Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PEG-Alendronate
2.3. Preparation of Fe3O4 Nanoparticles and Their Modification with PEG-Ale
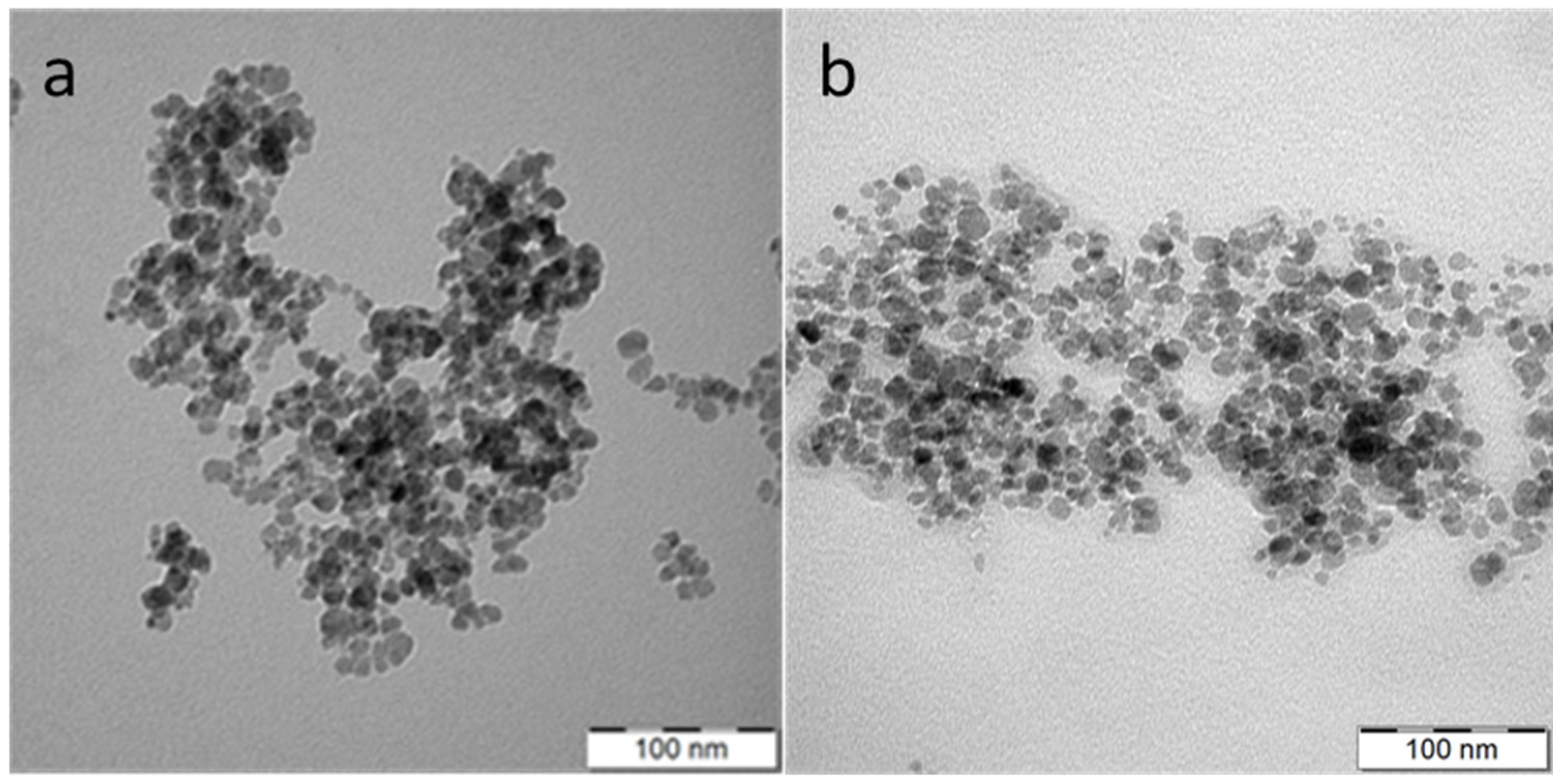
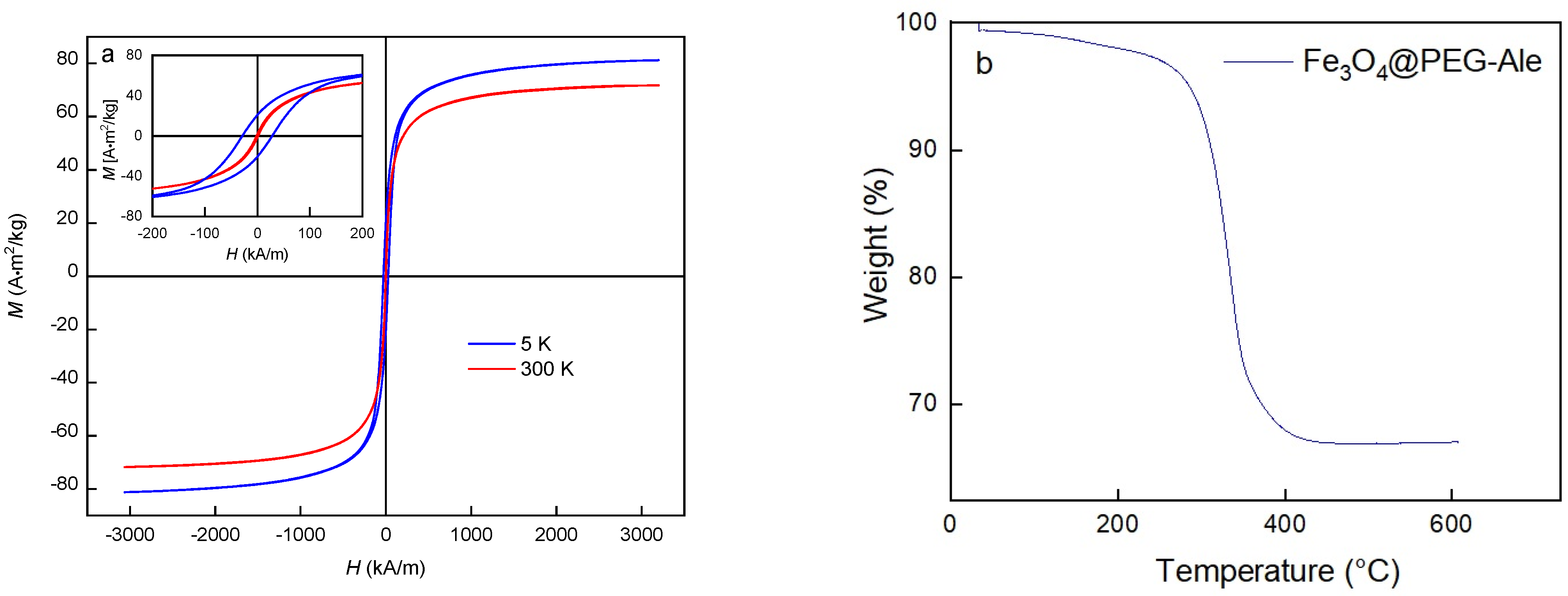
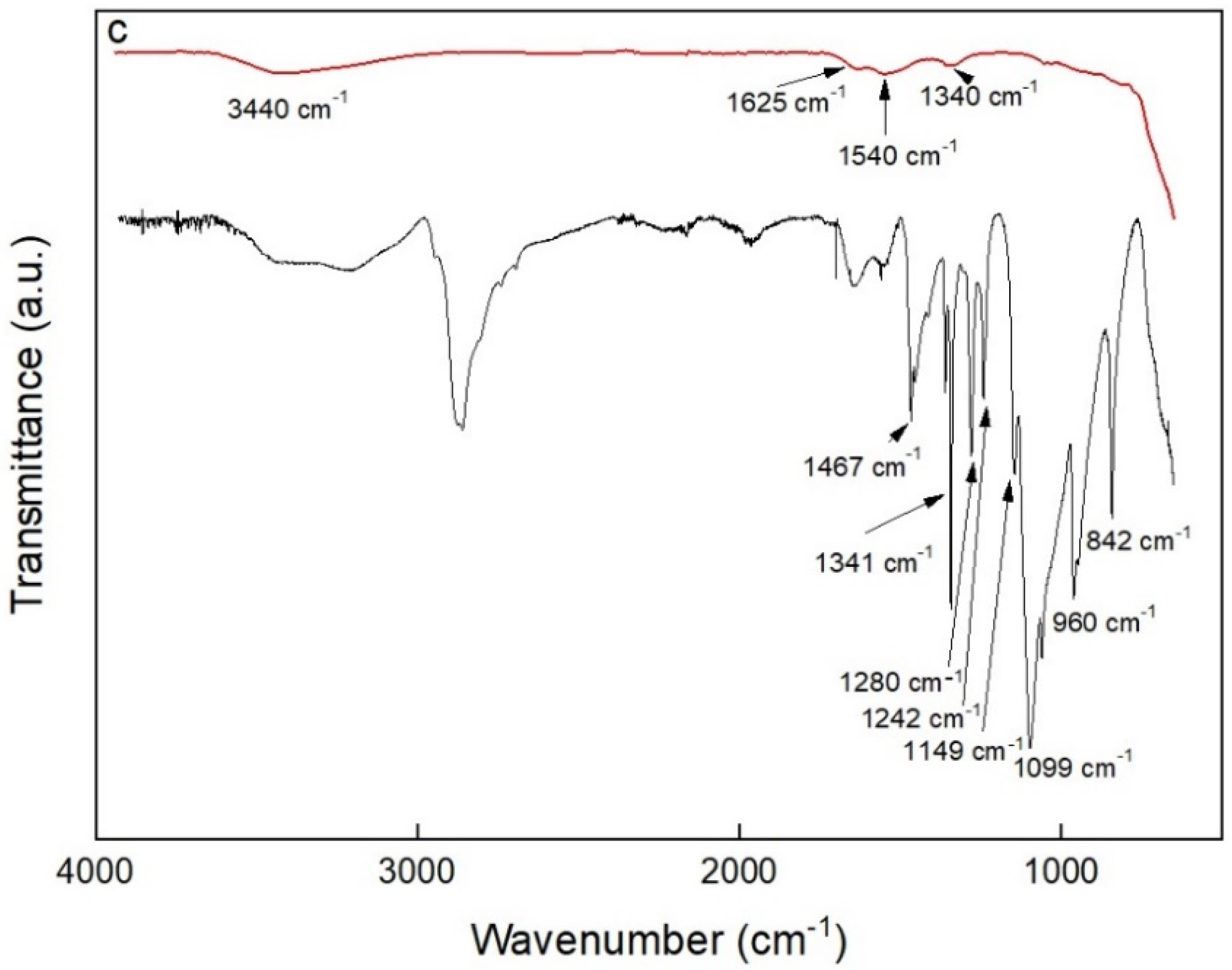
2.4. Characterization of Nanoparticles
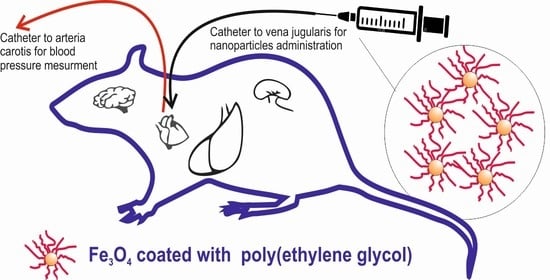
2.5. Animal Experiments
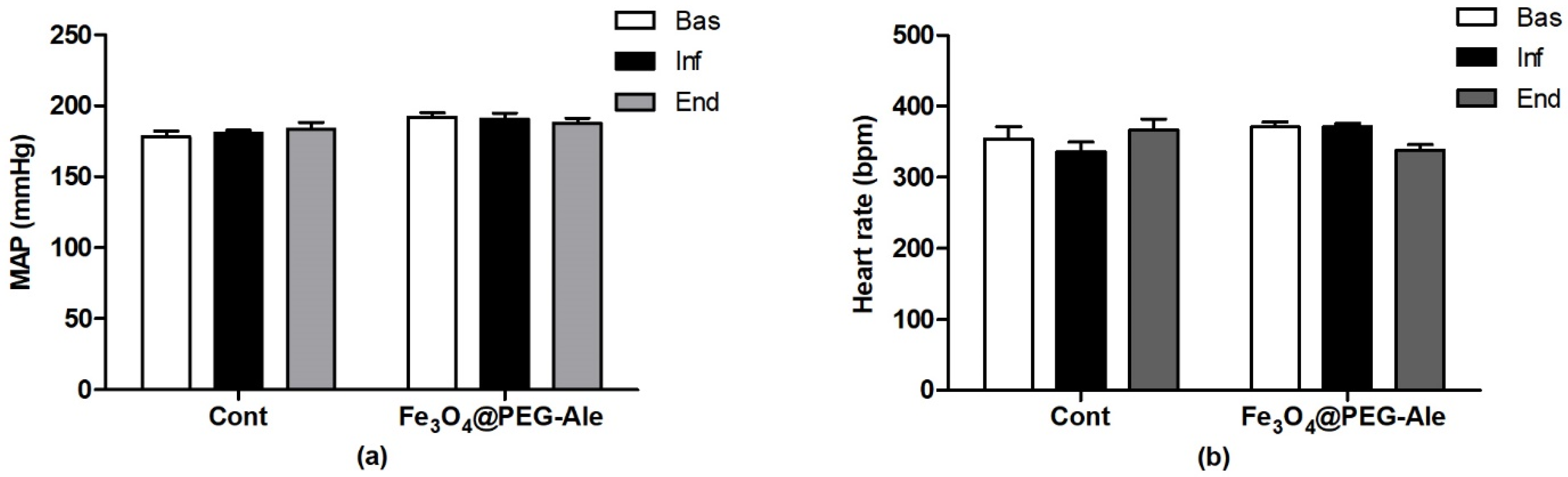
2.6. Measurement of Blood Pressure and Heart Rate
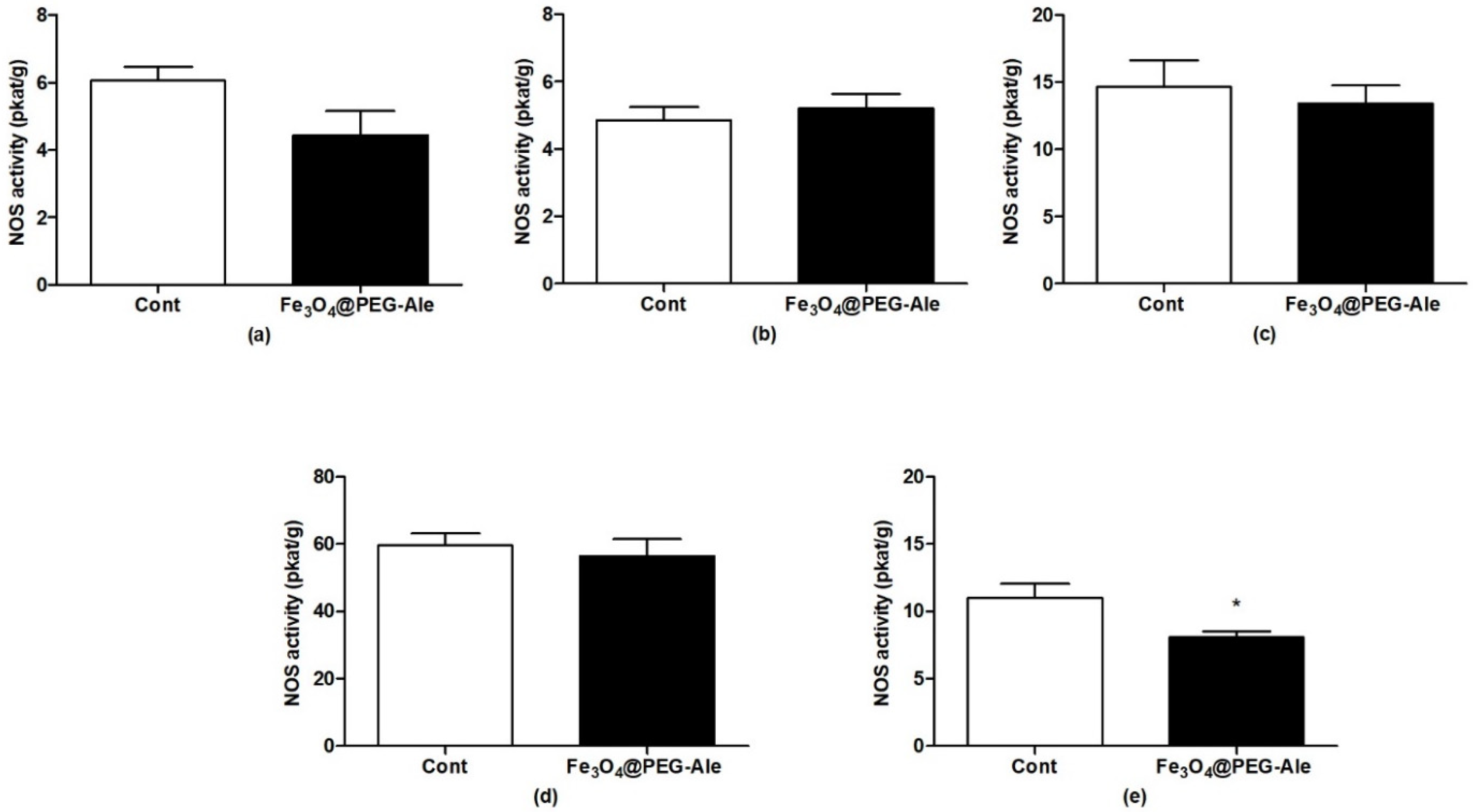
2.7. Determination of Activity of Nitric Oxide Synthase
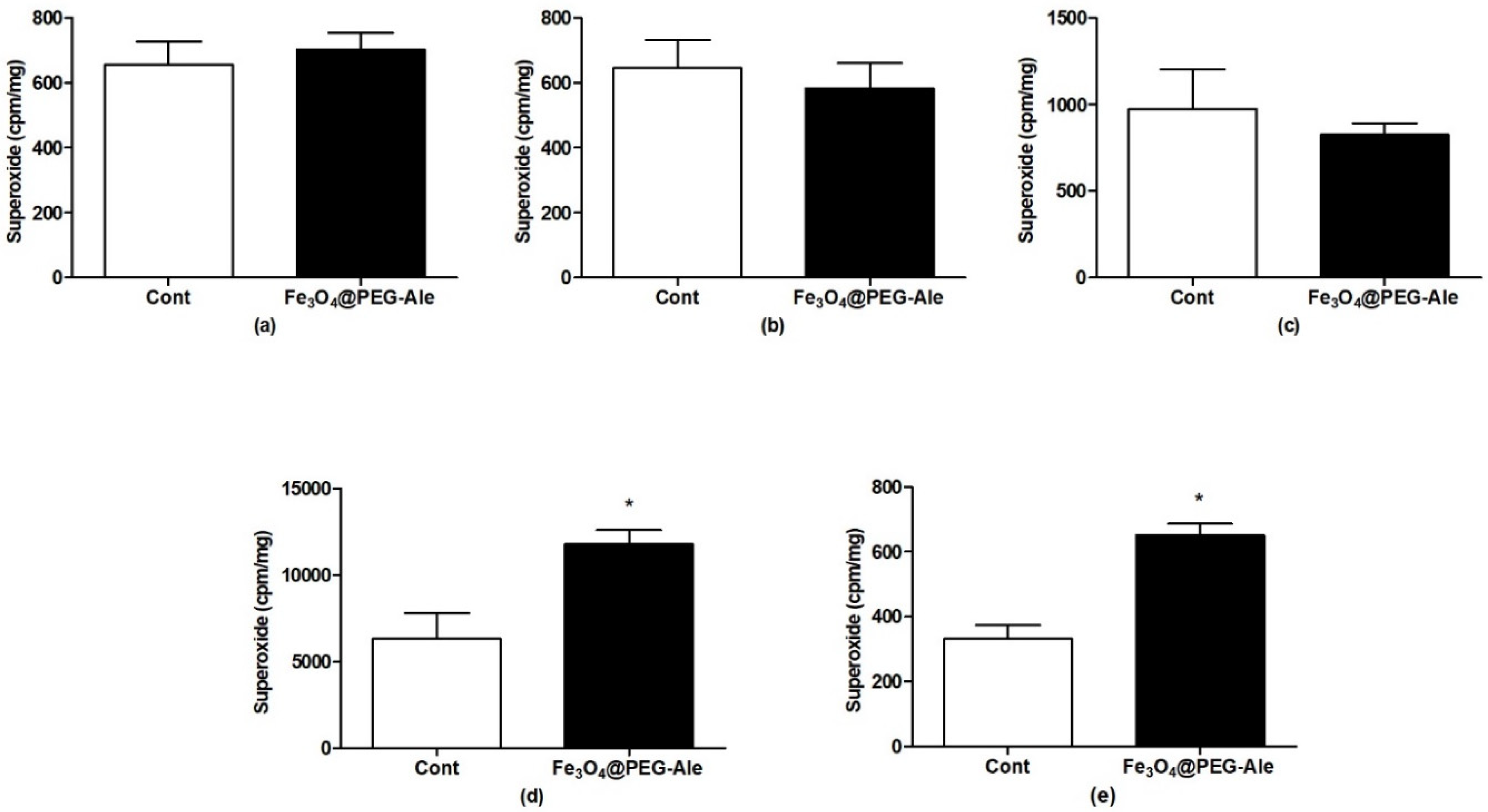
2.8. Production of Superoxide
2.9. Determination of Vascular Functions
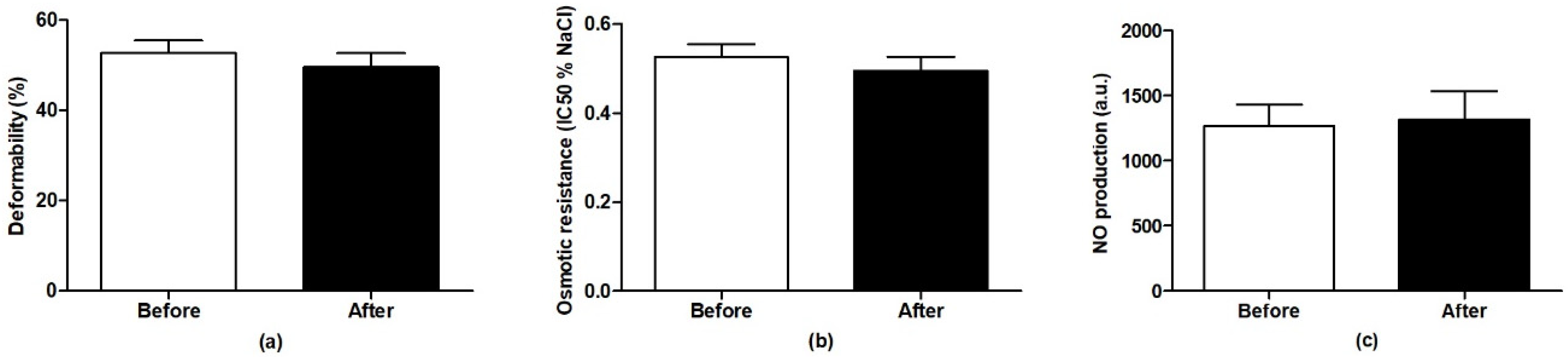
2.10. Determination of Red Blood Cell Parameters
2.11. Statistical Analyses
3. Results
3.1. Fe3O4 Nanoparticles Preparation
3.2. Blood Pressure, Heart Rate and Red Blood Cell Parameters
3.3. Determination of Nitric Oxide Synthase Activity
3.4. Production of Superoxide
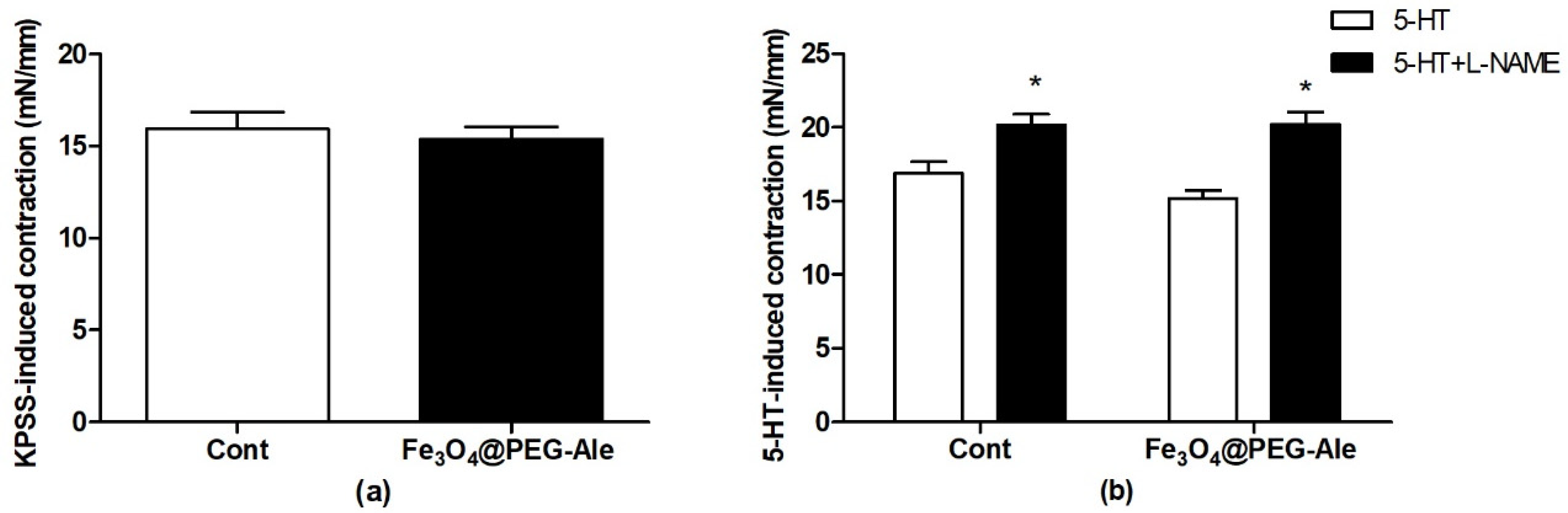
3.5. Examination of Contractions of the Femoral Artery
3.6. Examination of Relaxations of the Femoral Artery
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine (serotonin) |
| ACh | acetylcholine |
| Ale | alendronate |
| ANOVA | analysis of variance |
| Bas | baseline |
| BP | arterial blood pressure |
| bpm | beats per minute |
| Cont | control |
| Ð | dispersity |
| Dh | hydrodynamic diameter |
| DLS | dynamic light scattering |
| Dn | number-average diameter |
| Dw | weight-average diameter |
| FDA | Food and Drug Administration |
| Fe3O4@PEG-Ale | magnetite coated with poly(ethylene glycol)-alendronate |
| FTIR | Fourier-transform infrared |
| HR | heart rate |
| i.v. | intravenous |
| KPSS | high concentration of potassium (125 mmol/l)-containing physiological saline solution |
| L-NAME | N(ω)-nitro-L-arginine methyl ester |
| MAP | mean arterial pressure |
| Mn | number-average molar mass |
| Mw | weight-average molar mass |
| MRI | magnetic resonance imaging |
| NMR | nuclear magnetic resonance |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| NPs | nanoparticles |
| PB | phosphate buffer |
| PD | polydispersity |
| PEG | poly(ethylene glycol) |
| PEG-NHS | α-methoxy poly(ethylene glycol) succinimidyl ester |
| PSS | physiological saline solution |
| RBC | red blood cell |
| SEM | standard error of mean |
| SHR | spontaneously hypertensive rat |
| SNP | sodium nitroprusside |
| TEM | transmission electron microscope |
| TGA | thermogravimetric analysis |
| WKY | Wistar-Kyoto rat |
| ZFC-FC | zero-field-cooled and field-cooled |
References
- Wu, Y.; Lu, Z.; Li, Y.; Yang, J.; Zhang, X. Surface modification of iron oxide-based magnetic nanoparticles for cerebral theranostics: Application and prospection. Nanomaterials 2020, 10, 1441. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimodal imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef] [Green Version]
- Stephen, Z.R.; Kievit, F.M.; Zhang, M. Magnetite nanoparticles for medical MR imaging. Mater. Today 2011, 14, 330–338. [Google Scholar] [CrossRef]
- Fütterer, S.; Andrusenko, I.; Kolb, U.; Hofmeister, W.; Langguth, P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD). J. Pharm. Biomed. 2013, 86, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Wáng, Y.X.J.; Idée, J.M. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 2017, 7, 88–122. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Líšková, S.; Bališ, P.; Mičurová, A.; Kluknavský, M.; Okuliarová, M.; Puzserová, A.; Škrátek, M.; Sekaj, I.; Maňka, J.; Valovič, P.; et al. Effect of iron oxide nanoparticles on vascular function and nitric oxide production in acute stress-exposed rats. Physiol. Res. 2020, 69, 1067–1083. [Google Scholar] [CrossRef]
- Kolenko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovni, K.; Lebedev, O.I.; et al. Large-scale synthesis of colloidal Fe3O4 nanoparticles exhibiting high heating efficiency in magnetic hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Morales, M.P.; Serna, C.J. Effect of nature and particle size on properties of uniform magnetite and maghemite nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Fuentes-Caparrós, A.M.; Sharkey, J.; Daniels, L.M.; Mandal, P.; Park, B.K.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. SPIONs for cell labelling and tracking using MRI: Magnetite or maghemite? Biomater. Sci. 2018, 6, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent folate-dendrimer-coated iron oxide theranostic nanoparticles for simultaneous magnetic resonance imaging and precise cancer cell targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- NDong, C.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E.M.S. Shape anisotropic iron oxide-based magnetic nanoparticles: Synthesis and biomedical applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [Green Version]
- Dalzon, B.; Torres, A.; Reymond, S.; Gallet, B.; Saint-Antonin, F.; Collin-Faure, V.; Moriscot, C.; Fenel, D.; Schoehn, G.; Aude-Garcia, C.; et al. Influences of nanoparticles characteristics on the cellular responses: The example of iron oxide and macrophages. Nanomaterials 2020, 10, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, I.; Mestach, D.; Leiza, J.R.; Asua, J.M. Adhesion enhancement in waterborne acrylic latex binders synthesized with phosphate methacrylate monomers. Prog. Org. Coat. 2008, 61, 38–44. [Google Scholar] [CrossRef]
- Guénin, E.; Hardouin, J.; Lalatonne, Y.; Motte, L. Bivalent alkyne-bisphosphonate as clickable and solid anchor to elaborate multifunctional iron oxide nanoparticles with microwave enhancement. J. Nanopart. Res. 2012, 14, 965. [Google Scholar] [CrossRef]
- Chen, B.W.; He, Y.C.; Sung, S.Y.; Le, T.T.H.; Hsieh, C.L.; Chen, J.Y.; Wei, Z.H.; Yao, D.J. Synthesis and characterization of magnetic nanoparticles coated with polystyrene sulfonic acid for biomedical applications. Sci. Technol. Adv. Mater. 2020, 21, 471–481. [Google Scholar] [CrossRef]
- Guibert, C.; Dupuis, V.; Peyre, V.; Fresnais, J. Hyperthermia of magnetic nanoparticles: Experimental study of the role of aggregation. J. Phys. Chem. C 2015, 119, 28148–28154. [Google Scholar] [CrossRef] [Green Version]
- Turcheniuk, K.; Tarasevych, A.V.; Kukhar, V.P.; Boukherroub, R.; Szunerits, S. Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale 2013, 5, 10729–10752. [Google Scholar] [CrossRef]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Rydtoft, L.M.; Lokanathan, A.R.; Hansen, L.; Østergaard, L.; Kingshott, P.; Howard, K.A.; Besenbacher, F.; et al. Accumulation of magnetic iron oxide nanoparticles coated with variably sized polyethylene glycol in murine tumors. Nanoscale 2012, 4, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Ronnander, P.L.; Simon, H.; Spilgies, A.; Koch, A.; Scherr, S. Dissolving polyvinylpyrrolidone-based microneedle systems for in-vitro delivery of sumatriptan succinate. Eur. J. Pharm. Sci. 2018, 114, 84–92. [Google Scholar] [CrossRef]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual delivery of alendronate and E7-BMP-2 peptide via calcium chelation to mineralized nanofiber fragments for alveolar bone regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Endothelial dysfunction in experimental models of arterial hypertension: Cause or consequence? Biomed. Res. Int. 2014, 2014, 598271. [Google Scholar] [CrossRef]
- Gumienna-Kontecka, E.; Silvagni, R.; Lipinski, R.; Lecouvey, M.; Marincola, F.C.; Crisponi, G.; Nurchib, V.M.; Leroux, Y.; Kozlowski, H. Bisphosphonate chelating agents: Complexation of Fe(III) and Al(III) by 1-phenyl-1-hydroxymethylene bisphosphonate and its analogues. Inorg. Chim. Acta 2002, 339, 111–118. [Google Scholar] [CrossRef]
- Waterborg, J.H. The Lowry method for protein quantitation. In The Protein Protocols Handbook, 3rd ed.; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 7–10. [Google Scholar]
- Kluknavsky, M.; Balis, P.; Skratek, M.; Manka, J.; Bernatova, I. (–)-Epicatechin reduces the blood pressure of young borderline hypertensive rats during the post-treatment period. Antioxidants 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Radosinska, J.; Jasenovec, T.; Radosinska, D.; Balis, P.; Puzserova, A.; Skratek, M.; Manka, J.; Bernatova, I. Ultra-small superparamagnetic iron-oxide nanoparticles exert different effects on erythrocytes in normotensive and hypertensive rats. Biomedicines 2021, 9, 377. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; John Wiley: Weinheim, Germany, 2003. [Google Scholar] [CrossRef]
- Labouta, H.I.; Gomez-Garcia, M.; Sarsons, C.D.; Nguyen, T.; Kennard, J.; Ngo, W.; Terefe, K.; Iragorri, N.; Lai, P.; Rinker, K.D.; et al. Surface-grafted polyethylene glycol conformation impacts the transport of PEG-functionalized liposomes through a tumour extracellular matrix model. RSC Adv. 2018, 8, 7697–7708. [Google Scholar] [CrossRef] [Green Version]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, S.J.; Joy, M.; Ghosh, C.K.; Dey, S.; Kotnala, R.K.; Ghosh, S. Magnetic, X-ray and Mössbauer studies on magnetite/maghemite core-shell nanostructures fabricated through an aqueous route. RSC Adv. 2014, 4, 64919–64929. [Google Scholar] [CrossRef] [Green Version]
- Khanna, R.K.; Moore, M.H. Carbamic acid: Molecular structure and IR spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1999, 55, 961–967. [Google Scholar] [CrossRef]
- Patsula, V.; Horák, D.; Kučka, J.; Macková, H.; Lobaz, V.; Francová, P.; Herynek, V.; Heizer, T.; Páral, P.; Šefc, L. Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Santora, A.C.; Black, D.M.; Russell, R.G.G. History of alendronate. Bone 2020, 137, 115411. [Google Scholar] [CrossRef]
- Thomas, J.C. The determination of log normal particle size distributions by dynamic light scattering. J. Colloid Interface Sci. 1987, 117, 187–192. [Google Scholar] [CrossRef]
- Fischer, K.; Schmidt, M. Pitfalls and novel applications of particle sizing by dynamic light scattering. Biomaterials 2016, 98, 79–91. [Google Scholar] [CrossRef]
- Iversen, N.K.; Frische, S.; Thomsen, K.; Laustsen, C.; Pedersen, M.; Hansen, P.B.; Bie, P.; Fresnais, J.; Berret, J.F.; Baatrup, E.; et al. Superparamagnetic iron oxide polyacrylic acid coated γ-Fe2O3 nanoparticles do not affect kidney function but cause acute effect on the cardiovascular function in healthy mice. Toxicol. Appl. Pharmacol. 2013, 266, 276–288. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.T.; Wang, Y.; Feng, W.Y.; Wang, B.; Wang, M.; Ouyang, H.; Chai, Z.F. Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J. Nanosci. Nanotechnol. 2010, 10, 8584–8590. [Google Scholar] [CrossRef] [PubMed]
- Astanina, K.; Simon, Y.; Cavelius, C.; Petry, S.; Kraegeloh, A.; Kiemer, A.K. Superparamagnetic iron oxide nanoparticles impair endothelial integrity and inhibit nitric oxide production. Acta. Biomater. 2014, 10, 4896–4911. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef] [Green Version]
- Benyettou, F.; Hardouin, J.; Lecouvey, M.; Jouni, H.; Motte, L. PEGylated versus non-PEGylated γ-Fe2O3@alendronate nanoparticles. J. Bioanal. Biomed. 2012, 4, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID bio-magnetometry for determination and differentiation of biogenic iron and iron oxide nanoparticles in the biological samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleksa, V.; Bernátová, I.; Patsula, V.; Líšková, S.; Bališ, P.; Radošinská, J.; Mičurová, A.; Kluknavský, M.; Jasenovec, T.; Radošinská, D.; et al. Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials 2021, 11, 1238. https://doi.org/10.3390/nano11051238
Oleksa V, Bernátová I, Patsula V, Líšková S, Bališ P, Radošinská J, Mičurová A, Kluknavský M, Jasenovec T, Radošinská D, et al. Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials. 2021; 11(5):1238. https://doi.org/10.3390/nano11051238
Chicago/Turabian StyleOleksa, Viktoriia, Iveta Bernátová, Vitalii Patsula, Silvia Líšková, Peter Bališ, Jana Radošinská, Andrea Mičurová, Michal Kluknavský, Tomáš Jasenovec, Dominika Radošinská, and et al. 2021. "Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats" Nanomaterials 11, no. 5: 1238. https://doi.org/10.3390/nano11051238
APA StyleOleksa, V., Bernátová, I., Patsula, V., Líšková, S., Bališ, P., Radošinská, J., Mičurová, A., Kluknavský, M., Jasenovec, T., Radošinská, D., Macková, H., & Horák, D. (2021). Poly(ethylene glycol)-Alendronate-Coated Magnetite Nanoparticles Do Not Alter Cardiovascular Functions and Red Blood Cells’ Properties in Hypertensive Rats. Nanomaterials, 11(5), 1238. https://doi.org/10.3390/nano11051238