Highly Ordered SnO2 Nanopillar Array as Binder-Free Anodes for Long-Life and High-Rate Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. SnO2 Nanopillar Array Deposition
2.2. Electrochemical Characterization
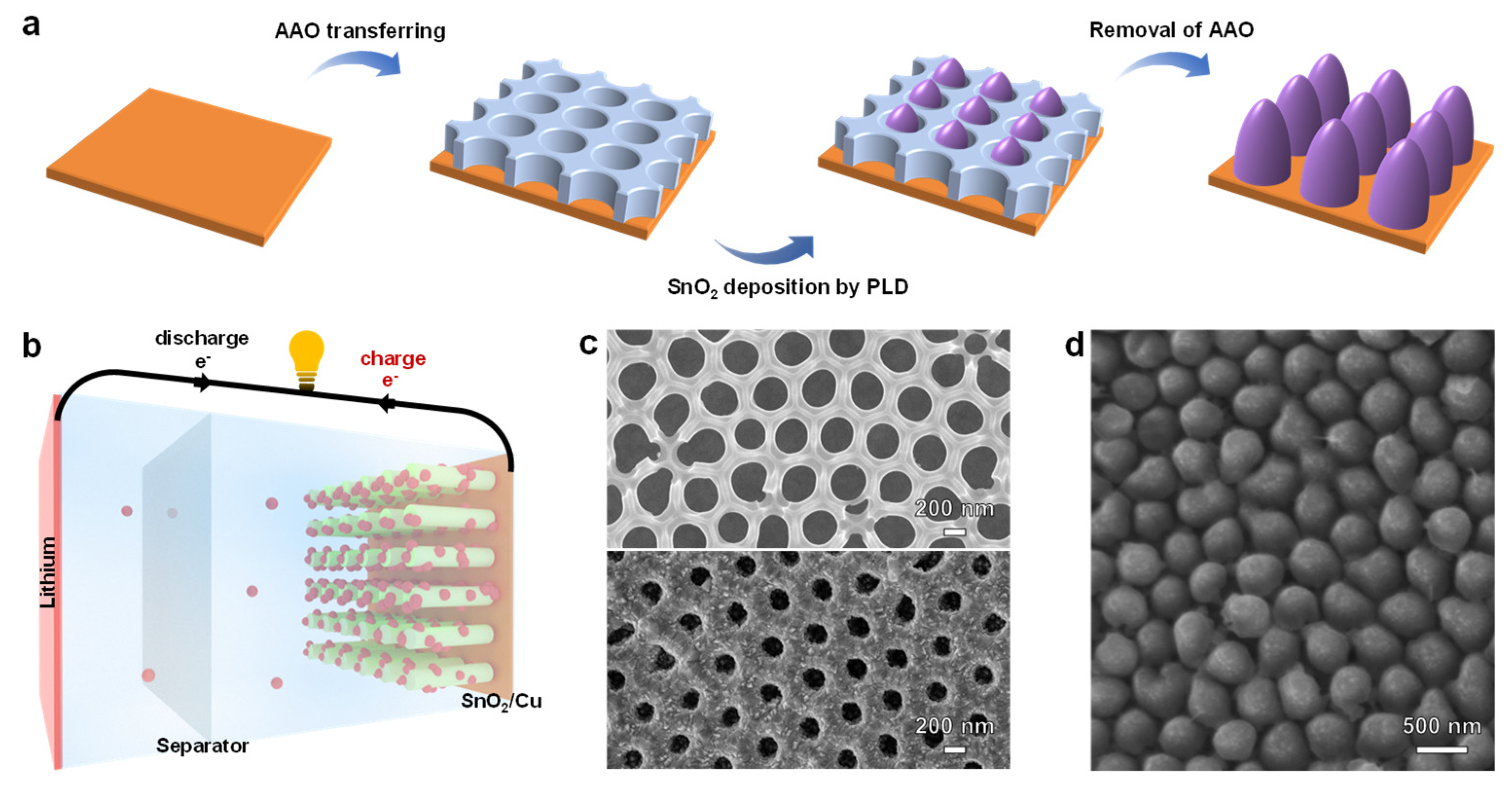
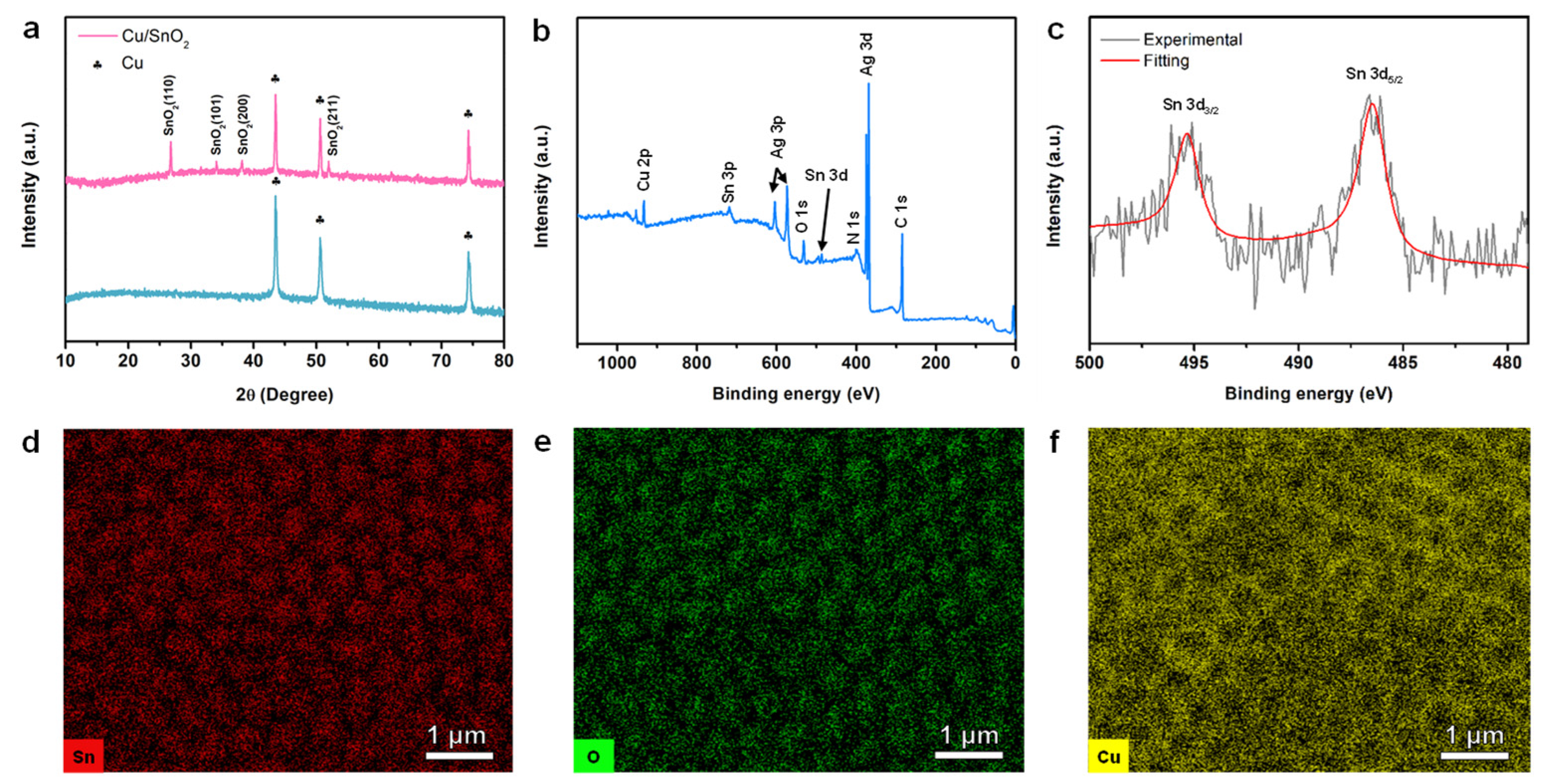
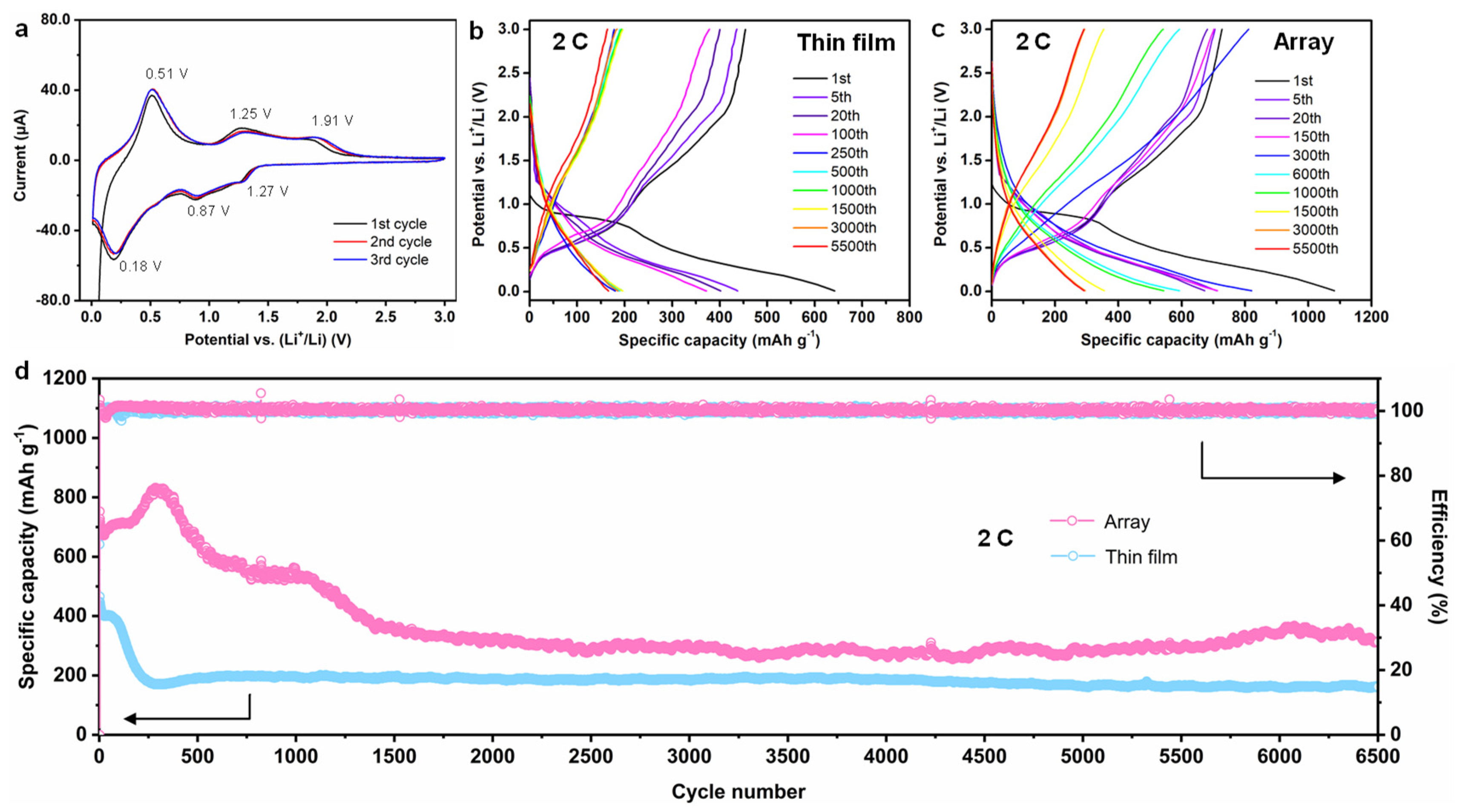
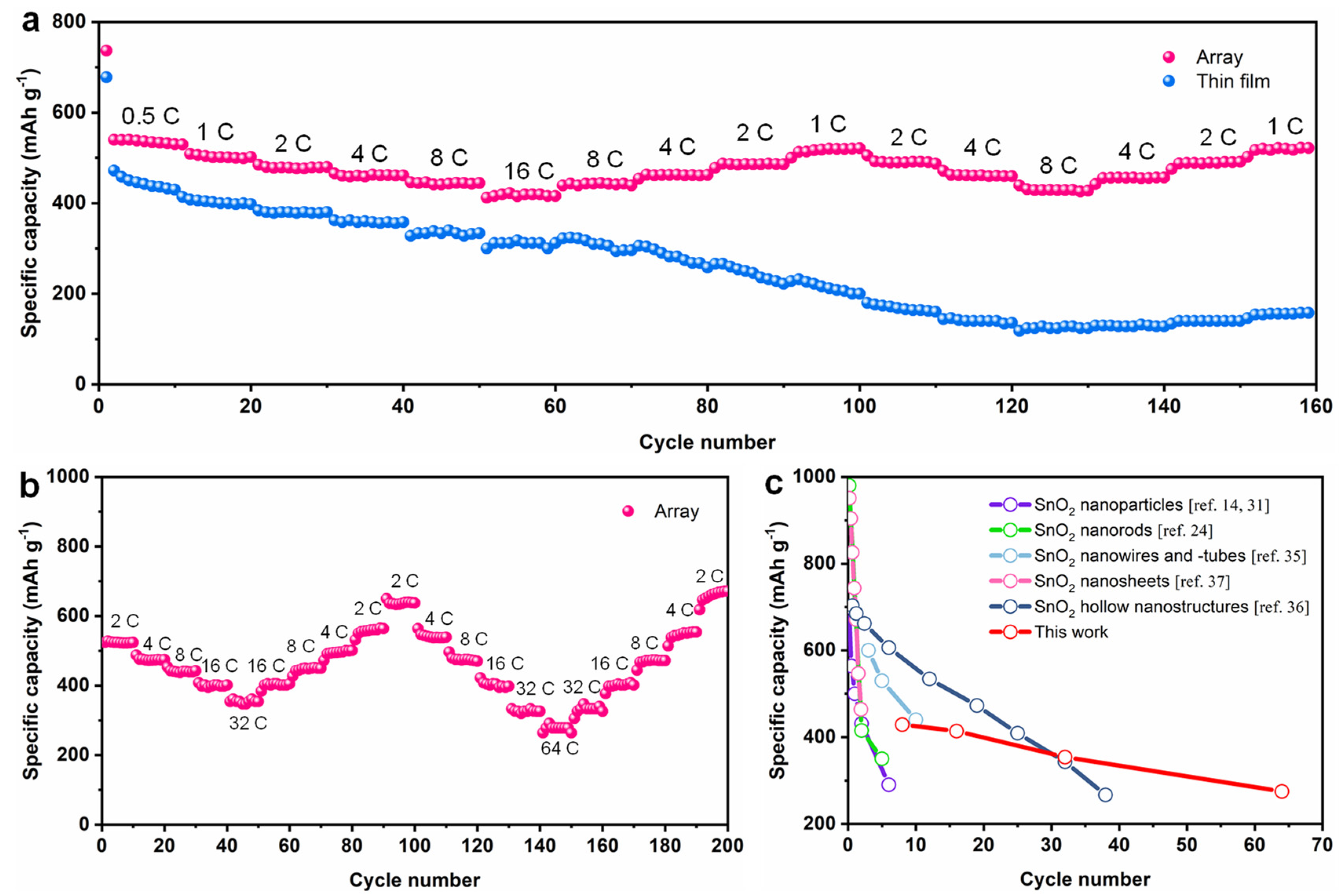
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Xu, X.; Qin, Y.; Ji, S.; Huo, Y.; Wang, Z.; Shen, J.; Liu, J. Recent progress in organic-inorganic composite solid electrolytes for all-solid-state lithium batteries. Chem. Eur. J. 2020, 26, 1720–1736. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Recham, N.; Armand, M.; Chotard, J.-N.; Barpanda, P.; Walker, W.; Dupont, L. Hunting for Better Li-Based Electrode Materials via Low Temperature Inorganic Synthesis. Chem. Mater. 2010, 22, 724–739. [Google Scholar] [CrossRef]
- Sui, Y.; Liu, C.; Masse, R.C.; Neale, Z.G.; Atif, M.; AlSalhi, M.; Cao, G. Dual-ion batteries: The emerging alternative rechargeable batteries. Energy Storage Mater. 2020, 25, 1–32. [Google Scholar] [CrossRef]
- Huang, X.; Yu, H.; Chen, J.; Lu, Z.; Yazami, R.; Hng, H.H. Ultrahigh Rate Capabilities of Lithium-Ion Batteries from 3D Ordered Hierarchically Porous Electrodes with Entrapped Active Nanoparticles Configuration. Adv. Mater. 2014, 26, 1296–1303. [Google Scholar] [CrossRef]
- Jiang, Y.; Hu, M.; Zhang, D.; Yuan, T.; Sun, W.; Xu, B.; Yan, M. Transition metal oxides for high performance sodium ion battery anodes. Nano Energy 2014, 5, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Tang, H.; Li, L.; Hu, Q.; Zhang, L.; Xue, H.; Pang, H. Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries. Adv. Sci. 2018, 5, 1700592. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Qu, K.; Ren, C.; Su, Y.; Fu, B.; Zi, M.; Dai, L.; Xiao, Q.; Xu, J.; Zhong, X.; et al. Epitaxial array of Fe3O4 nanodots for high rate high capacity conversion type lithium ion batteries electrode with long cycling life. Nano Energy 2020, 74, 104876. [Google Scholar] [CrossRef]
- Wu, Z.S.; Ren, W.; Wen, L.; Gao, L.; Zhao, J.; Chen, Z.; Zhou, G.; Li, F.; Cheng, H.M. Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance. ACS Nano 2010, 4, 3187–3194. [Google Scholar] [CrossRef]
- Hu, R.; Ouyang, Y.; Liang, T.; Wang, H.; Liu, J.; Chen, J.; Zhu, M. Stabilizing the nanostructure of SnO2 anodes by transition metals: A route to achieve high initial coulombic efficiency and stable capacities for lithium storage. Adv. Mater. 2017, 29, 1605006. [Google Scholar] [CrossRef]
- Courtney, I.A.; Dahn, J.R. Electrochemical and in situ X-ray diffraction studies of the reaction of lithium with tin oxide com-posites. J. Electrochem. Soc. 1997, 144, 2045–2052. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, L.-J.; Guo, Y.-G. Binding SnO2Nanocrystals in Nitrogen-Doped Graphene Sheets as Anode Materials for Lithium-Ion Batteries. Adv. Mater. 2013, 25, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Idota, Y.; Kubota, T.; Matsufuji, A.; Maekawa, Y.; Miyasaka, T. Tin-Based Amorphous Oxide: A High-Capacity Lithium-Ion-Storage Material. Science 1997, 276, 1395–1397. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; dos Santos, D.; Chew, S.; Wexler, D.; Wang, J.; Dou, S.; Liu, H. Polyol-mediated synthesis of ultrafine tin oxide nanoparticles for reversible Li-ion storage. Electrochem. Commun. 2007, 9, 915–919. [Google Scholar] [CrossRef]
- Etacheri, V.; Seisenbaeva, G.A.; Caruthers, J.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G.; Pol, V.G. Ordered Network of Interconnected SnO2 Nanoparticles for Excellent Lithium-Ion Storage. Adv. Energy Mater. 2015, 5, 1401289. [Google Scholar] [CrossRef]
- Wang, H.-G.; Wu, Q.; Wang, Y.; Wang, X.; Wu, L.; Song, S.; Zhang, H. Molecular Engineering of Monodisperse SnO2 Nanocrystals Anchored on Doped Graphene with High-Performance Lithium/Sodium-Storage Properties in Half/Full Cells. Adv. Energy Mater. 2019, 9, 1802993. [Google Scholar] [CrossRef]
- Zhong, G.; Bitla, Y.; Wang, J.; Zhong, X.; An, F.; Chin, Y.-Y.; Zhang, Y.; Gao, W.; Zhang, Y.; Eshghinejad, A.; et al. Tuning Fe concentration in epitaxial gallium ferrite thin films for room temperature multiferroic properties. Acta Mater. 2018, 145, 488–495. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Tian, G.; Li, P.; Zhao, L.; Zhang, F.; Yao, J.; Fan, H.; Song, X.; Chen, D.; et al. High-density array of ferroelectric nanodots with robust and reversibly switchable topological domain states. Sci. Adv. 2017, 3, e1700919. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; An, F.; Bitla, Y.; Wang, J.; Zhong, X.; Ye, M.; Zhang, Y.; Gao, W.; Pan, X.; Xie, S.; et al. Self-assembling epitaxial growth of a single crystalline CoFe2O4 nanopillar array via dual-target pulsed laser deposition. J. Mater. Chem. C 2018, 6, 4854–4860. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Ye, J.; Cai, J.; Ma, Y.; Zhou, H.; Qi, L. Kinetics-controlled growth of aligned mesocrystalline SnO2 nanorod arrays for lithium-ion batteries with superior rate performance. Nano Res. 2013, 6, 243–252. [Google Scholar] [CrossRef]
- Park, M.-S.; Wang, G.-X.; Kang, Y.-M.; Wexler, D.; Dou, S.-X.; Liu, H.-K. Preparation and Electrochemical Properties of SnO2 Nanowires for Application in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2007, 46, 750–753. [Google Scholar] [CrossRef]
- Ying, Z.; Wan, Q.; Cao, H.; Song, Z.T.; Feng, S.L. Characterization of SnO2 nanowires as an anode material for Li-ion batteries. Appl. Phys. Lett. 2005, 87, 113108. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, J.Y.; Zeng, H.C. Polycrystalline SnO2Nanotubes Prepared via Infiltration Casting of Nanocrystallites and Their Electrochemical Application. Chem. Mater. 2005, 17, 3899–3903. [Google Scholar] [CrossRef]
- Hwang, S.M.; Lim, Y.-G.; Kim, J.-G.; Heo, Y.-U.; Lim, J.H.; Yamauchi, Y.; Park, M.-S.; Kim, Y.-J.; Dou, S.X.; Kim, J.H. A case study on fibrous porous SnO2 anode for robust, high-capacity lithium-ion batteries. Nano Energy 2014, 10, 53–62. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Huang, X.; Ding, R.; Hu, Y.; Jiang, J.; Liao, L. Direct growth of SnO2 nanorod array electrodes for lithium-ion batteries. J. Mater. Chem. 2009, 19, 1859–1864. [Google Scholar] [CrossRef]
- Park, M.-S.; Kang, Y.-M.; Wang, G.-X.; Dou, S.-X.; Liu, H.-K. The Effect of Morphological Modification on the Electrochemical Properties of SnO2 Nanomaterials. Adv. Funct. Mater. 2008, 18, 455–461. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Li, K.; Xu, F.; Zhang, P.; Ding, Y. Electrospun free-standing N-doped C@SnO2 anode paper for flexible Li-ion batteries. Mater. Res. Bull. 2019, 109, 41–48. [Google Scholar] [CrossRef]
- Aravindan, V.; Jinesh, K.; Prabhakar, R.R.; Kale, V.S.; Madhavi, S. Atomic layer deposited (ALD) SnO2 anodes with exceptional cycleability for Li-ion batteries. Nano Energy 2013, 2, 720–725. [Google Scholar] [CrossRef]
- Li, N.; Martin, C.R. A High-Rate, High-Capacity, Nanostructured Sn-Based Anode Prepared Using Sol-Gel Template Synthesis. J. Electrochem. Soc. 2001, 148, A164–A170. [Google Scholar] [CrossRef]
- Shi, W.; Lu, B. Nanoscale Kirkendall effect synthesis of echinus-like SnO2@SnS2 nanospheres as high-performance anode ma-terial for lithium ion batteries. Electrochim. Acta 2014, 133, 247–253. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Xiang, C.; Ruan, G.; Yan, Z.; Natelson, D.; Tour, J.M. Graphene Nanoribbon and Nanostructured SnO2 Composite Anodes for Lithium Ion Batteries. ACS Nano 2013, 7, 6001–6006. [Google Scholar] [CrossRef]
- Yin, L.; Chai, S.; Wang, F.; Huang, J.; Li, J.; Liu, C.; Kong, X. Ultrafine SnO2 nanoparticles as a high performance anode material for lithium ion battery. Ceram. Int. 2016, 42, 9433–9437. [Google Scholar] [CrossRef]
- Ferraresi, G.; Villevieille, C.; Czekaj, I.; Horisberger, M.; Novák, P.; El Kazzi, M. SnO2 Model Electrode Cycled in Li-Ion Battery Reveals the Formation of Li2SnO3 and Li8SnO6 Phases through Conversion Reactions. ACS Appl. Mater. Interfaces 2018, 10, 8712–8720. [Google Scholar] [CrossRef]
- Nadimpalli, S.P.V.; Sethuraman, V.A.; Bucci, G.; Srinivasan, V.; Bower, A.F.; Guduru, P.R. On Plastic Deformation and Fracture in Si Films during Electrochemical Lithiation/Delithiation Cycling. J. Electrochem. Soc. 2013, 160, A1885–A1893. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Xin, G.; Hu, T.; Yu, M.; Shao, D.; Sun, X.; Lian, J. High-rate lithiation-induced reactivation of mesoporous hollow spheres for long-lived lithium-ion batteries. Nat. Commun. 2014, 5, 4526. [Google Scholar] [CrossRef] [Green Version]
- Ko, Y.-D.; Kang, J.-G.; Park, J.-G.; Lee, S.; Kim, D.-W. Self-supported SnO2 nanowire electrodes for high-power lithium-ion batteries. Nanotechnology 2009, 20, 455701. [Google Scholar] [CrossRef] [PubMed]
- Park, G.D.; Lee, J.-K.; Kang, Y.C. Synthesis of Uniquely Structured SnO2 Hollow Nanoplates and Their Electrochemical Properties for Li-Ion Storage. Adv. Funct. Mater. 2016, 27, 1603399. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, H.; Zhai, H.; Cao, C. Microwave-Assisted and Gram-Scale Synthesis of Ultrathin SnO2 Nanosheets with Enhanced Lithium Storage Properties. ACS Appl. Mater. Interfaces 2015, 7, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Zhong, X.; Zou, J.; Fu, B.; Su, Y.; Ren, C.; Wang, J.; Zhong, G. Highly Ordered SnO2 Nanopillar Array as Binder-Free Anodes for Long-Life and High-Rate Li-Ion Batteries. Nanomaterials 2021, 11, 1307. https://doi.org/10.3390/nano11051307
Dai L, Zhong X, Zou J, Fu B, Su Y, Ren C, Wang J, Zhong G. Highly Ordered SnO2 Nanopillar Array as Binder-Free Anodes for Long-Life and High-Rate Li-Ion Batteries. Nanomaterials. 2021; 11(5):1307. https://doi.org/10.3390/nano11051307
Chicago/Turabian StyleDai, Liyufen, Xiangli Zhong, Juan Zou, Bi Fu, Yong Su, Chuanlai Ren, Jinbin Wang, and Gaokuo Zhong. 2021. "Highly Ordered SnO2 Nanopillar Array as Binder-Free Anodes for Long-Life and High-Rate Li-Ion Batteries" Nanomaterials 11, no. 5: 1307. https://doi.org/10.3390/nano11051307





