Optimization of Hybrid Ink Formulation and IPL Sintering Process for Ink-Jet 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Schematic of Experimental Method
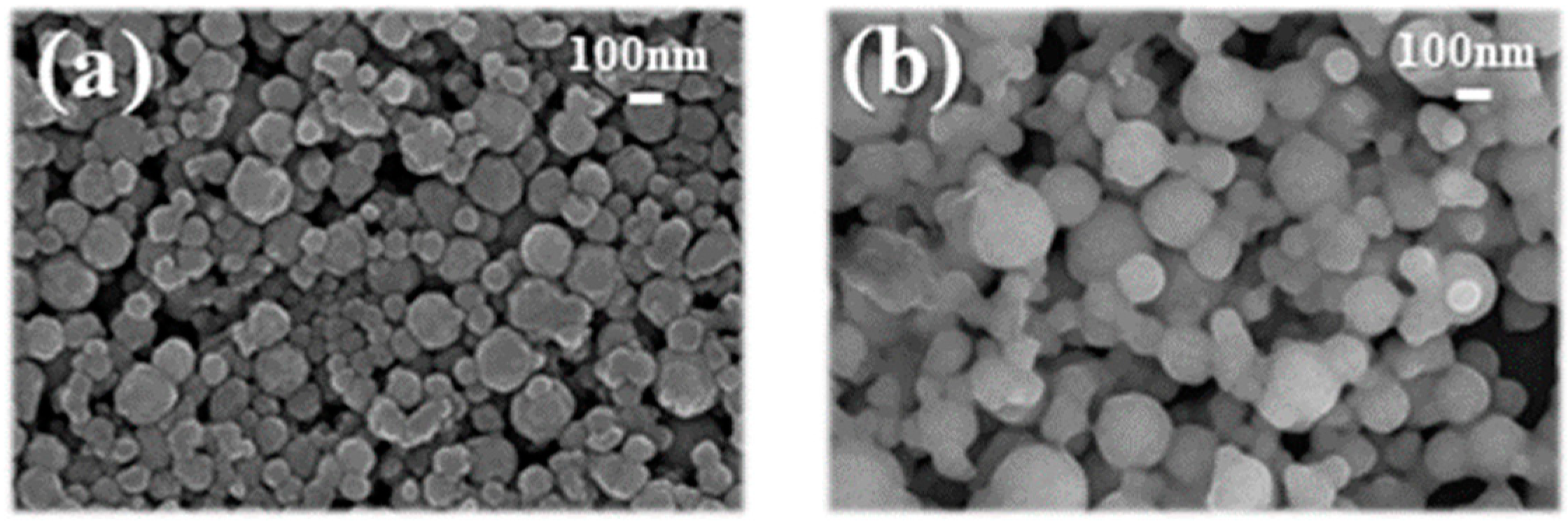
2.2. Synthesis of Photo-Curable Nano SiO2 Ink and IPL-Sinterable Nano Cu Ink
2.3. Multi-Material Ink-Jet 3D Printing
2.4. The Intense Pulsed Light Sintering Process
3. Results and Discussion
3.1. Rheological Properties of Photo-Curable Nano SiO2 Ink
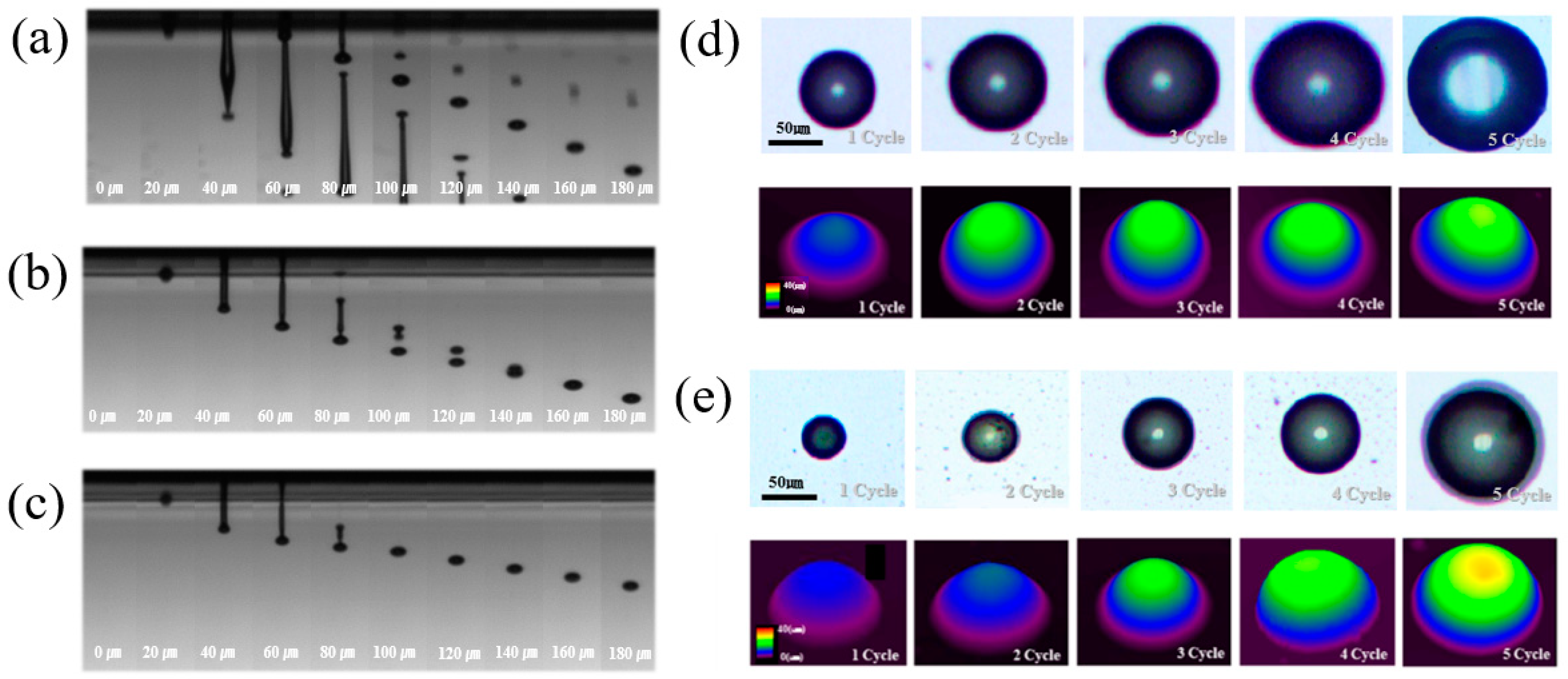
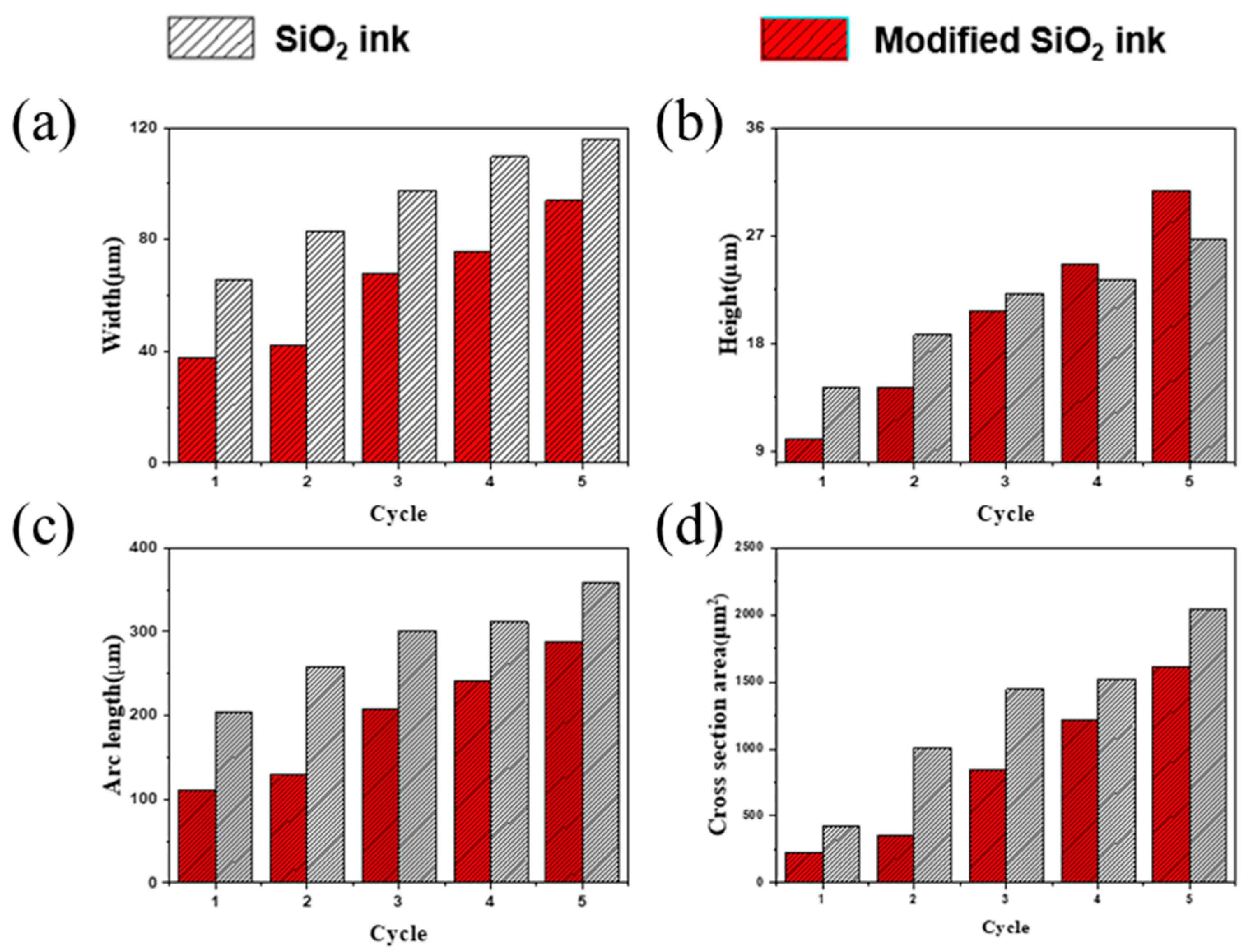
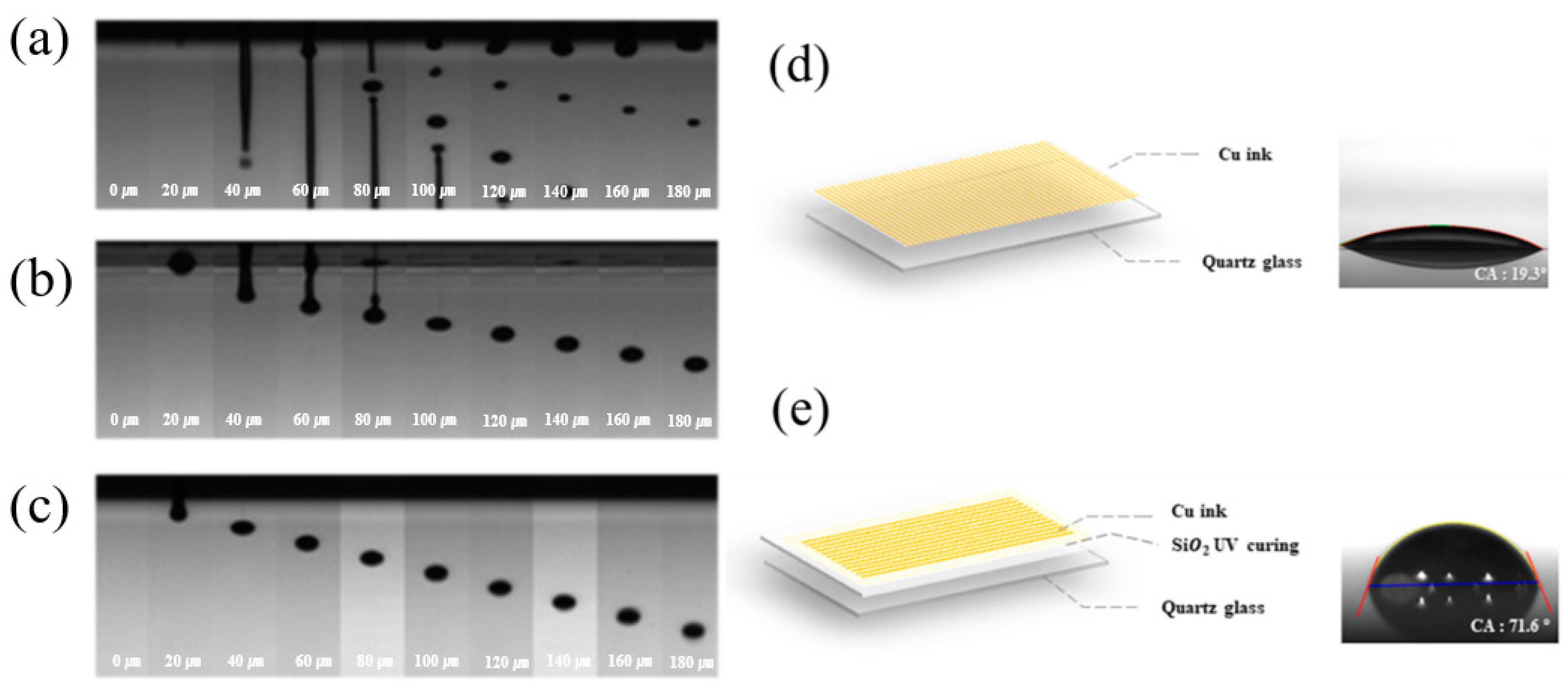
3.2. Ink-Jet 3D Printability of Photo-Curable Nano SiO2 Ink
3.3. Characterization of Ink-Jet 3D Printed Insulation Structure with Photo-Cured Nano SiO2
3.4. Rheological Properties and Printability of IPL-Sinterable Nano Cu Ink
3.5. Optimization of Annealing Temperature with IPL-Sinterable Nano Cu Ink
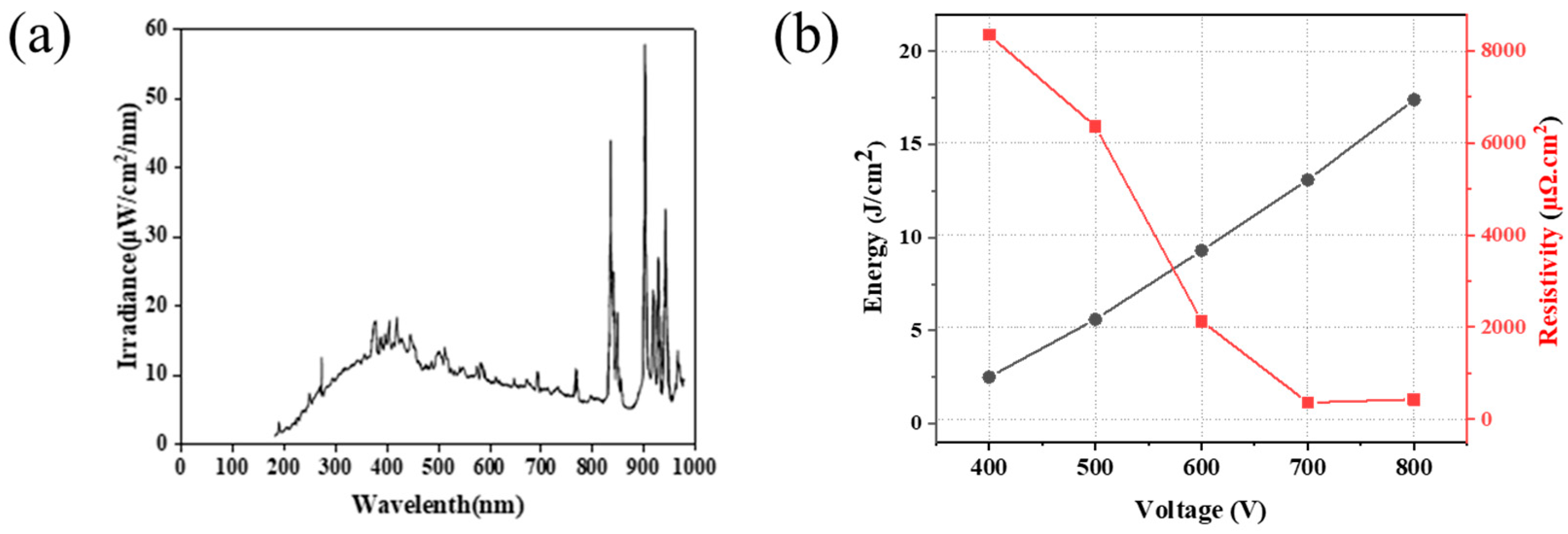
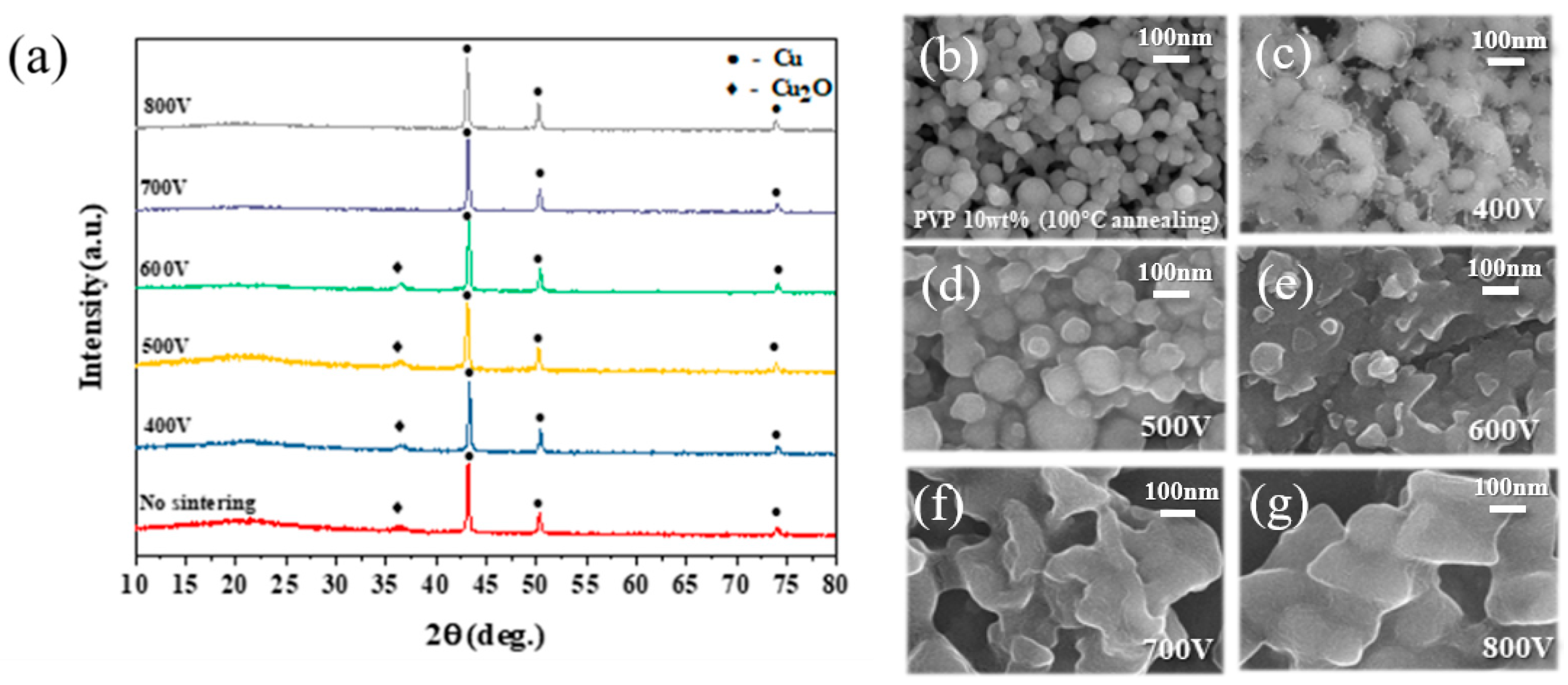
3.6. The Effect of Applied IPL Voltage to Sintering of Cu Conductive Layer
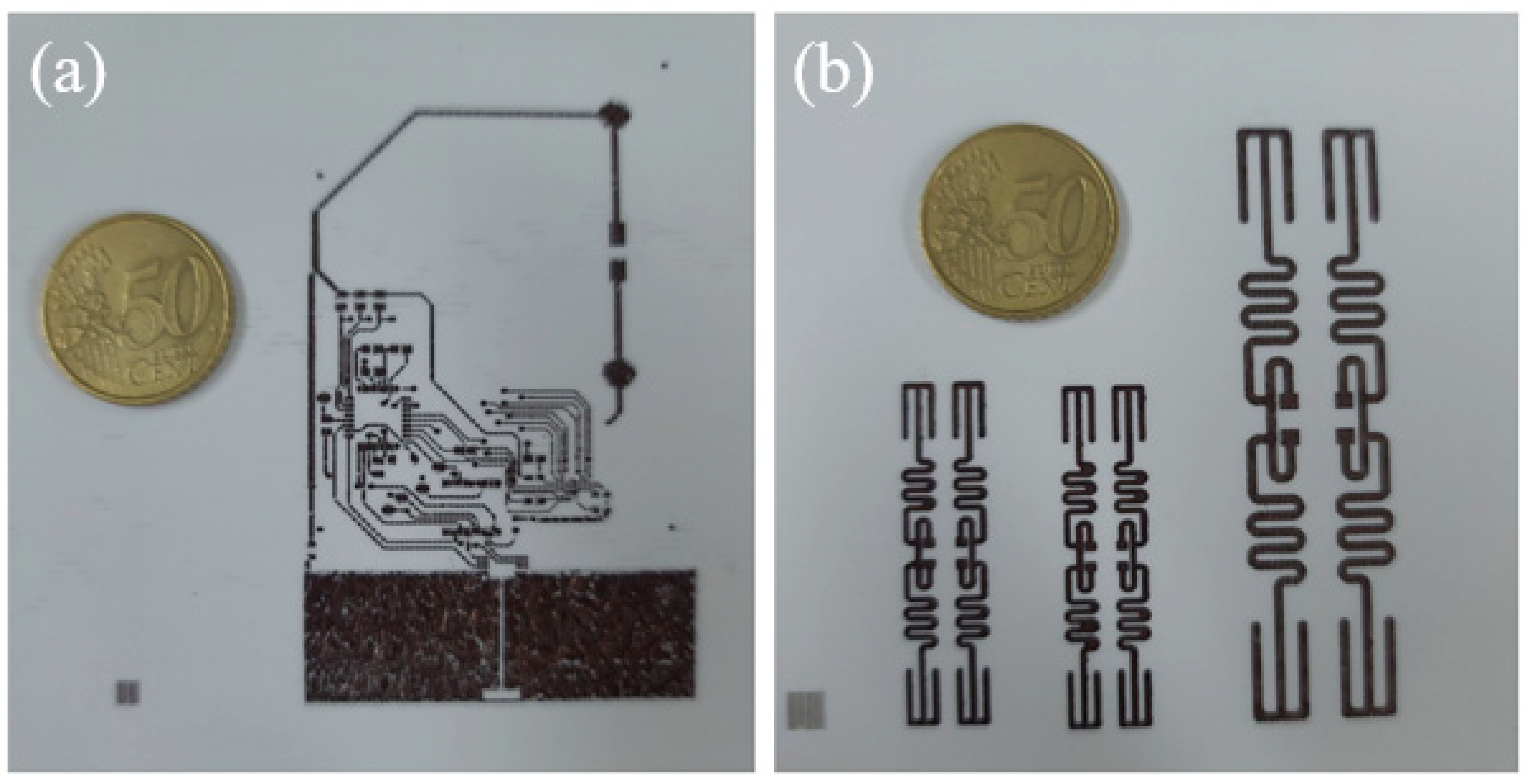
3.7. Electrical and Mechanical Properties of the Ink-Jet 3D Complex Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunders, R.E.; Gough, J.E.; Derby, B. Delivery of human fibroblast cells by piezoelectric drop-on-demand ink-jet printing. Biomaterials 2008, 29, 193–203. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, H.; Shu, X.; Xiao, J.; Cao, S. Multi-materials drop-on-demand ink-jet technology based on pneumatic diaphragm actuator. Sci. China Technol. Sci. 2010, 53, 1605–1611. [Google Scholar] [CrossRef]
- Kang, H.; Sowade, E.; Baumann, R.R. Direct intense pulsed light sintering of inkjet-printed copper oxide layers within six milliseconds. ACS Appl. Mater. Interfaces 2014, 6, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Kamran, M. A Comprehensive Study on 3D Printing Technology. MIT Int. J. Mech. Eng. 2016, 6, 63–69. [Google Scholar]
- Brünahl, J.; Grishin, A.M. Piezoelectric shear mode drop-on-demand ink-jet actuator. Sens. Actuators A Phys. 2002, 101, 371. [Google Scholar] [CrossRef]
- Chen, A.U.; Basaran, O.A. A new method for significantly reducing drop radius without reducing nozzle radius in drop-on-demand drop production. Phys. Fluids 2002, 14, L1. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- MacDonald, E.; Wicker, R. Multiprocess 3D printing for increasing component functionality. Science 2016, 353, 6307. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar]
- Park, J.Y.; Hirata, Y.; Hamada, K. Relationship between the dye/additive interaction and inkjet ink droplet formation. Dyes Pigment. 2012, 95, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Saleh, E.; Woolliams, P.; Clarke, B.; Gregory, A.; Greedy, S.; Smartt, C.; Tuck, C. 3D inkjet-printed UV-curable inks for multi-functional electromagnetic applications. Addit. Manuf. 2017, 13, 143–148. [Google Scholar] [CrossRef]
- Leigh, S.J.; Bradley, R.J.; Purssell, C.P.; Billson, D.R.; Hutchins, D.A. A simple, low-cost conductive composite material for 3D printing of electronic sensors. PLoS ONE 2012, 7, e49365. [Google Scholar] [CrossRef]
- Woo, K.; Kim, D.; Kim, J.S.; Lim, S.; Moon, J. Ink-Jet printing of Cu–Ag-based highly conductive tracks on a transparent substrate. Langmuir 2008, 25, 429–433. [Google Scholar] [CrossRef]
- Han, W.S.; Hong, J.M.; Kim, H.S.; Song, Y.W. Multi-pulsed white light sintering of printed Cu nano inks. Nanotechnology 2011, 22, 395705. [Google Scholar] [CrossRef] [PubMed]
- Nayak, L.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. A review on inkjet printing of nanoparticle inks for flexible electron-ics. J. Mater. Chem. C 2019, 7, 8771–8795. [Google Scholar] [CrossRef]
- Perelaer, J.; De Gans, B.J.; Schubert, U.S. Ink-jet printing and microwave sintering of conductive silver tracks. Adv. Mater. 2006, 18, 2101–2104. [Google Scholar] [CrossRef]
- Gardini, D.; Blosi, M.; Zanelli, C.; Dondi, M. Ceramic ink-jet printing for digital decoration: Physical constraints for ink design. J. Nanosci. Nanotechnol. 2015, 15, 3552–3561. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Lu, W.; Zhang, Y.; Lin, G.; Wei, T.; Jiang, L. Gold nanoparticle ink suitable for electric-conductive pattern fabrication using in ink-jet printing technology. Colloids Surf. 2010, 358, 35–41. [Google Scholar] [CrossRef]
- Chung, W.Y.; Lai, Y.C.; Yonezawa, T.; Liao, Y.C. Sintering Copper Nanoparticles with Photonic Additive for Printed Conductive Patterns by Intense Pulsed Light. Nanomaterials 2019, 9, 1071. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.Y.; Yoo, T.H.; Song, Y.W.; Lim, D.S.; Oh, Y.J. Cu ion ink for a flexible substrate and highly conductive patterning by intensive pulsed light sintering. ACS Appl. Mater. Interfaces 2013, 5, 4113–4119. [Google Scholar] [CrossRef] [PubMed]
- Niittynen, J.; Sowade, E.; Kang, H.; Baumann, R.R.; Mäntysalo, M. Comparison of laser and intense pulsed light sintering (IPL) for inkjet-printed copper nanoparticle layers. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, H.S.; Hahn, H.T. Reactive sintering of copper nanoparticles using intense pulsed light for printed electronics. J. Electron. Mater. 2011, 40, 42–50. [Google Scholar] [CrossRef]
- Song, S.K.; Kim, J.H.; Hwang, K.S.; Ha, K.R. Spectroscopic analysis of silica nanoparticles modified with silane coupling agent. Korean Chem. Soc. 2011, 49, 181. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.H.; Lim, S.Y. Precise size control of inkjet-printed droplets on a flexible polymer substrate using plasma surface treatment. J. Micromech. Microeng. 2009, 20, 015030. [Google Scholar] [CrossRef]
- Guo, Y.; Patanwala, H.S.; Bognet, B.; Ma, A.W. Inkjet and inkjet-based 3D printing: Connecting fluid properties and printing performance. Rapid Prototyp. J. 2017, 23, 562. [Google Scholar] [CrossRef]
- Chen, K.; Fu, Q.; Ye, S.; Ge, J. Multicolor printing using electric field-responsive and photocurable photonic crystals. Adv. Funct. Mater. 2017, 27, 1702825. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Y.; Zhou, Y.; Gao, W.; Zhao, H.; Wang, W. Preparation of superhydrophobic nanocomposite fiber membranes by electrospinning poly (vinylidene fluoride)/silane coupling agent modified SiO2 nanoparticles. J. Appl. Polym. Sci. 2017, 134, 13. [Google Scholar] [CrossRef]
- Son, Y.; Kim, C.; Yang, D.H.; Ahn, D.J. Spreading of an ink-jet droplet on a solid surface with a controlled contact angle at low Weber and Reynolds numbers. Langmuir 2008, 24, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Derby, B. Experimental study of the parameters for stable drop-on-demand inkjet performance. Phys. Fluids 2019, 31, 032004. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.C.; Halloran, J.W. Photopolymerization monitoring of ceramic stereolithography resins by FTIR methods. J. Mater. Sci. 2005, 40, 71–76. [Google Scholar] [CrossRef]
- Halloran, J.W. Ceramic stereolithography: Additive manufacturing for ceramics by photopolymerization. Annu. Rev. Mater. Res. 2016, 46, 19. [Google Scholar] [CrossRef]
- Joo, M.; Lee, B.; Jeong, S.; Lee, M. Laser sintering of Cu paste film printed on polyimide substrate. Appl. Surf. Sci. 2011, 258, 521–524. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choa, Y.H. Adhesion enhancement of ink-jet printed conductive copper patterns on a flexible substrate. J. Mater. Chem. 2012, 22, 12517–12522. [Google Scholar] [CrossRef]
- Bidoki, S.M.; Lewis, D.M.; Clark, M.; Vakorov, A.; Millner, P.A.; McGorman, D. Ink-jet fabrication of electronic components. J. Micromech. Microeng. 2007, 17, 967. [Google Scholar] [CrossRef]
- Hossain, S.Z.; Luckham, R.E.; Smith, A.M.; Lebert, J.M.; Davies, L.M.; Pelton, R.H.; Brennan, J.D. Development of a bioactive paper sensor for detection of neurotoxins using piezoelectric ink-jet printing of sol gel-derived bioinks. Anal. Chem. 2009, 81, 5474–5483. [Google Scholar] [CrossRef]
- Wu, C.H.; Hwang, W.S. The effect of the echo-time of a bipolar pulse waveform on molten metallic droplet formation by squeeze mode piezoelectric inkjet printing. Microelectron. Reliab. 2015, 55, 630–636. [Google Scholar] [CrossRef]
- Reis, N.; Ainsley, C.; Derby, B. Ink-jet delivery of particle suspensions by piezoelectric droplet ejectors. J. Appl. Phys. 2005, 97, 094903. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395. [Google Scholar] [CrossRef]
- Jianfeng, Y.; Guisheng, Z.; Anming, H.; Zhou, Y.N. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2011, 21, 15981. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.R.; Joo, S.J.; Chu, J.H.; Uhm, H.J.; Park, J.W.; Ryu, C.H.; Kim, H.S. A Review on Intense Pulsed Light Sintering Technologies for Conductive Electrodes in Printed Electronics. Int. J. Precis. Eng. Manuf.-Green Technol. 2020, 8, 1–37. [Google Scholar] [CrossRef]
- Soltman, D.; Subramanian, V. Inkjet-printed line morphologies and temperature control of the coffee ring effect. Langmuir 2008, 24, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Dhage, S.R.; Shim, D.E.; Hahn, H.T. Intense pulsed light sintering of copper nanoink for printed electronics. Appl. Phys. A 2009, 97, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Oh, G.H.; Hwang, H.J.; Kim, H.S. Effect of copper oxide shell thickness on flash light sintering of copper nanoparticle ink. RSC Adv. 2017, 7, 17724–17731. [Google Scholar] [CrossRef] [Green Version]





| Viscosity (mPas) | Density (g/mL) | Surface Tension (mN/m) | Inverse of Ohnesorge Number (z) | |
|---|---|---|---|---|
| SiO2 ink | 5.68 | 0.94 | 24.21 | 5.82 |
| ModifiedSiO2 ink | 4.83 | 0.98 | 27.91 | 7.50 |
| Solvent Content (Weight Fraction) | Viscosity (mPas) | Density (g/mL) | Surface Tension (mN/m) | PVP (wt%) | Inverse of Ohnesorge Number (z) |
|---|---|---|---|---|---|
| Cu NP (0.40) + DEG (0.60) | 23.12 | 1/39 | 34.39 | 0 | 1.83 |
| Cu NP (0.40) + DEG (0.60) | 48.45 | 1.45 | 34.78 | 10 | 1.07 |
| Cu NP (0.20) + DEG (0.30) + EtOH(0.50) | 4.54 | 1.22 | 29.46 | 0 | 9.73 |
| Cu NP (0.20) + DEG (0.30) + EtOH (0.50) | 9.21 | 1.23 | 30.22 | 10 | 4.84 |
| Cu NP (0.20) + DEG (0.30) + EtOH (0.50) | 24.13 | 1.25 | 31.96 | 20 | 1.85 |
| 400 V | 500 V | 600 V | 700 V | 800 V | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cycle | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Adhesion | 4B | 3B | 1B | 4B | 4B | 3B | 5B | 5B | 4B | 5B | 5B | 5B | 3B | 1B | 0B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-Y.; Choi, C.-S.; Hwang, K.-T.; Han, K.-S.; Kim, J.-H.; Nahm, S.; Kim, B.-S. Optimization of Hybrid Ink Formulation and IPL Sintering Process for Ink-Jet 3D Printing. Nanomaterials 2021, 11, 1295. https://doi.org/10.3390/nano11051295
Lee J-Y, Choi C-S, Hwang K-T, Han K-S, Kim J-H, Nahm S, Kim B-S. Optimization of Hybrid Ink Formulation and IPL Sintering Process for Ink-Jet 3D Printing. Nanomaterials. 2021; 11(5):1295. https://doi.org/10.3390/nano11051295
Chicago/Turabian StyleLee, Jae-Young, Cheong-Soo Choi, Kwang-Taek Hwang, Kyu-Sung Han, Jin-Ho Kim, Sahn Nahm, and Bum-Seok Kim. 2021. "Optimization of Hybrid Ink Formulation and IPL Sintering Process for Ink-Jet 3D Printing" Nanomaterials 11, no. 5: 1295. https://doi.org/10.3390/nano11051295





