Contribution of Nano-Zero-Valent Iron and Arbuscular Mycorrhizal Fungi to Phytoremediation of Heavy Metal-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil
2.2. AM Inoculum and nZVI
2.3. Experimental Design and Procedure
2.4. Sample Analysis
2.5. Data Analysis
3. Results
3.1. Root Colonization
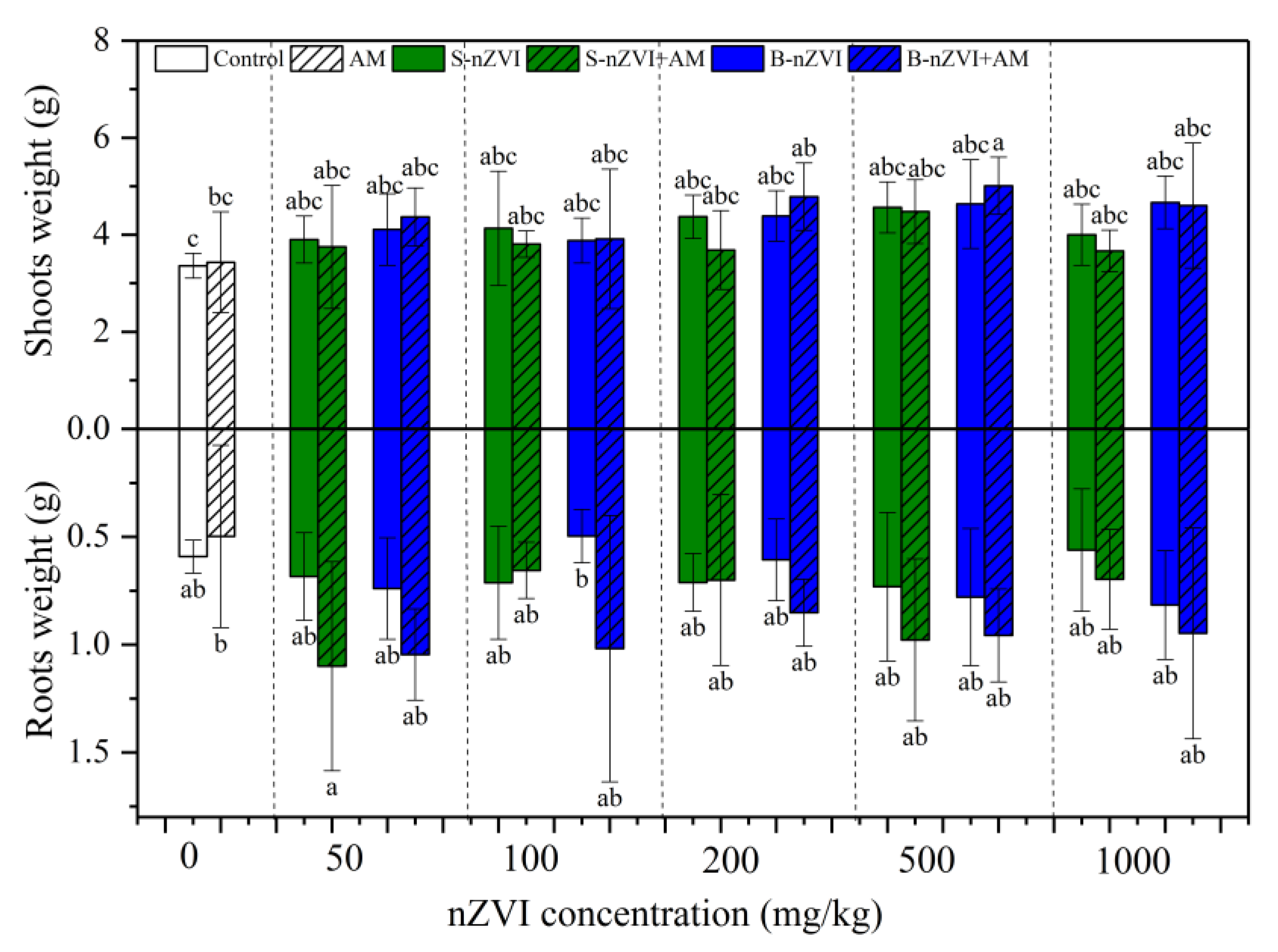
3.2. Pant Biomass
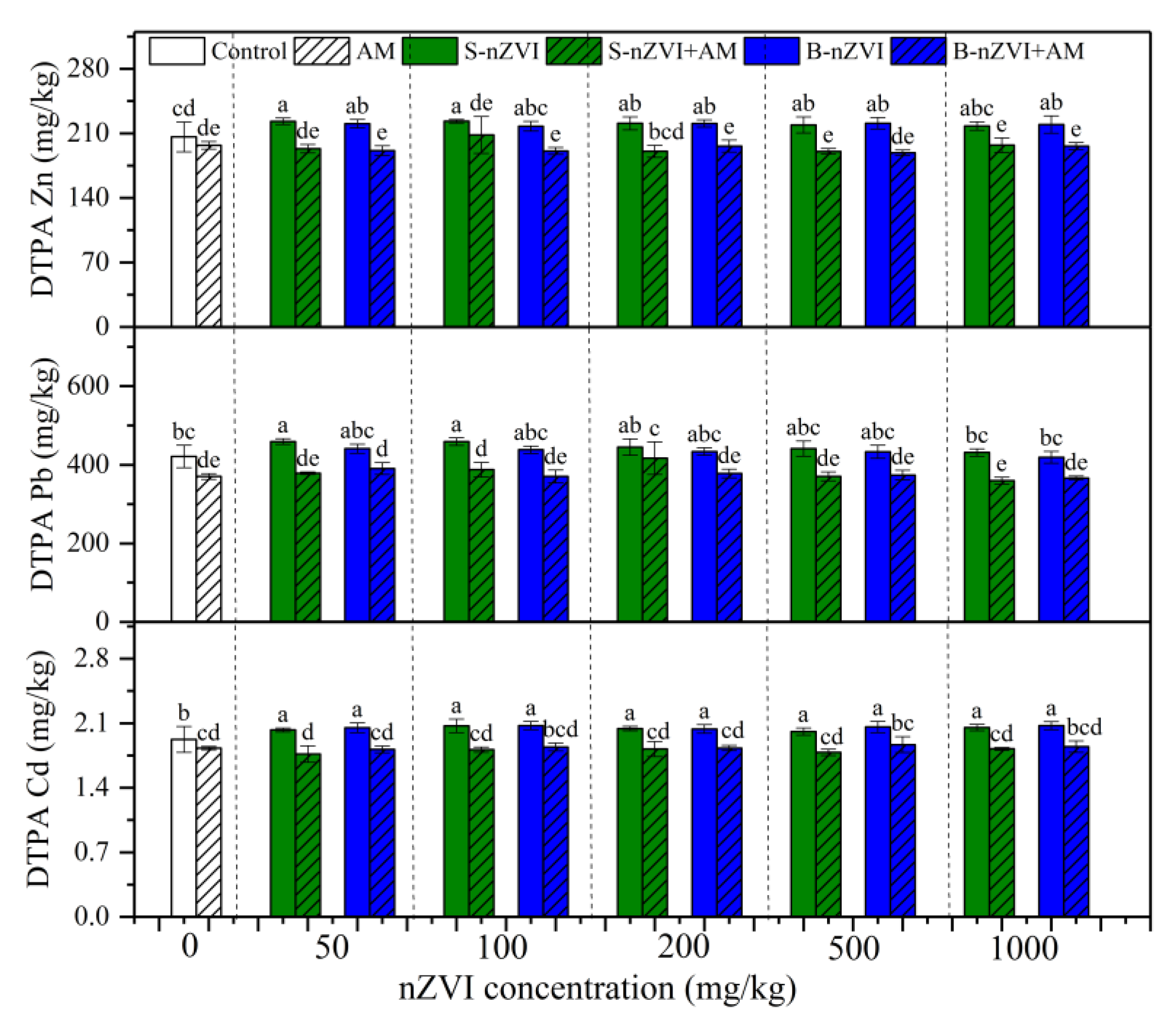
3.3. Bioavailability of Metals in Soil
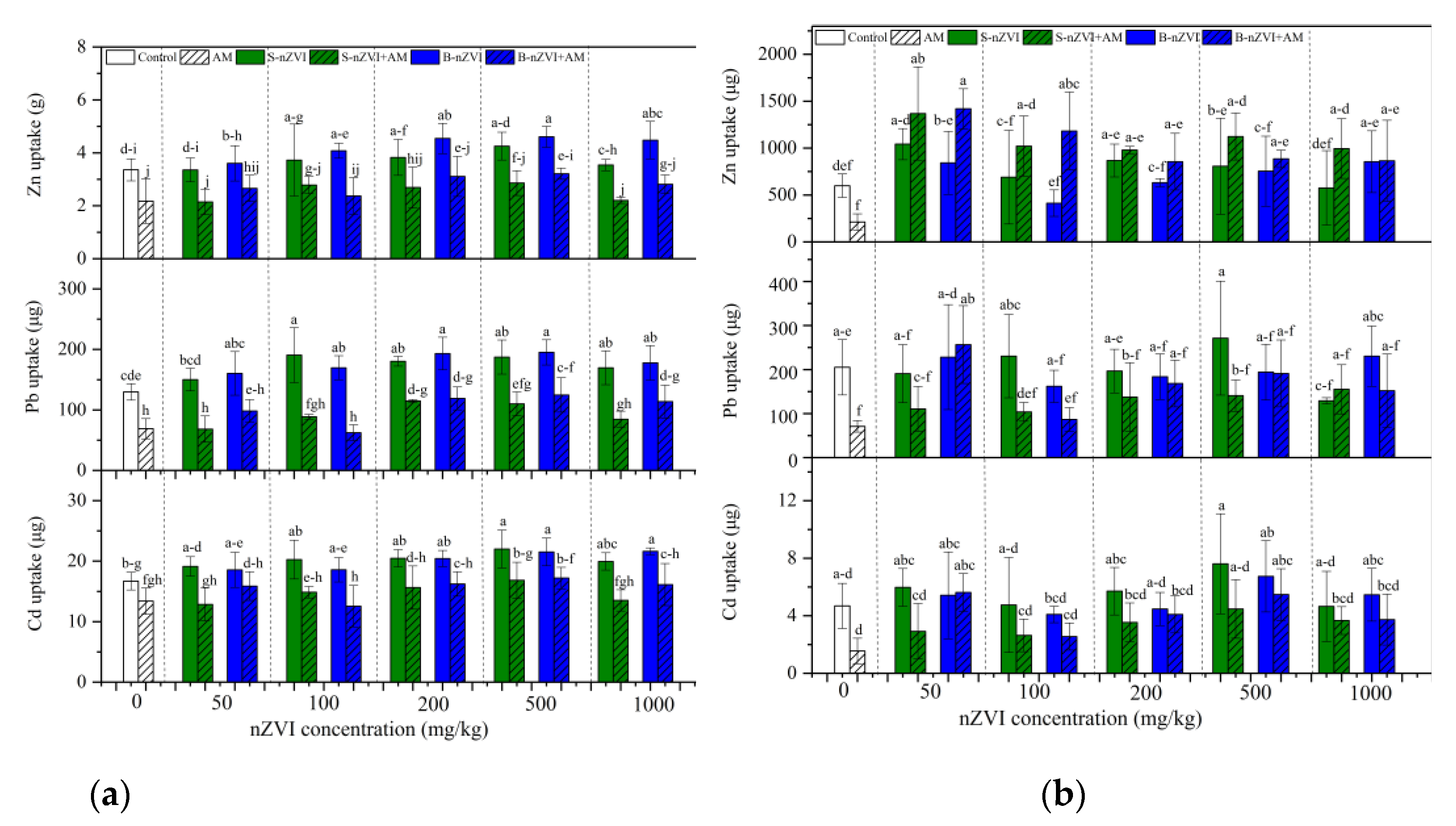
3.4. Heavy Metal Concentrations and Uptake in Plants
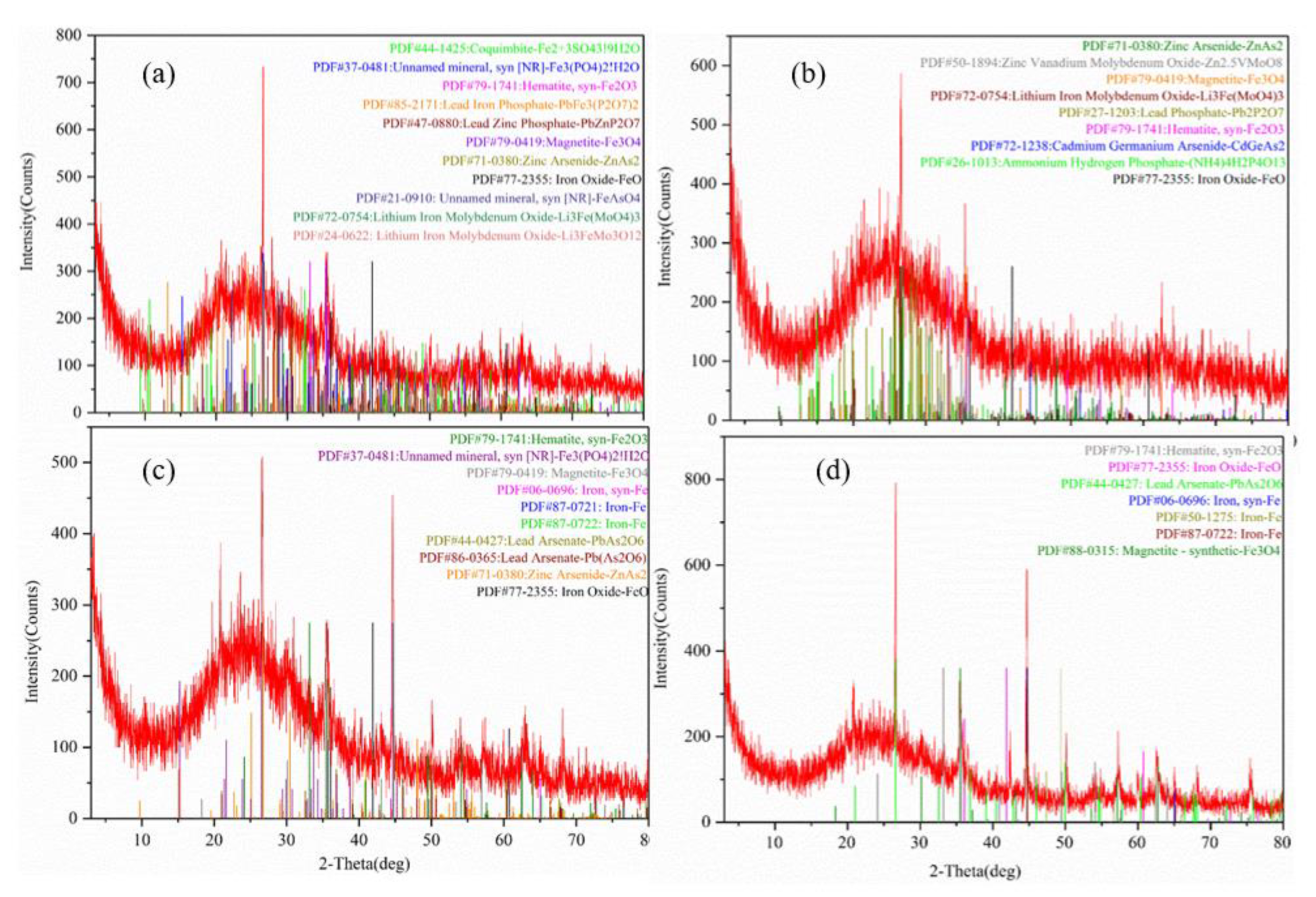
3.5. XRD, EDS and Mapping Analyses of nZVI
3.6. Mycorrhizal Response
4. Discussion
4.1. Effect of AM Fungi on Phytoremediation
4.2. Effect of nZVI on Phytoremediation
4.3. AM Fungi and nZVI Interactions on Phytoremediation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xu, P.; Zeng, G.; Yang, C.; Huang, D.; Zhang, J. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: Applications, microbes and future research needs. Biotechnol. Adv. 2015, 33, 745–755. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, F.; Wang, W.; Zhang, S.; Wang, F. Remediation of Cr(VI)-contaminated soil by nano-zero-valent iron in combination with biochar or humic acid and the consequences for plant performance. Toxics 2020, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Gil-Diaz, M.; Pinilla, P.; Alonso, J.; Lobo, M.C. Viability of a nanoremediation process in single or multi-metal(loid) contaminated soils. J. Hazard. Mater. 2017, 321, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Wu, J.; Xu, Y.; Wang, F.; Tang, X.; Xu, J.; Ok, Y.S.; Meng, J.; Liu, X. Zeolite-supported nanoscale zero-valent iron for immobilization of cadmium, lead, and arsenic in farmland soils: Encapsulation mechanisms and indigenous microbial responses. Environ. Pollut. 2020, 260, 114098. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M. The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI): Effect of relative parameters and soil experiments. Micropor. Mesopor. Mater. 2017, 239, 60–69. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Z.; Kang, Y.; Tsang, E.P. Immobilization and phytotoxicity of chromium in contaminated soil remediated by CMC-stabilized nZVI. J. Hazard. Mater. 2014, 275, 230–237. [Google Scholar] [CrossRef]
- Huang, D.; Qin, X.; Peng, Z.; Liu, Y.; Gong, X.; Zeng, G.; Huang, C.; Cheng, M.; Xue, W.; Wang, X.; et al. Nanoscale zero-valent iron assisted phytoremediation of Pb in sediment: Impacts on metal accumulation and antioxidative system of Lolium perenne. Ecotoxicol. Environ. Saf. 2018, 153, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized nanoscale zerovalent iron mediated cadmium accumulation and oxidative damage of Boehmeria nivea (L.) Gaudich cultivated in cadmium contaminated sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Huang, D.; Zeng, G.; Wan, J.; Cheng, M.; Zhang, C.; Hu, C.; Li, J. Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: A review. Chemosphere 2018, 210, 1145–1156. [Google Scholar] [CrossRef]
- Pawlett, M.; Ritz, K.; Dorey, R.A.; Rocks, S.; Ramsden, J.; Harris, J.A. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent. Environ. Sci. Pollut. Res. 2013, 20, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Adams, C.A.; Yang, W.; Sun, Y.; Shi, Z. Benefits of arbuscular mycorrhizal fungi in reducing organic contaminant residues in crops: Implications for cleaner agricultural production. Crit. Rev. Environ. Sci. Technol. 2019, 50, 1580–1612. [Google Scholar] [CrossRef]
- Wang, F. Occurrence of arbuscular mycorrhizal fungi in mining-impacted sites and their contribution to ecological restoration: Mechanisms and applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1901–1957. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Pinto, V.; Montereali, M.R.; Marconi, P.; Tasso, F.; Turnau, K.; De Giudici, G.; Goralska, K.; Bevilacqua, M.; et al. Assessment of the applicability of a “toolbox” designed for microbially assisted phytoremediation: The case study at Ingurtosu mining site (Italy). Environ. Sci. Pollut. Res. 2014, 21, 6939–6951. [Google Scholar] [CrossRef]
- Gonzalez-Chavez, C.; D’Haen, J.; Vangronsveld, J.; Dodd, J.C. Copper sorption and accumulation by the extraradical mycelium of different Glomus spp. (arbuscular mycorrhizal fungi) isolated from the same polluted soil. Plant. Soil 2002, 240, 287–297. [Google Scholar] [CrossRef]
- Wu, S.; Vosatka, M.; Vogel-Mikus, K.; Kavcic, A.; Kelemen, M.; Sepec, L.; Pelicon, P.; Skala, R.; Valero Powter, A.R.; Teodoro, M.; et al. Nano zero-valent iron mediated metal(loid) uptake and translocation by arbuscular mycorrhizal symbioses. Environ. Sci. Technol. 2018, 52, 7640–7651. [Google Scholar] [CrossRef]
- Zhuang, D.; Jiang, D.; Liu, L.; Huang, Y. Assessment of bioenergy potential on marginal land in China. Renew. Sustain. Energy Rev. 2011, 15, 1050–1056. [Google Scholar] [CrossRef]
- Zhuang, P.; Shu, W.; Li, Z.; Liao, B.; Li, J.; Shao, J. Removal of metals by sorghum plants from contaminated land. J. Environ. Sci. 2009, 21, 1432–1437. [Google Scholar] [CrossRef]
- Sathya, A.; Kanaganahalli, V.; Rao, P.S.; Gopalakrishnan, S. Cultivation of sweet sorghum on heavy metal-contaminated soils by phytoremediation approach for production of bioethanol. In Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 271–292. [Google Scholar]
- Shi, Z.; Zhang, J.; Lu, S.; Li, Y.; Wang, F. Arbuscular mycorrhizal fungi improve the performance of sweet sorghum grown in a Mo-contaminated soil. J. Fungi 2020, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, S.; Cheng, P.; Zhang, S.; Sun, Y. Effects of soil amendments on heavy metal immobilization and accumulation by maize grown in a multiple-metal-contaminated soil and their potential for safe crop production. Toxics 2020, 8, 102. [Google Scholar] [CrossRef]
- Wang, F.Y.; Lin, X.G.; Yin, R. Inoculation with arbuscular mycorrhizal fungus Acaulospora mellea decreases Cu phytoextraction by maize from Cu-contaminated soil. Pedobiologia 2007, 51, 99–109. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Shi, Z. Arbuscular mycorrhiza enhances biomass production and salt tolerance of sweet sorghum. Microorganisms 2019, 7, 289. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jing, R.; Zheng, F.; Zhang, S.; Jiao, W.; Wang, F. Evaluating phytotoxicity of bare and starch-stabilized zero-valent iron nanoparticles in mung bean. Chemosphere 2019, 236, 124336. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pütter, J.; Becker, R. Peroxidases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 286–293. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wang, F.; Jin, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased ZnO nanoparticles phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef]
- Wu, S.; Hu, Y.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Lv, J.; Li, J.; Zhang, J.; Zheng, L.; et al. Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ. Exp. Bot. 2018, 147, 43–52. [Google Scholar] [CrossRef]
- Wang, F.; Adams, C.A.; Shi, Z.; Sun, Y. Combined effects of ZnO NPs and Cd on sweet sorghum as influenced by an arbuscular mycorrhizal fungus. Chemosphere 2018, 209, 421–429. [Google Scholar] [CrossRef]
- Jia, W.; Lv, S.; Feng, J.; Li, J.; Li, Y.; Li, S. Morphophysiological characteristic analysis demonstrated the potential of sweet sorghum (Sorghum bicolor (L.) Moench) in the phytoremediation of cadmium-contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 18823–18831. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolan, N.S. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant Soil 1991, 134, 189–207. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Merlos, M.A.; Zitka, O.; Vojtech, A.; Azcón-Aguilar, C.; Ferrol, N. The arbuscular mycorrhizal fungus Rhizophagus irregularis differentially regulates the copper response of two maize cultivars differing in copper tolerance. Plant Sci. 2016, 253, 68–76. [Google Scholar] [CrossRef]
- Repetto, O.; Bestel-Corre, G.; Dumas-Gaudot, E.; Berta, G.; Gianinazzi-Pearson, V.; Gianinazzi, S. Targeted proteomics to identify cadmium-induced protein modifications in Glomus mosseae-inoculated pea roots. New Phytol. 2003, 157, 555–567. [Google Scholar] [CrossRef]
- Ma, X.M.; Gurung, A.; Deng, Y. Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci. Total Environ. 2013, 443, 844–849. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef]
- Dar, R.A.; Dar, E.A.; Kaur, A.; Phutela, U.G. Sweet sorghum-a promising alternative feedstock for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 4070–4090. [Google Scholar]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Cadmium (Cd2+) removal by nano zerovalent iron: Surface analysis, effects of solution chemistry and surface complexation modeling. Environ. Sci. Pollut. Res. 2013, 20, 6210–6221. [Google Scholar] [CrossRef]
- Mar Gil-Díaz, M.; Pérez-Sanz, A.; Ángeles Vicente, M.; Carmen Lobo, M. Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: Effects on soil properties. CLEAN Soil Air Water 2014, 42, 1776–1784. [Google Scholar] [CrossRef]
- Vítková, M.; Rákosová, S.; Michálková, Z.; Komárek, M. Metal(loid)s behaviour in soils amended with nano zero-valent iron as a function of pH and time. J. Environ. Manag. 2017, 186, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.; Han, B.; Qian, T.; Zhao, D. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental remediation and application of nanoscale zero-valent iron and its composites for the removal of heavy metal ions: A review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef]
- Wang, F.Y.; Shi, Z.Y.; Tong, R.J.; Xu, X.F. Dynamics of phoxim residues in green onion and soil as influenced by arbuscular mycorrhizal fungi. J. Hazard. Mater. 2011, 185, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Miao, Y.F. Effects of different arbuscular mycorrhizal fungi on the growth and yield of soybean in coal mine spoil. World J. Agric. Sci. 2006, 2, 383–389. [Google Scholar]
- Cao, J.; Feng, Y.; Lin, X.; Wang, J.; Xie, X. Iron oxide magnetic nanoparticles deteriorate the mutual interaction between arbuscular mycorrhizal fungi and plant. J. Soils Sed. 2016, 17, 841–851. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, X.; He, S.; Dong, G.; Chen, M.; Wang, J.; Lin, X. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef]
- Mokarram-Kashtiban, S.; Hosseini, S.M.; Tabari Kouchaksaraei, M.; Younesi, H. The impact of nanoparticles zero-valent iron (nZVI) and rhizosphere microorganisms on the phytoremediation ability of white willow and its response. Environ. Sci. Pollut. Res. 2019, 26, 10776–10789. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Oleszczuk, P.; Ok, Y.S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Engin. J. 2016, 287, 618–632. [Google Scholar] [CrossRef]
- Lefevre, E.; Bossa, N.; Wiesner, M.R.; Gunsch, C.K. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities. Sci. Total Environ. 2016, 565, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Kang, Y.G.; Chang, Y.S.; Kim, J.H. Effects of zerovalent iron nanoparticles on photosynthesis and biochemical adaptation of soil-grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Wu, T.; White, J.C.; Lin, D. A new strategy using nanoscale zero-valent iron to simultaneously promote remediation and safe crop production in contaminated soil. Nat. Nanotechnol. 2021, 16, 197–205. [Google Scholar] [CrossRef]
- Hansel, C.M.; Fendorf, S.; Sutton, S.; Newville, M. Characterization of Fe plaque and associated metals on the roots of mine-waste impacted aquatic plants. Environ. Sci. Technol. 2002, 35, 3863–3868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, Z.; Cheng, W.; Yan, X.; Tsang, P.E.; Zhao, D. Higher concentrations of nanoscale zero-valent iron (nZVI) in soil induced rice chlorosis due to inhibited active iron transportation. Environ. Pollut. 2016, 210, 338–345. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Z.Q.; Cheng, W.; Tsang, P.E.; Zhao, D.Y. Ageing decreases the phytotoxicity of zero-valent iron nanoparticles in soil cultivated with Oryza sativa. Ecotoxicology 2016, 25, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Gil-Díaz, M.; Lobo, M.C. Phytotoxicity of nanoscale zerovalent iron (nZVI) in remediation strategies. In Phytotoxicity of Nanoparticles; Faisal, M., Saquib, Q., Alatar, A., Al-Khedhairy, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 301–333. [Google Scholar]
- El-Temsah, Y.S.; Sevcu, A.; Bobcikova, K.; Cernik, M.; Joner, E.J. DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere 2016, 144, 2221–2228. [Google Scholar] [CrossRef] [Green Version]



| Item | Value |
|---|---|
| pH | 5.0 |
| Total Cd | 2.6 mg/kg |
| Total Pb | 1796 mg/kg |
| Total Zn | 1603 mg/kg |
| DTPA-Cd | 1.90 mg/kg |
| DTPA-Pb | 412.6 mg/kg |
| DTPA-Zn | 237.9 mg/kg |
| DTPA-Cu | 7.5 mg/kg |
| DTPA-Mn | 68.5 mg/kg |
| DTPA-Fe | 154.6 mg/kg |
| DTPA-Mg | 84.2 mg/kg |
| Organic matter | 25.8 g/kg |
| Available P | 27 mg/kg |
| Available K | 26.3 mg/kg |
| Available N | 118 mg/kg |
| Cation exchange capacity | 3.15 cmol/kg |
| Soil type | Paddy soil |
| Variable | AM | nZVI Type | nZVI Conc. | AM×Type | AM×Conc. | Types ×Dose | AM×Type×Conc. |
|---|---|---|---|---|---|---|---|
| Colonization rate | - | 6.75 *** | 5.37 * | - | - | 1.06 NS | - |
| Root biomass | 3.32 NS | 0.58 NS | 0.84 NS | 0.43 NS | 0.44 NS | 0.63 NS | 1.03 NS |
| Shoot biomass | 0.00 NS | 4.36 * | 2.03 NS | 1.07 NS | 0.12 NS | 0.62 NS | 0.08 NS |
| DTPA-Cd | 225.02 *** | 5.03 * | 1.03 NS | 0.64 NS | 0.38 NS | 0.70 NS | 0.04 NS |
| DTPA-Pb | 245.21 *** | 8.39 ** | 6.36 *** | 0.99 NS | 1.78 NS | 1.77 NS | 1.78 NS |
| DTPA-Zn | 155.19 *** | 1.34 NS | 1.29 NS | 0.37 NS | 0.54 NS | 1.73 NS | 1.39 NS |
| DTPA-Fe | 349.83 *** | 17.05 *** | 19.91 *** | 4.53 * | 3.70 ** | 3.81 ** | 2.02 NS |
| DTPA-Cu | 614.76 *** | 2.16 NS | 0.87 NS | 0.81 NS | 0.13 NS | 1.26 NS | 0.28 NS |
| DTPA-Mn | 4.95 * | 7.36 ** | 2.05 NS | 1.62 NS | 0.51 NS | 0.81 NS | 0.84 NS |
| DTPA-Mg | 252.83 *** | 1.45 NS | 0.68 NS | 0.74 NS | 0.87 NS | 0.69 NS | 0.79 NS |
| Shoot Cd conc. | 81.53 *** | 2.45 NS | 0.25 NS | 0.06 NS | 0.85 NS | 0.42 NS | 0.38 NS |
| Shoot Pb conc. | 447.99 *** | 0.73 NS | 3.44 * | 0.04 NS | 0.58 NS | 0.48 NS | 2.79 * |
| Shoot Zn conc. | 96.16 *** | 2.10 NS | 1.64 NS | 3.94 NS | 0.01 NS | 0.25 NS | 0.94 NS |
| Shoot Fe conc. | 0.04 NS | 10.94 ** | 0.75 NS | 29.82 *** | 0.40 NS | 0.70 NS | 3.38 * |
| Shoot Ca conc. | 22.18 *** | 5.65 * | 0.58 NS | 4.18 * | 1.54 NS | 6.41 * | 0.74 NS |
| Shoot Cu conc, | 81.02 *** | 3.46 NS | 0.52 NS | 6.61 * | 0.34 NS | 1.01 NS | 0.35 NS |
| Shoot Mn conc. | 73.18 *** | 0.16 NS | 2.79 * | 4.94 * | 1.50 NS | 0.85 NS | 0.70 NS |
| Shoot Mg conc. | 19.45 *** | 2.98 NS | 1.70 NS | 0.08 NS | 0.30 NS | 0.73 NS | 1.52 NS |
| Shoot P conc. | 22.96 *** | 0.11 NS | 0.97 NS | 0.60 NS | 0.21 NS | 1.06 NS | 0.49 NS |
| Root Cd conc. | 122.17 *** | 0.13 NS | 1.29 NS | 10.34 ** | 0.73 NS | 1.07 NS | 0.37 NS |
| Root Pb conc. | 164.67 *** | 3.56 NS | 0.95 NS | 0.00 NS | 3.45 * | 2.78 * | 0.55 NS |
| Root Zn conc. | 8.41 ** | 21.94 *** | 5.19 ** | 0.73 NS | 2.28 NS | 2.57 * | 5.22 ** |
| Root Fe conc. | 212.21 *** | 10.17 ** | 1.08 NS | 0.27 NS | 2.34 NS | 1.82 NS | 1.02 NS |
| Root Ca conc. | 68.51 *** | 2.71 NS | 3.36 * | 11.48 ** | 3.09 * | 2.08 NS | 1.03 NS |
| Root Cu conc. | 236.01 *** | 3.19 NS | 4.40 ** | 0.00 NS | 1.23 NS | 3.95 ** | 0.83 NS |
| Root Mn conc. | 162.02 *** | 3.56 NS | 3.40 * | 4.49 * | 1.10 NS | 3.02 * | 3.01 * |
| Root Mg conc. | 33.62 *** | 0.00 NS | 6.32 *** | 4.48 ** | 1.21 NS | 3.05 * | 1.23 NS |
| Root P conc. | 145.40 *** | 0.08 NS | 4.07 ** | 7.07 * | 0.59 NS | 2.70 * | 4.12 ** |
| Shoot Cd uptake | 67.38 *** | 0.35 NS | 3.79 ** | 0.99 NS | 0.34 NS | 1.70 NS | 0.42 NS |
| Shoot Pb uptake | 183.87 *** | 1.74 NS | 6.67 *** | 0.41 NS | 1.41 NS | 2.18 NS | 0.35 NS |
| Shoot Zn uptake | 85.16 *** | 9.07 ** | 3.97 ** | 0.69 NS | 0.27 NS | 0.97 NS | 0.41 NS |
| Root Cd uptake | 16.64 *** | 0.14 NS | 3.22 * | 2.17 NS | 0.14 NS | 0.35 NS | 0.55 NS |
| Root Pb uptake | 16.79 *** | 1.33 NS | 1.47 NS | 1.95 NS | 0.79 NS | 2.15 NS | 1.54 NS |
| Root Zn uptake | 4.87 * | 1.05 * | 3.01 * | 0.08 NS | 1.02 NS | 0.36 NS | 1.08 NS |
| CAT | 5.64 * | 0.03 NS | 1.84 NS | 1.72 NS | 1.18 NS | 0.19 NS | 0.72 NS |
| POD | 5.51 * | 7.26 ** | 1.83 NS | 0.02 NS | 2.74 * | 0.91 NS | 1.27 NS |
| Variable | S-nZVI | B-nZVI | ||||
|---|---|---|---|---|---|---|
| AM | Conc. | AM×Conc. | AM | Conc. | AM×Conc. | |
| Root biomass | 1.43 NS | 1.49 NS | 0.80 NS | 3.85 NS | 1.52 NS | 0.92 NS |
| Shoot biomass | 0.92 NS | 1.82 NS | 0.12 NS | 0.53 NS | 3.00 * | 0.10 NS |
| DTPA-Cd | 145.18 *** | 1.50 NS | 1.97 NS | 117.76 *** | 2.11 NS | 1.42 NS |
| DTPA-Pb | 133.42 *** | 5.09 ** | 2.12 NS | 181.83 *** | 2.54 * | 0.42 NS |
| DTPA-Zn | 74.47 *** | 2.17 NS | 1.95 NS | 131.76 *** | 1.17 NS | 2.29 NS |
| DTPA-Fe | 150.72 *** | 9.38 *** | 2.19 NS | 213.99 *** | 20.10 *** | 1.64 NS |
| DTPA-Cu | 276.96 *** | 2.13 NS | 0.74 NS | 242.35 *** | 2.20 NS | 0.39 NS |
| DTPA-Mn | 0.97 NS | 1.49 NS | 0.54 NS | 9.43 ** | 1.14 NS | 0.96 NS |
| DTPA-Mg | 186.55 *** | 2.13 NS | 0.41 NS | 123.03 *** | 2.66 * | 0.84 NS |
| Shoot Cd conc. | 58.01 *** | 0.20 NS | 0.54 NS | 47.65 *** | 0.48 NS | 0.50 NS |
| Shoot Pb conc. | 305.09 *** | 2.43 NS | 0.56 NS | 308.35 *** | 2.16 NS | 2.20 NS |
| Shoot Zn conc. | 49.81 *** | 1.07 NS | 0.94 NS | 66.68 *** | 1.05 NS | 0.37 NS |
| Shoot Fe conc. | 12.00 ** | 0.50 NS | 2.81 * | 13.65 ** | 1.51 NS | 1.80 NS |
| Shoot Ca conc. | 5.77 * | 5.10 ** | 0.54 NS | 28.18 *** | 5.43 ** | 1.74 NS |
| Shoot Cu conc. | 34.62 *** | 0.64 NS | 0.95 NS | 65.14 *** | 0.78 NS | 0.41 NS |
| Shoot Mn conc. | 25.62 *** | 2.65 * | 0.97 NS | 68.42 *** | 3.29 * | 0.85 NS |
| Shoot Mg conc. | 11.25 ** | 0.96 NS | 1.41 NS | 10.30 ** | 2.18 NS | 0.50 NS |
| Shoot P conc. | 11.41 ** | 1.02 NS | 0.52 NS | 17.80 ** | 0.98 NS | 0.10 NS |
| Root Cd conc. | 104.52 *** | 2.88 * | 0.61 NS | 63.76 *** | 2.37 NS | 0.65 NS |
| Root Pb conc. | 102.97 *** | 3.68 ** | 1.60 NS | 125.51 *** | 3.93 ** | 2.89 * |
| Root Zn conc. | 8.45 ** | 5.24 ** | 5.71 ** | 6.10 * | 9.35 *** | 5.34 ** |
| Root Fe conc. | 131.67 *** | 5.81 ** | 2.75 * | 138.39 *** | 2.72 * | 0.81 NS |
| Root Ca conc. | 70.48 *** | 2.97 * | 0.71 NS | 25.13 *** | 1.48 NS | 3.76 ** |
| Root Cu conc. | 143.91 *** | 9.24 *** | 0.74 NS | 140.62 *** | 6.77 *** | 0.96 NS |
| Root Mn conc. | 124.93 *** | 6.11 *** | 1.92 NS | 101.28 *** | 5.15 ** | 2.80 * |
| Root Mg conc. | 32.41 *** | 5.35 ** | 2.54 * | 18.52 *** | 1.09 NS | 2.50 NS |
| Root P conc. | 94.11 *** | 6.66 *** | 4.46 ** | 94.64 *** | 0.92 NS | 1.54 NS |
| Shoot Cd uptake | 57.37 *** | 3.29 * | 0.42 NS | 39.69 *** | 4.28 ** | 0.58 NS |
| Shoot Pb uptake | 157.45 *** | 7.09 *** | 0.86 NS | 106.13 *** | 7.25 *** | 0.97 NS |
| Shoot Zn uptake | 42.60 *** | 2.13 NS | 0.13 NS | 68.17 *** | 4.69 ** | 0.51 NS |
| Root Cd uptake | 15.40 *** | 1.70 NS | 0.33 NS | 5.99 * | 3.12 * | 0.84 NS |
| Root Pb uptake | 18.05 *** | 0.97 NS | 1.63 NS | 4.75 * | 2.79 * | 1.36 NS |
| Root Zn uptake | 3.42 NS | 4.17 ** | 1.43 NS | 6.61 * | 5.02 ** | 3.78 ** |
| CAT | 0.67 NS | 0.86 NS | 2.02 NS | 6.10 * | 1.24 NS | 2.05 NS |
| POD | 3.93 NS | 1.13 NS | 0.83 NS | 2.88 NS | 1.66 NS | 2.69 * |
| Parameter | No nZVI | 50 | 100 | 200 | 500 | 1000 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-nZVI | B-nZVI | S-nZVI | B-nZVI | S-nZVI | B-nZVI | S-nZVI | B-nZVI | S-nZVI | B-nZVI | |||
| Cd conc. | Shoot | −25 | −28 | −20 | −23 | −32 | −14 | −22 | −22 | −23 | −27 | −24 |
| Root | −30 | −40 | −31 | −52 | −32 | −52 | −37 | −49 | −29 | −41 | −26 | |
| Cd uptake | Shoot | −20 | −33 | −27 | −24 | −23 | −32 | −14 | −32 | −21 | −20 | −25 |
| Root | −67 | −51 | −45 | −38 | −41 | −21 | 4 | −38 | −9 | −19 | −32 | |
| Pb conc. | Shoot | −52 | −53 | −42 | −41 | −55 | −39 | −44 | −40 | −43 | −46 | −34 |
| Root | −40 | −34 | −18 | −50 | −50 | −28 | −37 | −31 | −32 | −30 | −27 | |
| Pb uptake | Shoot | −55 | −55 | −39 | −53 | −54 | −45 | −38 | −41 | −47 | −50 | −36 |
| Root | −65 | −42 | −55 | −30 | −48 | 20 | 13 | −47 | −8 | −2 | −34 | |
| Zn conc. | Shoot | −29 | −32 | −31 | −23 | −42 | −22 | −38 | −32 | −30 | −32 | −33 |
| Root | −24 | −13 | 32 | 59 | 38 | 12 | 22 | 24 | 1 | 44 | −2 | |
| Zn uptake | Shoot | −35 | −36 | −25 | −30 | −33 | −38 | −26 | −42 | −32 | −30 | −37 |
| Root | −65 | 31 | 48 | 13 | 39 | 73 | 68 | 186 | 35 | 17 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Zhang, S.; Wang, Q.; Feng, X.; Zhang, S.; Sun, Y.; Wang, F. Contribution of Nano-Zero-Valent Iron and Arbuscular Mycorrhizal Fungi to Phytoremediation of Heavy Metal-Contaminated Soil. Nanomaterials 2021, 11, 1264. https://doi.org/10.3390/nano11051264
Cheng P, Zhang S, Wang Q, Feng X, Zhang S, Sun Y, Wang F. Contribution of Nano-Zero-Valent Iron and Arbuscular Mycorrhizal Fungi to Phytoremediation of Heavy Metal-Contaminated Soil. Nanomaterials. 2021; 11(5):1264. https://doi.org/10.3390/nano11051264
Chicago/Turabian StyleCheng, Peng, Shuqi Zhang, Quanlong Wang, Xueying Feng, Shuwu Zhang, Yuhuan Sun, and Fayuan Wang. 2021. "Contribution of Nano-Zero-Valent Iron and Arbuscular Mycorrhizal Fungi to Phytoremediation of Heavy Metal-Contaminated Soil" Nanomaterials 11, no. 5: 1264. https://doi.org/10.3390/nano11051264







