Effect of Substrate Types on the Structure of Vertical Graphene Prepared by Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of VG
2.2. Characterizations of VG
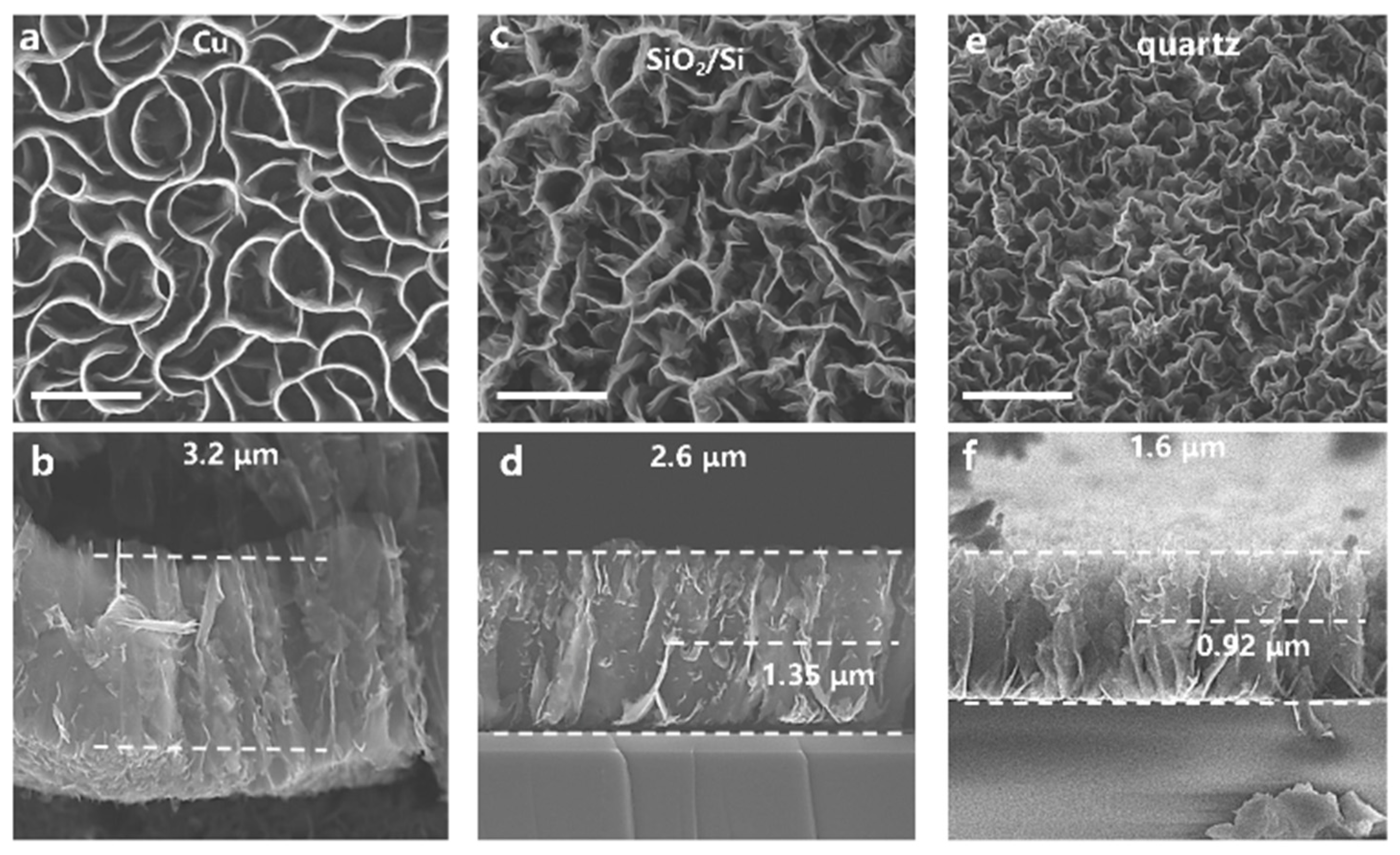
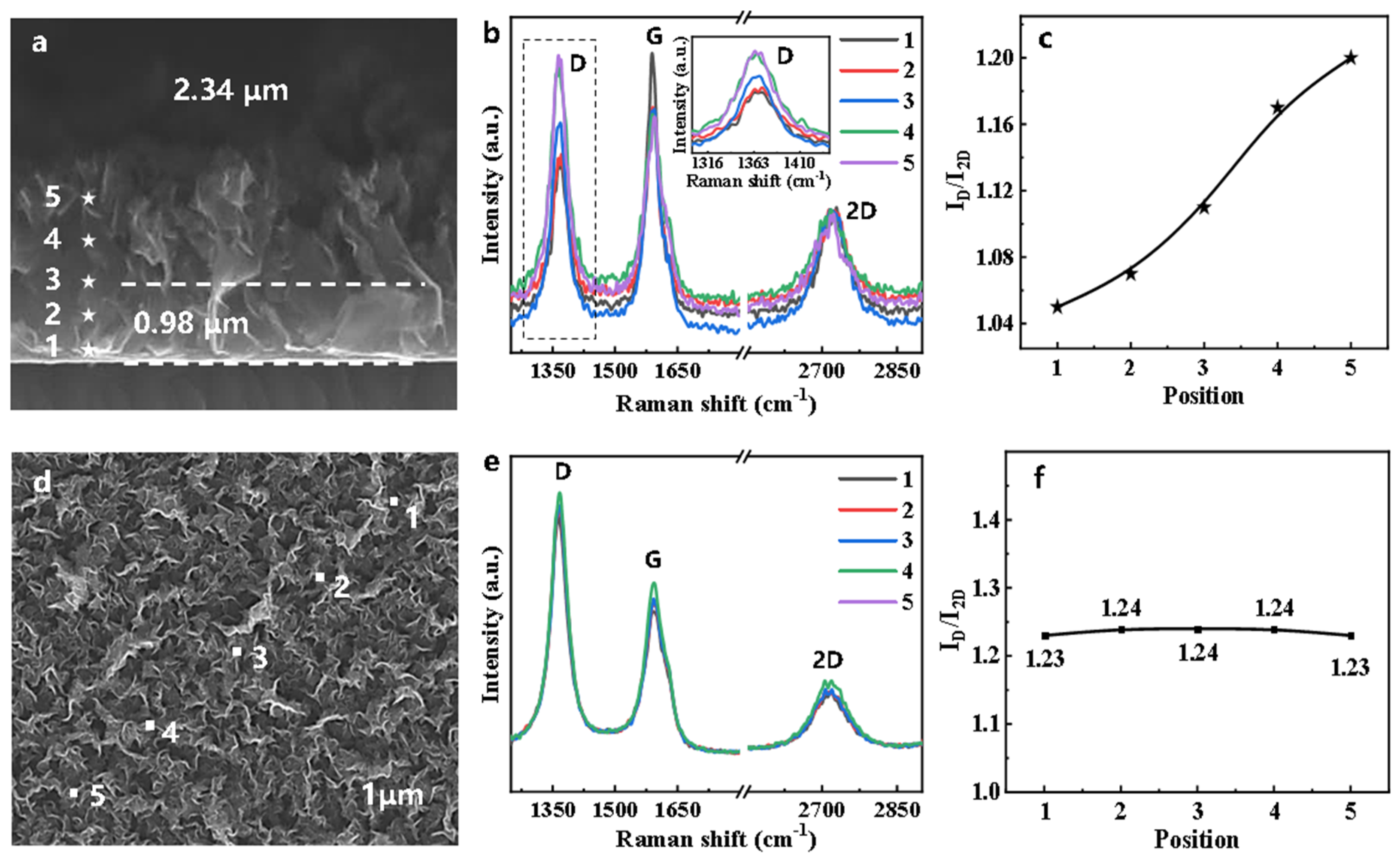
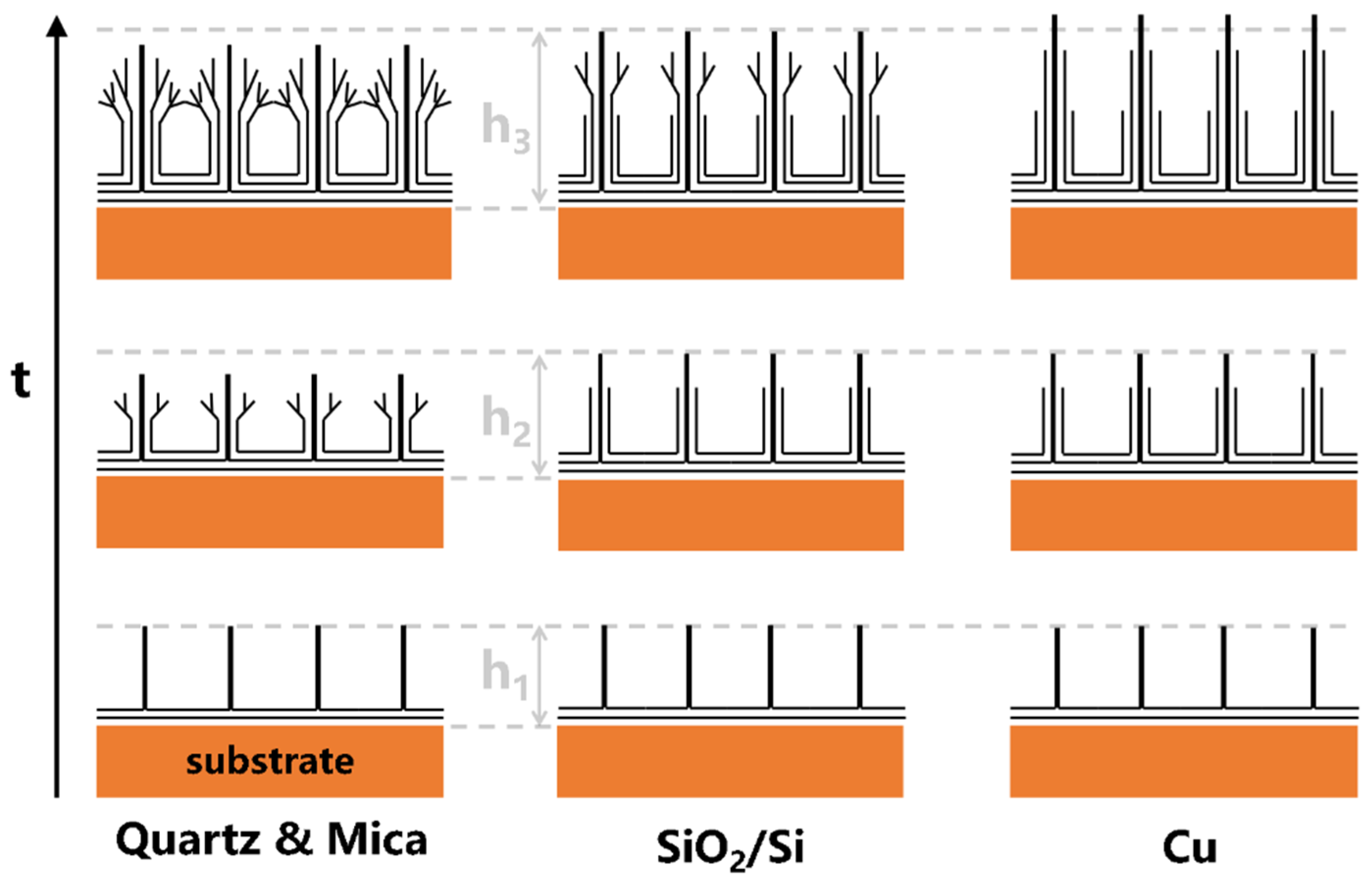
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.Z.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Service, R.F. MATERIALS SCIENCE Carbon Sheets an Atom Thick Give Rise to Graphene Dreams. Science 2009, 324, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, Y.; Song, Y.; Zhang, X.Y.; Ma, Y.F.; Liang, J.J.; Chen, Y.S. Room-Temperature Ferromagnetism of Graphene. Nano Lett. 2009, 9, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Liang, J.X.; Zhou, Y.; Sha, Z.; Lim, S.; Huang, F.; Han, Z.J.; Brown, S.A.; Cao, L.Y.; Wang, D.W.; et al. A vertical graphene enhanced Zn-MnO2 flexible battery towards wearable electronic devices. J. Mater. Chem. A 2021, 9, 575–584. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, S.S.; Zeng, J.; Yu, J. In-situ formation of 3D vertical graphene by carbonizing organic precursor in ammonia. Carbon 2021, 171, 111–118. [Google Scholar] [CrossRef]
- Xie, D.; Xia, X.H.; Zhong, Y.; Wang, Y.D.; Wang, D.H.; Wang, X.L.; Tu, J.P. Exploring Advanced Sandwiched Arrays by Vertical Graphene and N-Doped Carbon for Enhanced Sodium Storage (vol 7, 1601804, 2017). Adv. Energy Mater. 2021, 11. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, X.Y.; Huang, F.; Sha, Z.; Han, Z.J.; Chen, J.Y.; Yang, W.M.; Yu, Y.Y.; Zhang, J.; Peng, S.H.; et al. Hierarchically structured electrodes for moldable supercapacitors by synergistically hybridizing vertical graphene nanosheets and MnO2. Carbon 2021, 172, 272–282. [Google Scholar] [CrossRef]
- Yang, C.Y.; Bi, H.; Wan, D.Y.; Huang, F.Q.; Xie, X.M.; Jiang, M.H. Direct PECVD growth of vertically erected graphene walls on dielectric substrates as excellent multifunctional electrodes. J. Mater. Chem. A 2013, 1, 770–775. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Lee, C.S.; Zhang, W.J. Vertically Aligned Graphene Nanosheet Arrays: Synthesis, Properties and Applications in Electrochemical Energy Conversion and Storage. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.C.; Wang, S.S.; Chen, Z.; Sun, Y.Y.; Gao, Z.F.; Zhang, H.; Zhang, J. Electric-Field-Assisted Growth of Vertical Graphene Arrays and the Application in Thermal Interface Materials. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Miller, J.R.; Outlaw, R.A.; Holloway, B.C. Graphene double-layer capacitor with ac line-filtering performance. Science 2010, 329, 1637–1639. [Google Scholar] [CrossRef] [PubMed]
- Bo, Z.; Mao, S.; Han, Z.J.; Cen, K.; Chen, J.; Ostrikov, K.K. Emerging energy and environmental applications of vertically-oriented graphenes. Chem. Soc. Rev. 2015, 44, 2108–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Peng, S.; Han, Z.J.; Zhu, H.; Wang, C.H. Ultrasensitive and Stretchable Strain Sensors Based on Mazelike Vertical Graphene Network. ACS Appl. Mater. Interfaces 2018, 10, 36312–36322. [Google Scholar] [CrossRef]
- Roy, P.K.; Haider, G.; Chou, T.C.; Chen, K.H.; Chen, L.C.; Chen, Y.F.; Liang, C.T. Ultrasensitive Gas Sensors Based on Vertical Graphene Nanowalls/SiC/Si Heterostructure. ACS Sens. 2019, 4, 406–412. [Google Scholar] [CrossRef]
- Ci, H.; Chang, H.; Wang, R.; Wei, T.; Wang, Y.; Chen, Z.; Sun, Y.; Dou, Z.; Liu, Z.; Li, J.; et al. Enhancement of Heat Dissipation in Ultraviolet Light-Emitting Diodes by a Vertically Oriented Graphene Nanowall Buffer Layer. Adv. Mater. 2019, 31, e1901624. [Google Scholar] [CrossRef]
- Li, M.L.; Liu, D.H.; Wei, D.C.; Song, X.F.; Wei, D.P.; Wee, A.T.S. Controllable Synthesis of Graphene by Plasma-Enhanced Chemical Vapor Deposition and Its Related Applications. Adv. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Chugh, S.; Mehta, R.; Lu, N.; Dios, F.D.; Kim, M.J.; Chen, Z.H. Comparison of graphene growth on arbitrary non-catalytic substrates using low-temperature PECVD. Carbon 2015, 93, 393–399. [Google Scholar] [CrossRef]
- Qi, Y.; Deng, B.; Guo, X.; Chen, S.; Gao, J.; Li, T.; Dou, Z.; Ci, H.; Sun, J.; Chen, Z.; et al. Switching Vertical to Horizontal Graphene Growth Using Faraday Cage-Assisted PECVD Approach for High-Performance Transparent Heating Device. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, S.D.; Lu, Z.Y.; Chen, X.C.; Chen, Q.X.; Gao, P.Q.; Yu, T.B.; Peng, Z.J.; Ye, J.C. Efficient and controllable growth of vertically oriented graphene nanosheets by mesoplasma chemical vapor deposition. Carbon 2019, 147, 341–347. [Google Scholar] [CrossRef]
- Zhao, J.; Shaygan, M.; Eckert, J.; Meyyappan, M.; Rummeli, M.H. A growth mechanism for free-standing vertical graphene. Nano Lett. 2014, 14, 3064–3071. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Wang, J.J.; Holloway, B.C.; Outlaw, R.A.; Zhao, X.; Hou, K.; Shutthanandan, V.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon 2007, 45, 2229–2234. [Google Scholar] [CrossRef]
- Zhang, L.X.; Sun, Z.; Qi, J.L.; Shi, J.M.; Hao, T.D.; Feng, J.C. Understanding the growth mechanism of vertically aligned graphene and control of its wettability. Carbon 2016, 103, 339–345. [Google Scholar] [CrossRef]
- Gromov, D.; Borgardt, N.; Grishina, Y.; Dedkova, A.; Kirilenko, E.; Dubkov, S. Study of growth kinetics of amorphous carbon nanopillars formed by PECVD. In Proceedings of the International Conference on Micro- and Nano-Electronics 2014, Lausanne, Switzerland, 22–26 September 2014. [Google Scholar]
- Wu, Y.H.; Qiao, P.W.; Chong, T.C.; Shen, Z.X. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Al-Hagri, A.; Li, R.; Rajput, N.S.; Lu, J.Y.; Cong, S.; Sloyan, K.; Almahri, M.A.; Tamalampudi, S.R.; Chiesa, M.; Al Ghaferi, A. Direct growth of single-layer terminated vertical graphene array on germanium by plasma enhanced chemical vapor deposition. Carbon 2019, 155, 320–325. [Google Scholar] [CrossRef]
- Yu, K.H.; Wang, P.X.; Lu, G.H.; Chen, K.H.; Bo, Z.; Chen, J.H. Patterning Vertically Oriented Graphene Sheets for Nanodevice Applications. J. Phys. Chem. Lett. 2011, 2, 537–542. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Huang, J.; Zhang, Y.; Cai, W.; Zhang, X. Effect of Substrate Types on the Structure of Vertical Graphene Prepared by Plasma-Enhanced Chemical Vapor Deposition. Nanomaterials 2021, 11, 1268. https://doi.org/10.3390/nano11051268
Xie S, Huang J, Zhang Y, Cai W, Zhang X. Effect of Substrate Types on the Structure of Vertical Graphene Prepared by Plasma-Enhanced Chemical Vapor Deposition. Nanomaterials. 2021; 11(5):1268. https://doi.org/10.3390/nano11051268
Chicago/Turabian StyleXie, Siyi, Junjie Huang, Yufeng Zhang, Weiwei Cai, and Xueao Zhang. 2021. "Effect of Substrate Types on the Structure of Vertical Graphene Prepared by Plasma-Enhanced Chemical Vapor Deposition" Nanomaterials 11, no. 5: 1268. https://doi.org/10.3390/nano11051268




