---|---|---|
| Flat mp-TiO2 | 7.98 | 0.971 | 45.17 | 3.50 | 37.21 | 1272.73 |
| 1D GNP mp-TiO2 | 8.88 | 0.975 | 49.73 | 4.31 | 25.98 | 1773.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-P.; Kim, W.H.; Kwon, S.M.; Kim, J.Y.; Do, Y.S.; Woo, S. Enhanced Light Absorption by Facile Patterning of Nano-Grating on Mesoporous TiO2 Photoelectrode for Cesium Lead Halide Perovskite Solar Cells. Nanomaterials 2021, 11, 1233. https://doi.org/10.3390/nano11051233
Kim K-P, Kim WH, Kwon SM, Kim JY, Do YS, Woo S. Enhanced Light Absorption by Facile Patterning of Nano-Grating on Mesoporous TiO2 Photoelectrode for Cesium Lead Halide Perovskite Solar Cells. Nanomaterials. 2021; 11(5):1233. https://doi.org/10.3390/nano11051233
Chicago/Turabian StyleKim, Kang-Pil, Wook Hyun Kim, Soo Min Kwon, Jun Yong Kim, Yun Seon Do, and Sungho Woo. 2021. "Enhanced Light Absorption by Facile Patterning of Nano-Grating on Mesoporous TiO2 Photoelectrode for Cesium Lead Halide Perovskite Solar Cells" Nanomaterials 11, no. 5: 1233. https://doi.org/10.3390/nano11051233







