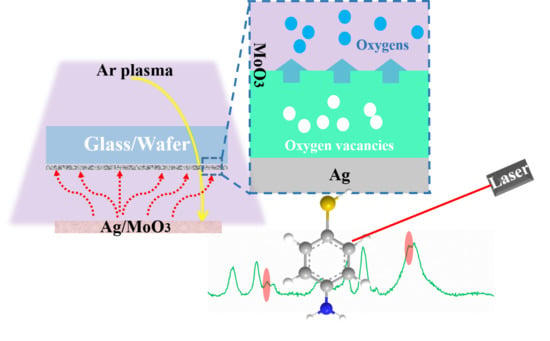
Charge-Transfer Induced by the Oxygen Vacancy Defects in the Ag/MoO3 Composite System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
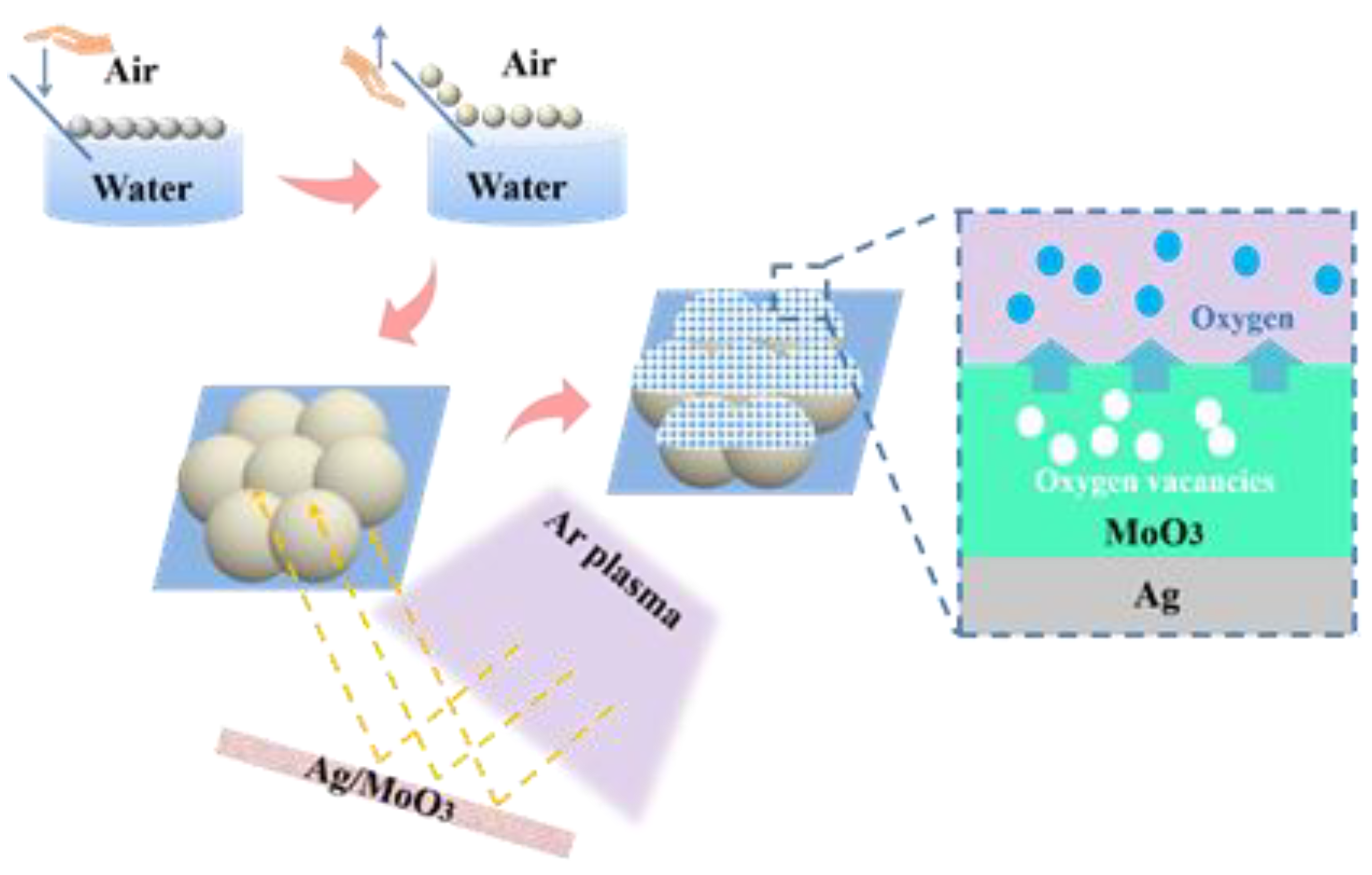
2.2. Preparation of the Ag/MoO3 Two-Dimensional Arrays
2.3. Characterization of the Ag/MoO3 Two-Dimensional Arrays
3. Results
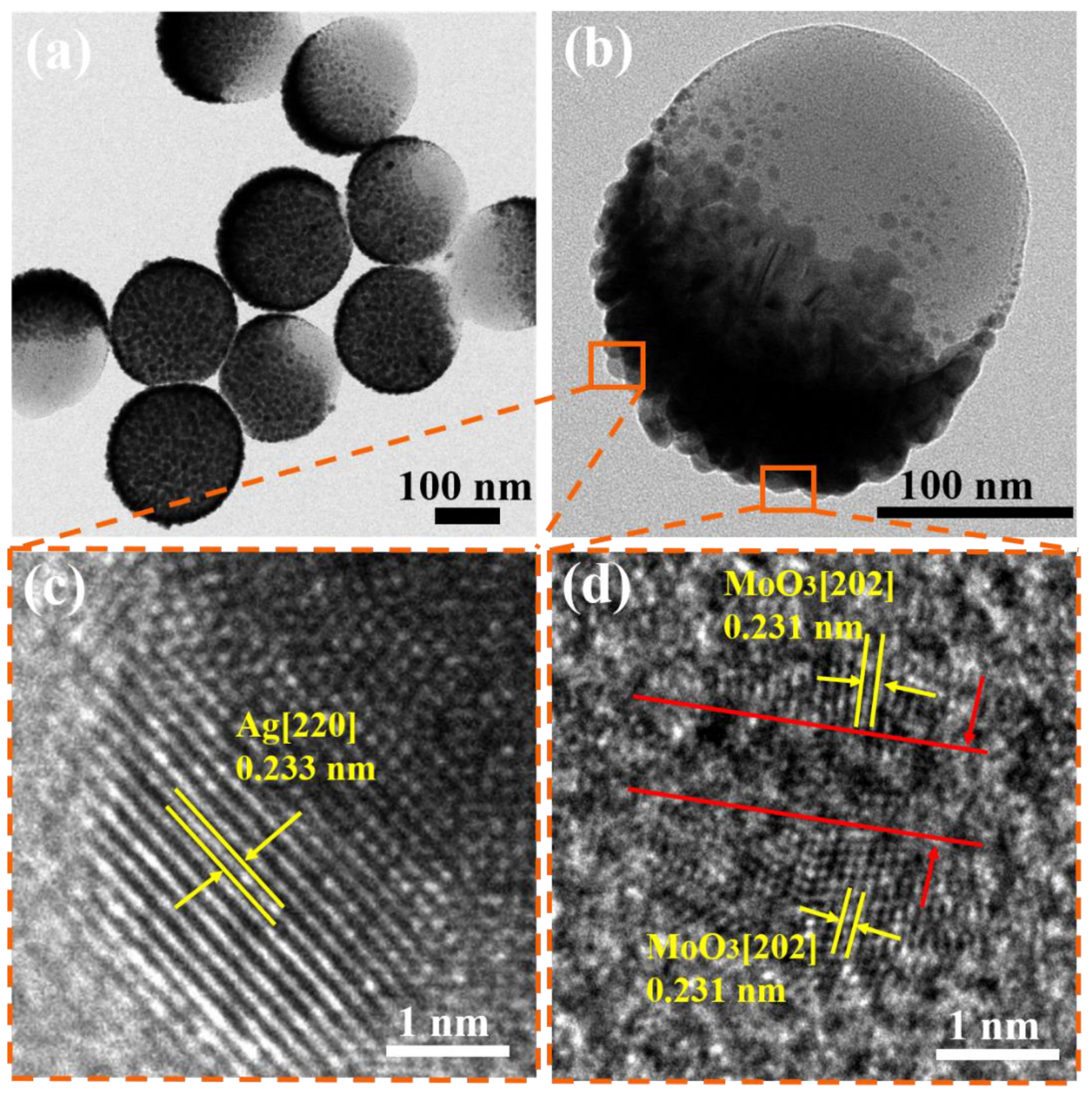
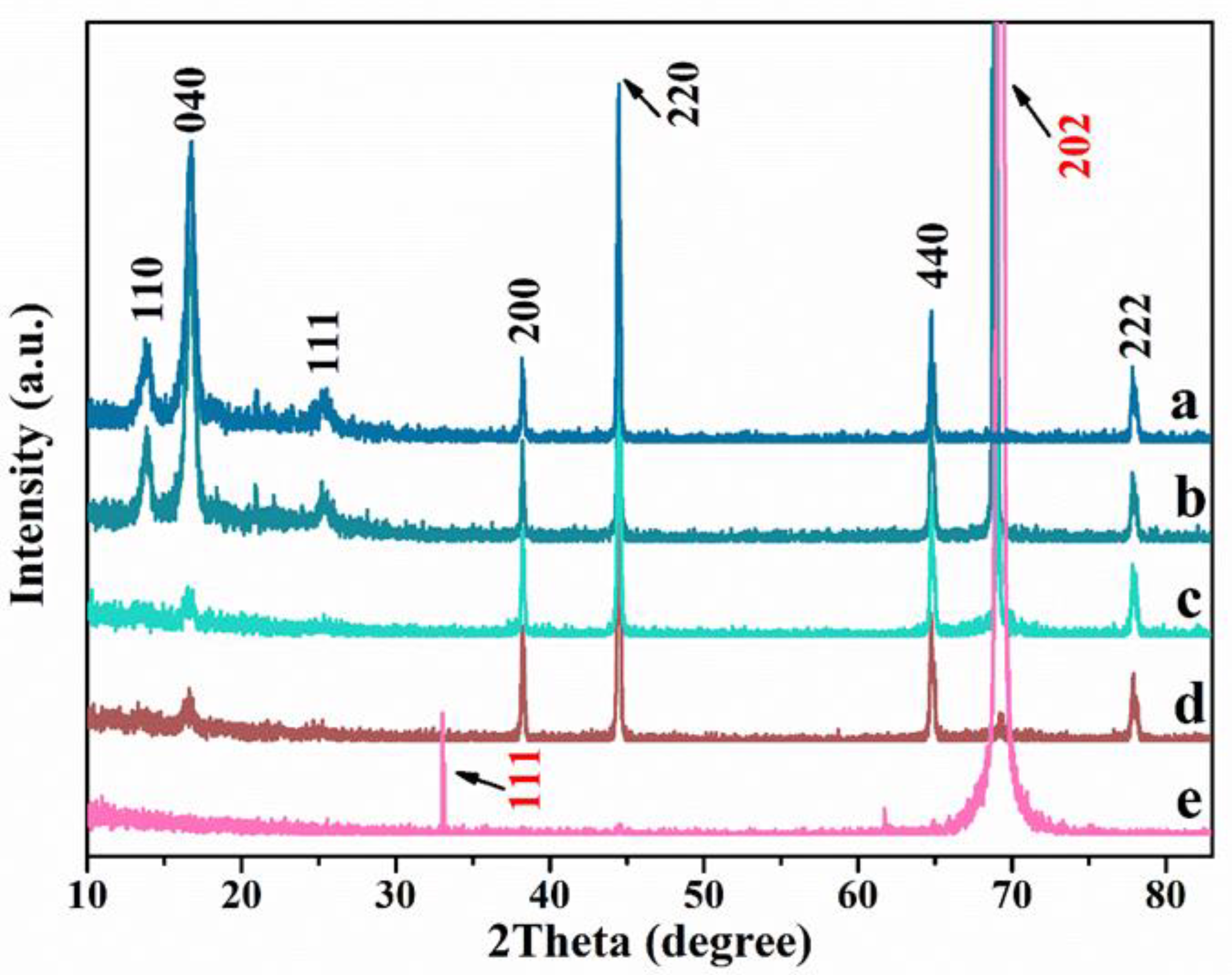
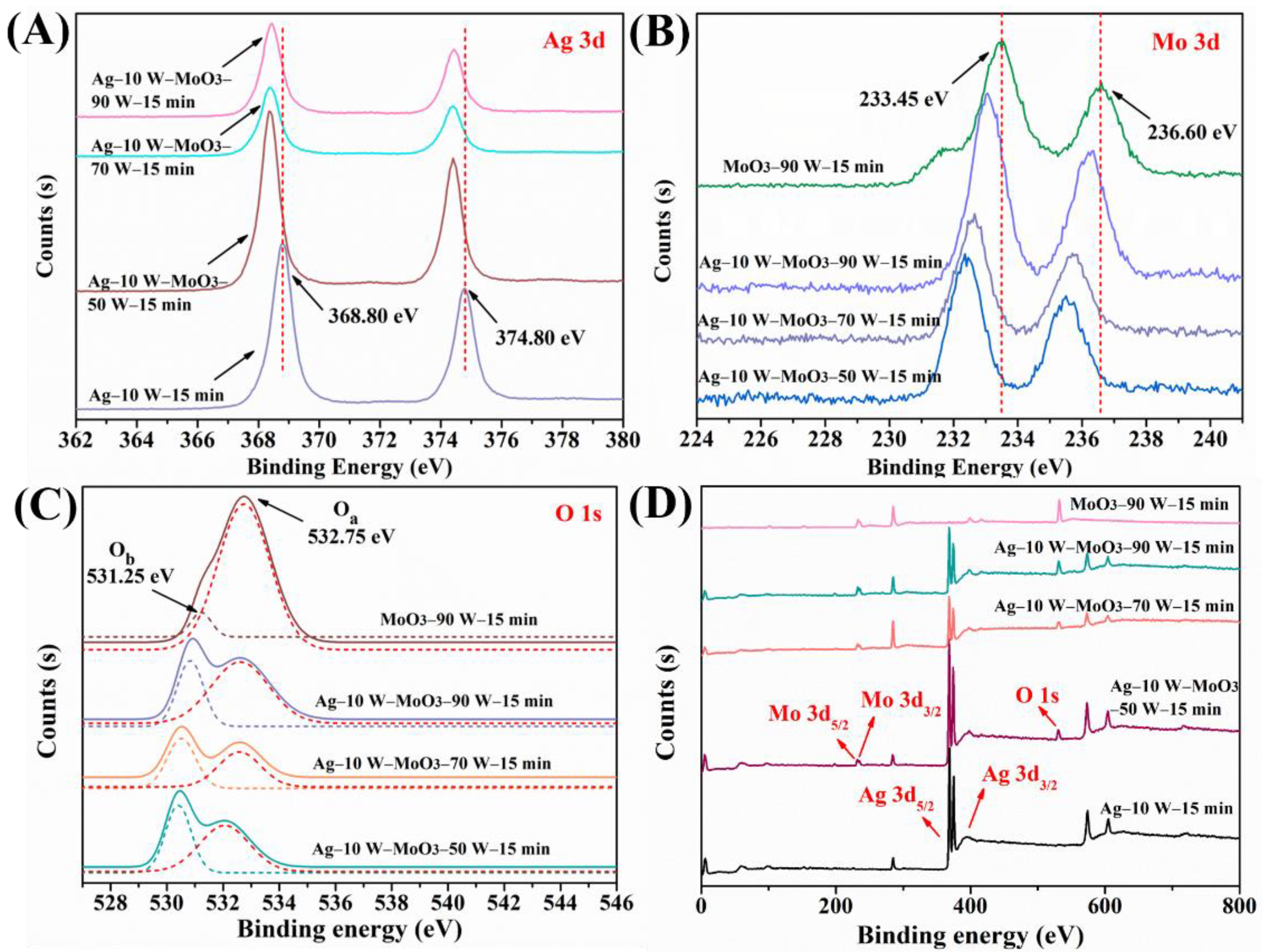
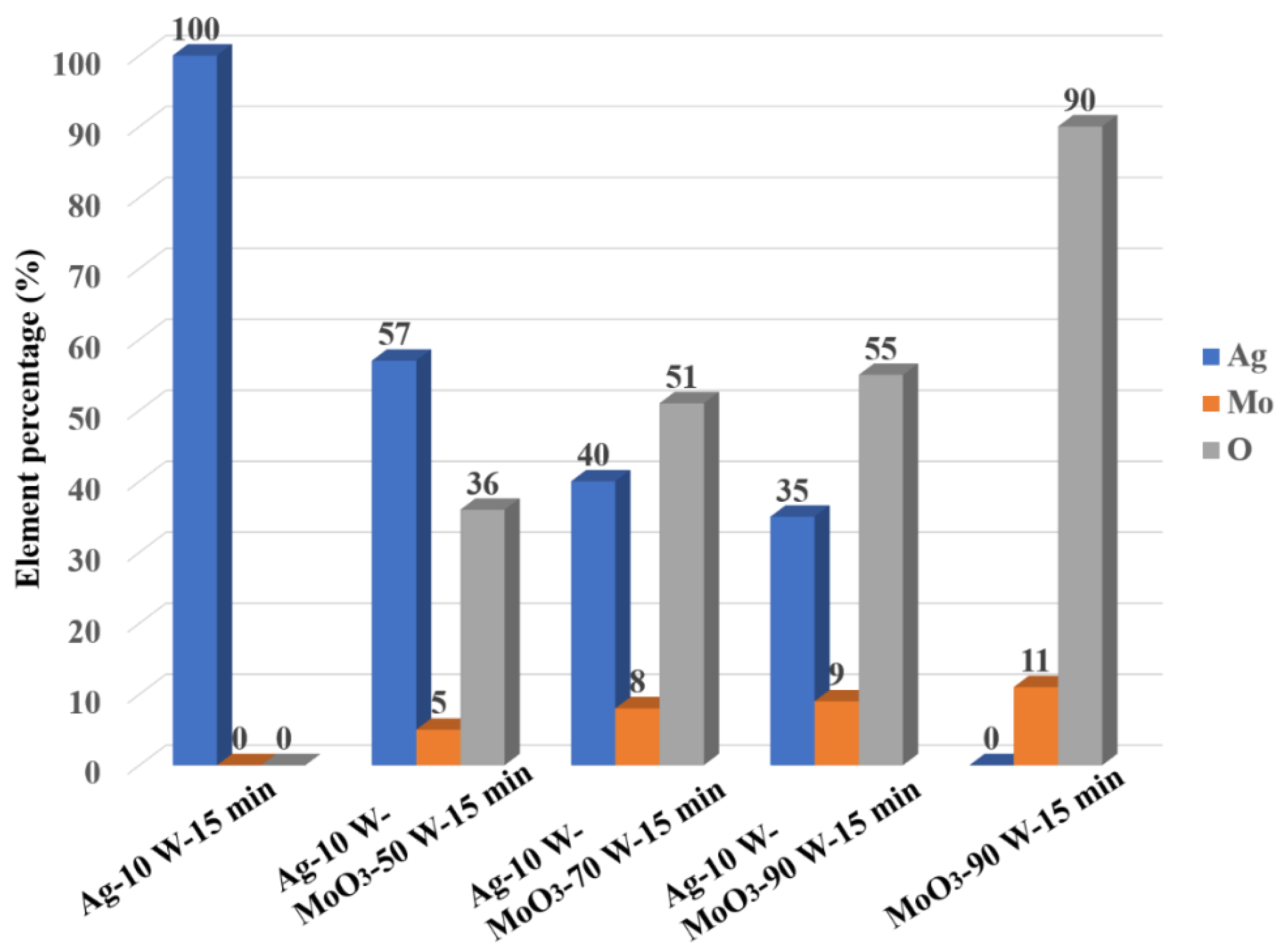
3.1. Characterization of the Ag/MoO3-Coated PS Templates
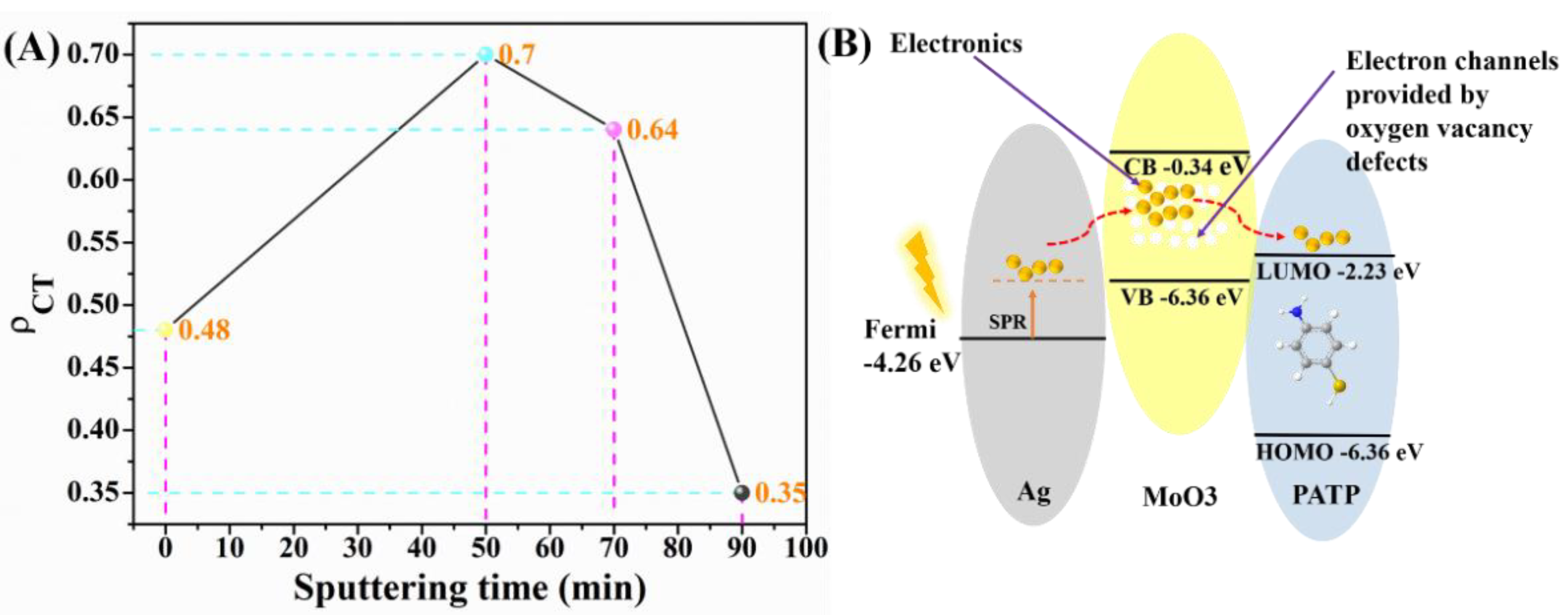
3.2. SERS Characteristics of the Ag/MoO3-Coated PS Templates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Conden. Mat. 2017, 29, 203002. [Google Scholar] [CrossRef]
- Ding, S.Y.; You, E.M.; Tian, Z.Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef]
- Han, B.; Guo, S.; Jin, S.; Park, E.; Xue, X.; Chen, L. Jung, Y.M. Improved charge transfer contribution by cosputtering Ag and ZnO. Nanomaterials 2020, 10, 1455. [Google Scholar] [CrossRef]
- Bernatová, S.; Donato, M.G.; Ježek, J.; Pilát, Z.; Samek, O.; Magazzù, A.; Maragò, O.M.; Zemánek, P.; Gucciardi, P.G. Wavelength-dependent optical force aggregation of gold nanorods for SERS in a microfluidic chip. J. Phys. Chem. C 2019, 9, 5608–5615. [Google Scholar] [CrossRef]
- Yan, X.; Wang, M.; Sun, X.; Wang, Y.; Shi, G.; Ma, W.; Hou, P. Sandwich-like Ag@Cu@CW SERS substrate with tunable nanogaps and component based on the Plasmonic nanonodule structures for sensitive detection crystal violet and 4-aminothiophenol. Appl. Surf. Sci. 2019, 479, 879–886. [Google Scholar] [CrossRef]
- He, L.; Liu, C.; Tang, J.; Zhou, Y.; Yang, H.; Liu, R.; Hu, J. Self-catalytic stabilized Ag-Cu nanoparticles with tailored SERS response for plasmonic photocatalysis. Appl. Surf. Sci. 2018, 434, 265–272. [Google Scholar] [CrossRef]
- Fleischman, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmarie, D.C.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Sun, M.; Xu, H. A novel application of plasmonics: Plasmon-driven surface-catalyzed reactions. Small Mol. 2012, 18, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Cai, L.; Ruan, W.; Bing, Z. Surface-enhanced Raman spectroscopy (SERS): Protein application. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, UK, 2014. [Google Scholar]
- Sadovnikov, A.V.; Gubanov, V.A.; Sheshukova, S.E.; Sharaevskii, Y.P.; Nikitov, S.A. Spin-wave drop filter based on asymmetric side-coupled magnonic crystals. Phys. Rev. Appl. 2018, 9, 051002. [Google Scholar] [CrossRef]
- Sadovnikov, A.V.; Beginin, E.N.; Sheshukova, S.E.; Sharaevskii, Y.P.; Stognij, A.I.; Novitski, N.N.; Sakharov, V.K.; Khivintsev, Y.V.; Nikitov, S.A. Route toward semiconductor magnonics: Light-induced spin-wave nonreciprocity in a YIG/GaAs structure. Phys. Rev. B 2019, 99, 054424. [Google Scholar] [CrossRef]
- Lin, J.; Hao, W.; Shang, Y.; Wang, X.; Qiu, D.; Ma, G.; Chen, C.; Li, S.; Guo, L. Direct experimental observation of facet-dependent SERS of Cu2O polyhedra. Small Mol. 2018, 14, 8. [Google Scholar]
- Wu, H.; Wang, H.; Li, G. Metal oxide semiconductor SERS-active substrates by defect engineering. Analyst 2017, 142, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Xu, J.; Ren, H.; Sun, L.; Xu, Q.C.; Zhang, H.; Li, J.F.; Tian, Z.Q. In situ SERS study of surface plasmon resonance enhanced photocatalytic reactions using bifunctional Au@CdS core-shell nanocomposites. Nanoscale Res. Lett. 2017, 9, 6254–6258. [Google Scholar] [CrossRef]
- Mitsai, E.; Kuchmizhak, A.; Pustovalov, E.; Sergeev, A.; Mironenko, A.; Bratskaya, S.; Linklater, D.P.; Balcytis, A.; Ivanova, E.; Juodkazis, S. Chemically non-perturbing SERS detection of a catalytic reaction with black silicon. Nanoscale Res. Lett. 2018, 10, 9780–9787. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Han, D.; Pang, Z.; Sun, Y.; Wang, Y.; Zhang, Y.; Yang, J.; Chen, L. Charge transfer in an ordered Ag/Cu2S/4-MBA system based on surface-enhanced raman scattering. J. Phys. Chem. C 2018, 122, 5599–5605. [Google Scholar] [CrossRef]
- Su, S.; Zhang, C.; Yuwen, L.; Chao, J.; Zuo, X.; Liu, X.; Song, C.; Fan, C.; Wang, L. Creating SERS hot spots on MoS2 nanosheets with in situ grown gold nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 18735–18741. [Google Scholar] [CrossRef]
- Ye, J.; Hutchison, J.A.; Uji-i, H.; Hofkens, J.; Lagae, L.; Maes, G.; Borghs, G.; Van Dorpe, P. Excitation wavelength dependent surface enhanced Raman scattering of 4-aminothiophenol on gold nanorings. Nanoscale Res. Lett. 2012, 4, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ray, C.; Sarkar, S.; Pradhan, M.; Negishi, Y.; Pal, T. Silver nanoparticle decorated reduced graphene oxide (rGO) nanosheet: A platform for SERS based low-level detection of uranyl ion. ACS Appl. Mater. Interfaces 2013, 5, 8724–8732. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, H.; Ren, X.; Tang, J.; Li, Y.; Zhang, X.; Cheng, Z. Au-ZnO hybrid nanoparticles exhibiting strong charge-transfer-induced SERS for recyclable SERS-active substrates. Nanoscale Res. Lett. 2015, 7, 5147–5151. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sang, Q.; Du, J. A Ag synchronously deposited and doped TiO2 hybrid as an ultrasensitive SERS substrate: A multifunctional platform for SERS detection and photocatalytic degradation. Phys. Chem. Chem. Phys. 2018, 20, 15149–15157. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Kitahama, Y.; Xue, X. Generation of pronounced resonance profile of charge-transfer contributions to surface-enhanced raman scattering. J. Phys. Chem. C 2012, 116, 2515–2520. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, X.; Yin, D. Recyclable Au-TiO2 nanocomposite SERS-active substrates contributed by synergistic charge transfer effect. Phys. Chem. Chem. Phys. 2017, 19, 11212–11219. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3-x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Lee, Y.J.; Seo, Y.I.; Kim, S.H.; Kim, D.G.; Kim, Y.D. Optical properties of molybdenum oxide thin films deposited by chemical vapor transport of MoO3(OH)2. Appl. Phys. A 2009, 97, 237–241. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, S.; Ye, K.; Hu, W.; Zhang, J.; Niu, X.; Lin, Y.; Chen, S.; Song, L.; Zhang, Q.; et al. Hydrogen-doping-induced metal-like ultrahigh free-carrier concentration in metal-oxide material for giant and tunable plasmon resonance. Adv. Mater. 2020, 50, 2004059. [Google Scholar] [CrossRef]
- Prabhu, B.R.; Bramhaiah, K.; Singh, K.K.; John, N.S. Single sea urchin–MoO3 nanostructure for surface enhanced Raman spectroscopy of dyes. Nanoscale 2019, 1, 2426–2434. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Liang, P.; Zhang, X.; Zhang, D.; Shu, H.; Huang, J.; Yu, Z.; Xu, Y. Synergistic enhancement effect of MoO3@Ag hybrid nanostructures for boosting selective detection sensitivity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118611. [Google Scholar] [CrossRef]
- Chu, Q.; Han, B.; Jin, Y.; Guo, S.; Jin, S.; Park, E.; Chen, L.; Jung, Y.M. Surface plasmon resonance induced charge transfer effect on the Ag-ZnSe-PATP system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119167. [Google Scholar] [CrossRef]
- Yang, L.; Yin, D.; Shen, Y.; Yang, M.; Li, X.; Han, X.; Jiang, X.; Zhao, B. Highly-dispersed TiO2 nanoparticles with abundant active sites induced by surfactants as a prominent substrate for SERS: Charge transfer contribution. Phys. Chem. Chem. Phys. 2017, 19, 22302–22308. [Google Scholar] [CrossRef]
- Ding, S.Y.; Yi, J.; Li, J.F.; Ren, B.; Wu, D.Y.; Panneerselvam, R.; Tian, Z.Q. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016, 1, 6. [Google Scholar] [CrossRef]
- Yan, X.; Li, Y.; Zhao, J.; Li, Y.; Bai, G.; Zhu, S. Roles of grain boundary and oxygen vacancies in Ba0.6Sr0.4TiO3 films for resistive switching device application. Appl. Phys. Lett. 2016, 108, 033108. [Google Scholar] [CrossRef]
- Mao, Y.; Li, W.; Sun, X.; Ma, Y.; Xia, J.; Zhao, Y.; Lu, X.; Gan, J.; Liu, Z.; Chen, J.; et al. Room-temperature ferromagnetism in hierarchically branched MoO3 nanostructures. CrystEngComm 2012, 4, 1419–1424. [Google Scholar] [CrossRef]
- Niu, Z.; Zhou, C.; Wang, J.; Xu, Y.; Gu, C.; Jiang, T.; Zeng, S.; Zhang, Y.; Ang, D.S.; Zhou, J. UV-light-assisted preparation of MoO3−x/Ag NPs film and investigation on the SERS performance. Asian J. Mater. Sci. 2020, 55, 8868–8880. [Google Scholar] [CrossRef]
- Cortie, M.; Ford, M. A plasmon-induced current loop in gold semi-shells. Nanotechnology 2007, 23, 235704. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, B.; Ren, Y.; Wong, L.M.; Kong, L.; Tok, E.S.; Chim, W.K.; Chiam, S.Y. Detrimental effects of oxygen vacancies in electrochromic molybdenum oxide. J. Phys. Chem. C 2015, 119, 10592–10601. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Dewangan, K.; Gajbhiye, N.S. Synthesis and room temperature d0 ferromagnetic properties of α-MoO3 nanofibers. J. Mater. Sci. Technol. 2015, 31, 453–457. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A unified approach to surface-enhanced Raman spectroscopy. J. Phys. Chem. C 2008, 112, 5605–5617. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Xu, H.; Sun, M. Ascertaining p,p’-dimercaptoazobenzene produced from p-aminothiophenol by selective catalytic coupling reaction on silver nanoparticles. Langmuir 2010, 26, 7737–7746. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, M.; Zhao, L.B.; Feng, J.M.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Activation of oxygen on gold and silver nanoparticles assisted by surface plasmon resonances. Angew. Chem. Int. Ed. 2014, 53, 2353–2357. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Liu, X.M.; Huang, Y.F.; Ren, B.; Xu, X.; Tian, Z.Q. Surface catalytic coupling reaction of p-Mercaptoaniline linking to silver nanostructures responsible for abnormal SERS enhancement: A DTF study. J. Phys. Chem. C 2019, 113, 18212–18222. [Google Scholar] [CrossRef]


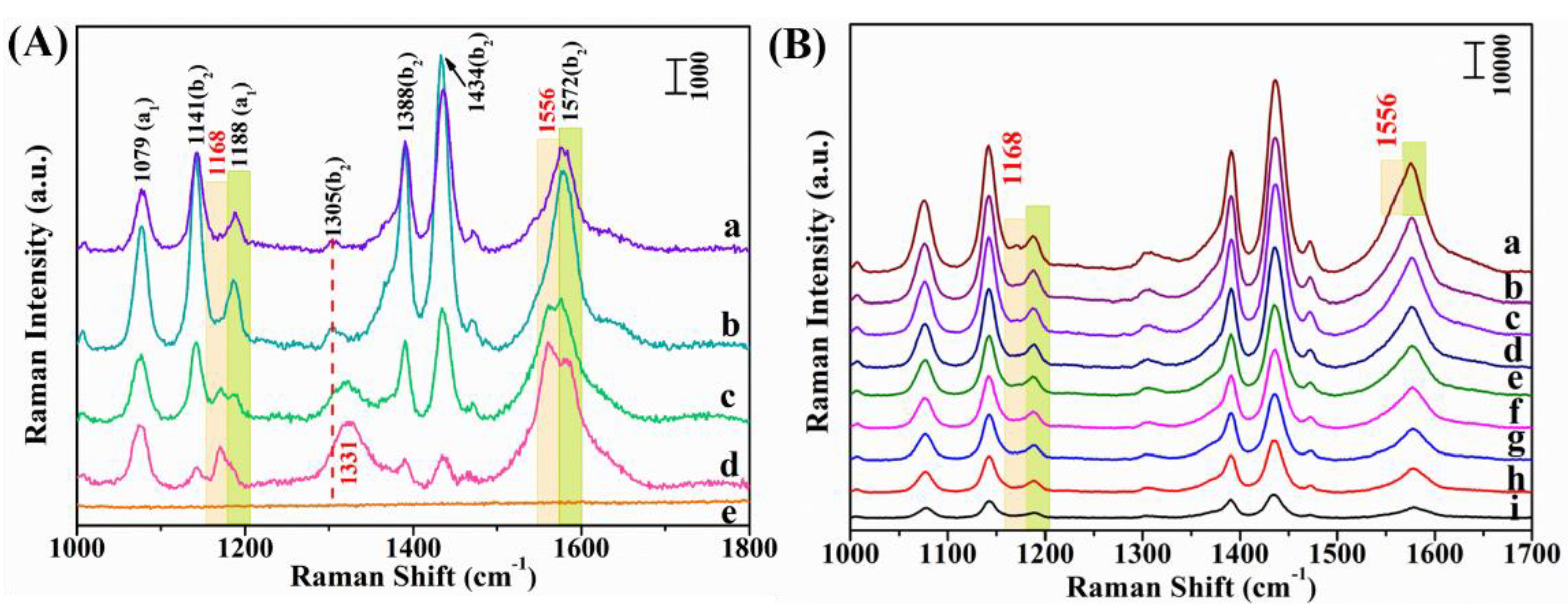
| Raman Shift/cm−1 | Band Assignments |
|---|---|
| 1079 | υ(CS) + υ(CS), 7a(a1) |
| 1141 | β(CH), 9b(b2) |
| 1168 | β(CH), 9a(a1) |
| 1188 | β(CH), 9a(a1) |
| 1305 | υ(CC), 15(b2) |
| 1331 | β(CH), 14(b2) |
| 1388 | β(CH), 14(b2) |
| 1434 | υ(CC) + β(CH), 19b(b2) |
| 1556 | υ(CC), 8(b2) |
| 1572 | υ(CC), 8(b2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Q.; Li, J.; Jin, S.; Guo, S.; Park, E.; Wang, J.; Chen, L.; Jung, Y.M. Charge-Transfer Induced by the Oxygen Vacancy Defects in the Ag/MoO3 Composite System. Nanomaterials 2021, 11, 1292. https://doi.org/10.3390/nano11051292
Chu Q, Li J, Jin S, Guo S, Park E, Wang J, Chen L, Jung YM. Charge-Transfer Induced by the Oxygen Vacancy Defects in the Ag/MoO3 Composite System. Nanomaterials. 2021; 11(5):1292. https://doi.org/10.3390/nano11051292
Chicago/Turabian StyleChu, Qi, Jingmeng Li, Sila Jin, Shuang Guo, Eungyeong Park, Jiku Wang, Lei Chen, and Young Mee Jung. 2021. "Charge-Transfer Induced by the Oxygen Vacancy Defects in the Ag/MoO3 Composite System" Nanomaterials 11, no. 5: 1292. https://doi.org/10.3390/nano11051292
APA StyleChu, Q., Li, J., Jin, S., Guo, S., Park, E., Wang, J., Chen, L., & Jung, Y. M. (2021). Charge-Transfer Induced by the Oxygen Vacancy Defects in the Ag/MoO3 Composite System. Nanomaterials, 11(5), 1292. https://doi.org/10.3390/nano11051292