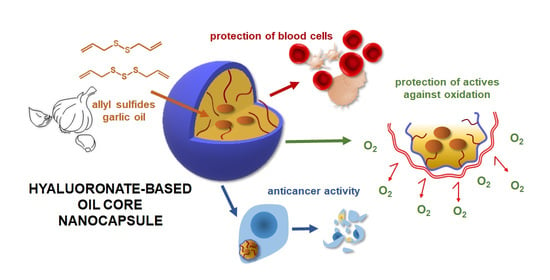
Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nanocapsules
2.2. Stability of Diallyl Disulfide (DADS) and Diallyl Trisulfide (DATS) in Oxidizing Conditions
2.3. Hemolysis Assay
2.4. Interaction with Human Serum
2.5. Interaction with Simulated Human Body Fluids
2.6. Cell Culture
2.7. Cytotoxicity
2.8. Scratch Assay
2.9. Isolation of Total RNA
2.10. Reverse Transcription of RNA
2.11. Sulfurtransferases Gene Expression Measurement
2.12. Cell Homogenization
2.13. Enzyme Assays
2.13.1. Rhodanese (TST) Activity
2.13.2. 3-Mercaptopyruvate Sulfurtransferase (MPST) Activity
2.13.3. Cystathionine γ-Lyase (CTH) Activity
2.13.4. Sulfane Sulfur
2.13.5. Protein Content
2.14. Determination of Concentrations of Low Molecular Weight Sulfur-Containing Compounds using Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
2.15. Statistical Analysis
3. Results and Discussions
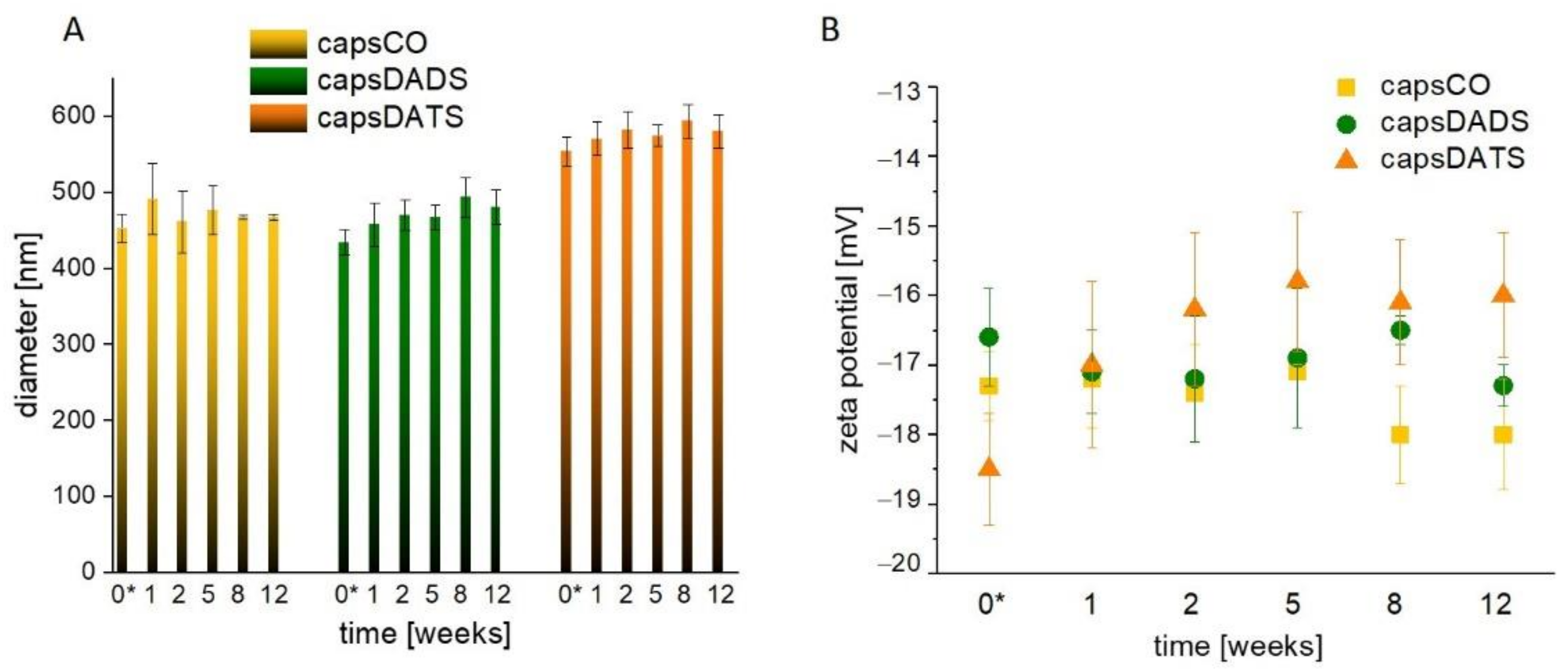
3.1. Nanocapsules as Delivery Systems of Hydrophobic Compounds
3.2. Stability of DADS and DATS in Oxidizing Conditions
3.3. Interaction with Blood and Digestive Track Components
3.3.1. Hemolysis
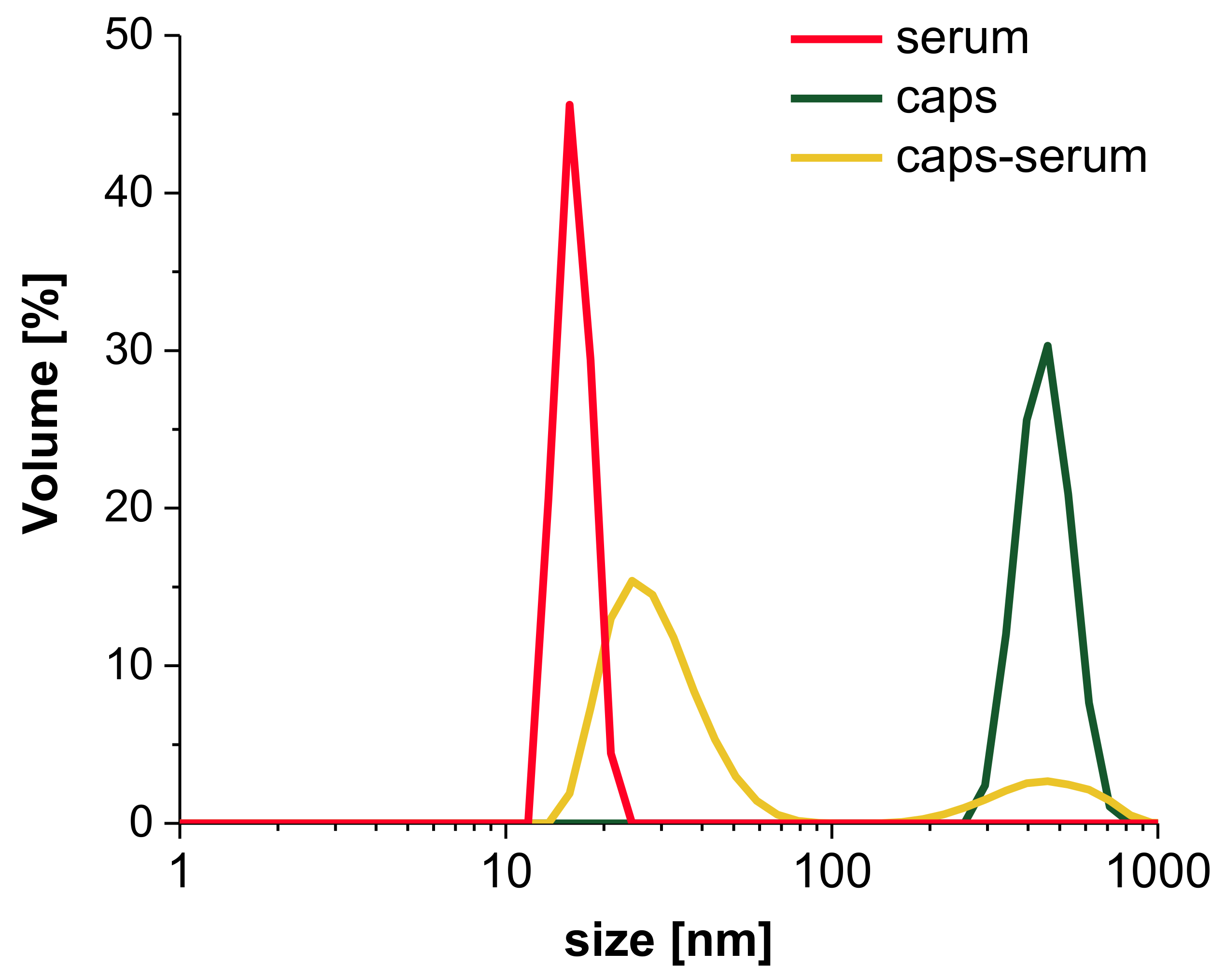
3.3.2. Interaction with Human Serum
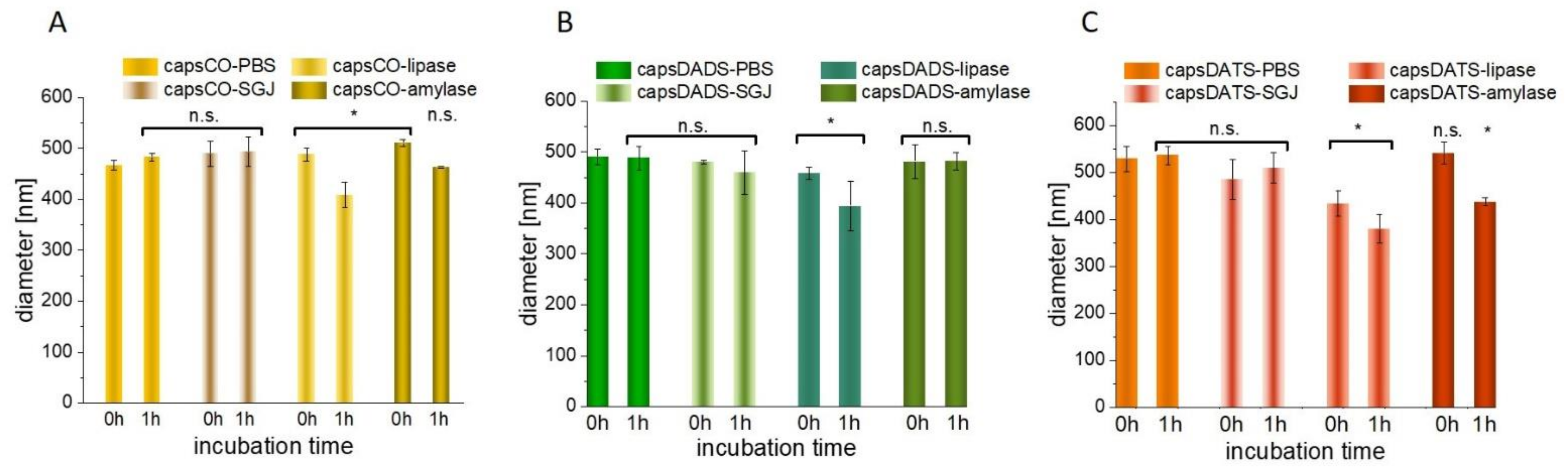
3.3.3. Interactions with Digestive Track’s Proteins and Influence of Low pH
3.4. Influence on Cancer Cells
3.4.1. Cytotoxicity
3.4.2. Cell Migration
3.4.3. Determination of Low Molecular Weight Sulfur-Containing Compounds
3.4.4. Sulfane Sulfur Level and Sulfurtransferases Activity and Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead phytochemicals for anticancer drug development. Front. Plant. Sci. 2016, 7, 1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Ruan, J.; Zhuang, X.; Zhang, X.; Li, Z. Phytochemicals of garlic: Promising candidates for cancer therapy. Biomed. Pharmacother. 2020, 123, 109730. [Google Scholar] [CrossRef]
- Yi, L.; Su, Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem. Toxicol. 2013, 57, 362–370. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, W.; Gao, C.; Jiang, X.; Wang, H. Drug Metabolism and Pharmacokinetics of Organosulfur Compounds from Garlic. J. Drug Metab. Toxicol. 2013, 4, 159. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.L.; Pinto, J.T. Cysteine S-conjugate β-lyases. Amino Acids 2006, 30, 1–15. [Google Scholar] [CrossRef]
- Oommen, S.; Anto, R.J.; Srinivas, G.; Karunagaran, D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur. J. Pharmacol. 2004, 485, 97–103. [Google Scholar] [CrossRef]
- Park, S.Y.; Cho, S.J.; Kwon, H.C.; Lee, K.R.; Rhee, D.K.; Pyo, S. Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA. Cancer Lett. 2005, 224, 123–132. [Google Scholar] [CrossRef]
- Liang, D.; Wu, H.; Wong, M.W.; Huang, D. Diallyl Trisulfide Is a Fast H2S Donor, but Diallyl Disulfide Is a Slow One: The Reaction Pathways and Intermediates of Glutathione with Polysulfides. Org. Lett. 2015, 17, 4196–4199. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Górny, M.; Sokołowska-Jeżewicz, M.; Kowalczyk-Pachel, D. The effects of different garlic-derived allyl sulfides on anaerobic sulfur metabolism in the mouse kidney. Antioxidants 2016, 5, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurkowska, H.; Wróbel, M.; Kaczor-Kamińska, M.; Jasek-Gajda, E. A possible mechanism of inhibition of U87MG and SH-SY5Y cancer cell proliferation by diallyl trisulfide and other aspects of its activity. Amino Acids 2017, 49, 1855–1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, H.; Tsuta, K.; Kiuchi, K.; Senzaki, H.; Tanaka, K.; Hioki, K.; Tsubura, A. Growth inhibitory effects of diallyl disulfide on human breast cancer cell lines. Carcinogenesis 2001, 22, 891–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.Y.; Yao, S.Q.; Zu, X.Y.; Huang, Z.X.; Liu, L.J.; Zhong, M.; Zhu, B.Y.; Tang, S.S.; Liao, D.F. Apoptosis induced by diallyl disulfide in human breast cancer cell line MCF-7. Acta Pharmacol. Sin. 2008, 29, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Na, H.K.; Kim, E.H.; Choi, M.A.; Park, J.M.; Kim, D.H.; Surh, Y.J. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem. Pharmacol. 2012, 84, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Gagnon, B.; Bélanger-Bouliga, M.; Trang Nguyen, P.; Nguyen, T.H.D.; Bourgault, S.; Nazemi, A. Degradable Spirocyclic Polyacetal-Based Core-Amphiphilic Assemblies for Encapsulation and Release of Hydrophobic Cargo. Nanomaterials 2021, 11, 161. [Google Scholar] [CrossRef]
- Adamczak, M.; Krok, M.; Pamuła, E.; Posadowska, U.; Szczepanowicz, K.; Barbasz, J.; Warszyński, P. Linseed oil based nanocapsules as delivery system for hydrophobic quantum dots. Colloids Surf. B Biointerfaces 2013, 110, 1–7. [Google Scholar] [CrossRef]
- Bazylińska, U.; Skrzela, R.; Szczepanowicz, K.; Warszyński, P.; Wilk, K.A. Novel approach to long sustained multilayer nanocapsules: Influence of surfactant head groups and polyelectrolyte layer number on the release of hydrophobic compounds. Soft Matter 2011, 7, 6113–6124. [Google Scholar] [CrossRef]
- Szafraniec-Szczęsny, J.; Janik-Hazuka, M.; Odrobińska, J.; Zapotoczny, S. Polymer capsules with hydrophobic liquid cores as functional nanocarriers. Polymers 2020, 12, 1–25. [Google Scholar] [CrossRef]
- Kuen, C.; Fakurazi, S.; Othman, S.; Masarudin, M. Increased Loading, Efficacy and Sustained Release of Silibinin, a Poorly Soluble Drug Using Hydrophobically-Modified Chitosan Nanoparticles for Enhanced Delivery of Anticancer Drug Delivery Systems. Nanomaterials 2017, 7, 379. [Google Scholar] [CrossRef] [Green Version]
- Shigehiro, T.; Masuda, J.; Saito, S.; Khayrani, A.; Jinno, K.; Seno, A.; Vaidyanath, A.; Mizutani, A.; Kasai, T.; Murakami, H.; et al. Practical Liposomal Formulation for Taxanes with Polyethoxylated Castor Oil and Ethanol with Complete Encapsulation Efficiency and High Loading Efficiency. Nanomaterials 2017, 7, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Novel Surface-Modified Bilosomes as Functional and Biocompatible Nanocarriers of Hybrid Compounds. Nanomaterials 2020, 10, 2472. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II—Production scales and clinically compliant production methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Yang, C.; Li, B.; Feng, L.; Hai, M.; Zhao, C.; Chen, D.; Liu, K.; Weitz, D.A. Active Encapsulation in Biocompatible Nanocapsules. Small 2020, 16, 2002716. [Google Scholar] [CrossRef] [PubMed]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.S.R.; Yamjala, K.; Wadhwani, A.; Madhunapantula, S.R.V.; Pindiprolu, S.S.S. Application of quality-by-design approach to optimize diallyl disulfide-loaded solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 474–488. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Sun, X.; Zhang, H.; Yang, C.; Liu, Y.; Yang, W.; Guo, C.; Wang, C. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury. Int. J. Nanomed. 2016, 11, 3255–3263. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yue, Y.; Zhou, Y.; Fan, Y.; Fan, C.; Huang, Y.; Wu, F.; Liu, Y. An oil-free microemulsion for intravenous delivery of diallyl trisulfide: Formulation and evaluation. Int. J. Pharm. 2011, 407, 158–166. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Chizzola, R.; Ramadan, A.A.; Edris, A.E. Chemical composition and antimicrobial activity of garlic essential oils evaluated in organic solvent, emulsifying, and self-microemulsifying water based delivery systems. Food Chem. 2017, 221, 196–204. [Google Scholar] [CrossRef]
- Albrecht, F.; Leontiev, R.; Jacob, C.; Slusarenko, A.J. An optimized facile procedure to synthesize and purify allicin. Molecules 2017, 22, 770. [Google Scholar] [CrossRef] [Green Version]
- Ju, X.; Zhang, S.; Wang, Q.; Li, X.; Yang, P. Preparation and Stability of Diallyl Trisulfide Self-Assembled Micellar Injection. PDA J. Pharm. Sci. Technol. 2010, 64, 92–96. [Google Scholar]
- Freeman, F.; Kodera, Y. Garlic Chemistry: Stability of S-(2-Propenyl) 2-Propene-l-sulfínothioate (Allicin) in Blood, Solvents, and Simulated Physiological Fluids. J. Agric. Food Chem. 1995, 43, 2332–2338. [Google Scholar] [CrossRef]
- Kim, S.M.; Kubota, K.; Kobayashi, A. Antioxidative activity of sulfur-containing flavor compounds in garlic. Biosci. Biotechnol. Biochem. 1997, 61, 1482–1485. [Google Scholar] [CrossRef]
- Yoo, M.; Kim, S.; Lee, S.; Shin, D. Validated HPLC Method and Temperature Stabilities for Oil-Soluble Organosulfur Compounds in Garlic Macerated Oil. J. Chromatogr. Sci. 2014, 52, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Bjerregaard, S.; Wulf-Andersen, L.; Stephens, R.W.; Roge Lund, L.; Vermehren, C.; Söderberg, I.; Frokjaer, S. Sustained elevated plasma aprotinin concentration in mice following intraperitoneal injections of w/o emulsions incorporating aprotinin. J. Control. Release 2001, 71, 87–98. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.I.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-N-vinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Błazejczyk, A.; Kus, E.; Janik, M.; Zajac, G.; Wietrzyk, J.; Chlopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 9, 18867–18880. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Salgado, B.S.; Monteiro, L.N.; Rocha, N.S. Allium species poisoning in dogs and cats. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011, 17, 4–11. [Google Scholar] [CrossRef]
- Kaczor-Kamińska, M.; Kamiński, K.; Stalińska, K.; Wróbel, M.; Feldman, A. Effect of glycosaminoglycans accumulation on the non-oxidative sulfur metabolism in mouse model of Sanfilippo syndrome, type B. Acta Biochim. Pol. 2019, 66, 567–576. [Google Scholar] [CrossRef] [Green Version]
- Kaczor-Kamińska, M.; Stalińska, K.; Kamiński, K.; Pisarek, A.; Maziarz, U.; Feldman, A.; Wróbel, M. Murine cellular model of mucopolysaccharidosis, type IIIB (MPS IIIB)—A preliminary study with particular emphasis on the non-oxidative L-cysteine metabolism. Biochimie 2020, 174, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Sörbo, B. Rhodanese Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academy Press: New York, NY, USA, 1955; Volume 2, pp. 334–337. [Google Scholar]
- Wróbel, M.; Jurkowska, H.; Śliwa, L.; Srebro, Z. Sulfurtransferases and cyanide detoxification in mouse liver, kidney, and brain. Toxicol. Mech. Methods 2004, 14, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Valentine, W.N.; Frankenfeld, J.K. 3-mercaptopyruvate sulfurtransferase (EC 2.8.1.2): A simple assay adapted to human blood cells. Clin. Chim. Acta 1974, 51, 205–210. [Google Scholar] [CrossRef]
- Matsuo, Y.; Greenberg, D.M. A crystalline enzyme that cleaves homoserine and cystathionine. II. Prosthetic group. J. Biol. Chem. 1958, 230, 561–571. [Google Scholar] [CrossRef]
- Czubak, J.; Wróbel, M.; Jurkowska, H. Cystathionine γ-lyase (EC 4.4.1.1): An enzymatic assay of α-ketobutyrate using lactate dehydrogenase. Acta Biol. Cracov. Ser. Zool. 2002, 44, 113–117. [Google Scholar]
- Wood, J.L. Sulfane Sulfur. Methods Enzymol. 1987, 143, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Rnadall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dominick, P.K.; Cassidy, P.B.; Roberts, J.C. A new and versatile method for determination of thiolamines of biological importance. J. Chromatogr. B Biomed. Sci. Appl. 2001, 761, 1–12. [Google Scholar] [CrossRef]
- Bronowicka-Adamska, P.; Zagajewski, J.; Czubak, J.; Wróbel, M. RP-HPLC method for quantitative determination of cystathionine, cysteine and glutathione: An application for the study of the metabolism of cysteine in human brain. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2005–2009. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Hardwick, C.; Hoare, K.; Owens, R.; Hohn, H.P.; Hook, M.; Moore, D.; Cripps, V.; Austen, L.; Nance, D.M.; Turley, E.A. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992, 117, 1343–1350. [Google Scholar] [CrossRef] [Green Version]
- Szafraniec, J.; Janik, M.; Odrobińska, J.; Zapotoczny, S. Nanocapsules templated on liquid cores stabilized by graft amphiphilic polyelectrolytes. Nanoscale 2015, 7, 5525–5536. [Google Scholar] [CrossRef] [PubMed]
- Janeesh, P.A.; Sami, H.; Dhanya, C.R.; Sivakumar, S.; Abraham, A. Biocompatibility and genotoxicity studies of polyallylamine hydrochloride nanocapsules in rats. RSC Adv. 2014, 4, 24484–24497. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munday, R. Harmful and beneficial effects of organic monosulfides, disulfides, and polysulfides in animals and humans. Chem. Res. Toxicol. 2012, 25, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bandyopadhyay, P. Polysaccharide-Protein Interactions and Their Relevance in Food Colloids. In The Complex World of Polysaccharides; InTech: London, UK, 2012. [Google Scholar]
- Peniche, C.; Howland, I.; Carrillo, O.; Zaldívar, C.; Argüelles-Monal, W. Formation and stability of shark liver oil loaded chitosan/calcium alginate capsules. Food Hydrocoll. 2004, 18, 865–871. [Google Scholar] [CrossRef]
- Czyzynska-Cichon, I.; Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Jasinski, K.; Węglarz, W.P.; Zapotoczny, S.; Chlopicki, S. Low Dose Curcumin Administered in Hyaluronic Acid-Based Nanocapsules Induces Hypotensive Effect in Hypertensive Rats. Int. J. Nanomed. 2021, 16, 1377–1390. [Google Scholar] [CrossRef]
- Atiroglu, V. Lipase immobilization on synthesized hyaluronic acid-coated magnetic nanoparticle-functionalized graphene oxide composites as new biocatalysts: Improved reusability, stability, and activity. Int. J. Biol. Macromol. 2020, 145, 456–465. [Google Scholar] [CrossRef]
- Laffleur, F.; Netsomboon, K.; Erman, L.; Partenhauser, A. Evaluation of modified hyaluronic acid in terms of rheology, enzymatic degradation and mucoadhesion. Int. J. Biol. Macromol. 2019, 123, 1204–1210. [Google Scholar] [CrossRef]
- Djordjevic, D.; Milovanovic, J.; Jurisevic, M.; Stojanovic, B.; Cvetkovic, O.; Pergal, M.; Ristanovic, E.; Vojvodic, D.; Simic, M.; Manojlovic, D.; et al. Antitumour effect of a mixture of n-propyl polysulfides In vitro. Serbian J. Exp. Clin. Res. 2019, 20, 295–300. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, V.M.; Bezerra, M.A., Jr.; Nascimento, J.C.; Amorim, L.M.F. Anticancer drug screening: Standardization of in vitro wound healing assay. J. Bras. Patol. Med. Lab. 2019, 55, 606–619. [Google Scholar] [CrossRef]
- Sadiq, I.Z.; Pankaj, T.; Khan, A.R.; Naziru, D.; Safiyanu, I.; Salisu, A.R. Cytoprotective, Conjugative and Antioxidant Activities of Glutathione; and Its Role in Removal of Toxic Metabolites and Protein Protection: A Review. Chem Res. J. 2016, 1, 147–153. [Google Scholar]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, H.T.; Goon, D.J.W.; Crankshaw, D.L.; Vince, R.; Patterson, S.E. Novel, orally effective cyanide antidotes. J. Med. Chem. 2007, 50, 6462–6464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandi, D.L.; Horowitz, P.M.; Westley, J. Rhodanese as a thioredoxin oxidase. Int. J. Biochem. Cell Biol. 2000, 32, 465–473. [Google Scholar] [CrossRef]
- Toohey, J.I. Sulphane sulphur in biological systems: A possible regulatory role. Biochem. J. 1989, 264, 625–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronowicka-Adamska, P.; Bentke, A.; Wróbel, M. Hydrogen sulfide generation from L-cysteine in the human glioblastoma-astrocytoma U-87 MG and neuroblastoma SHSY5Y cell lines. Acta Biochim. Pol. 2017, 64, 171–176. [Google Scholar] [CrossRef] [PubMed]






| Genes * | Forward (F) and Reverse (R) Primers (5′ → 3′) | RT-PCR Product Size (bp) |
|---|---|---|
| CTH | F: CAGCAAGACCCGATGCAAAG R: CAAAGCAACACCTGCCACTC | 304 [40,41] |
| MPST | F: AGCATTTATGAAGCCCGCCT R: CCTGGTCACTGTCGTCGTAG | 420 [40,41] |
| TST | F: AACCTGGGCATAAGCAACGA R: GGTCCACCTTCTTGTCCTGG | 460 [40,41] |
| GAPDH | F: GTCCCAGCTTAGGTTCATCAG R: TTTGGCTCCACCCTTCAAGT | 404 [40,41] |
| Group | Cysteine | GSH | GSSG | Total Glutathione | GSH/GSSG |
|---|---|---|---|---|---|
| nmole/mg Protein | |||||
| Control | 0.3 ± 0.2 | 23.6 ± 0.4 | 1.0 ± 0.2 | 25.6 ± 0.7 | 23.9 ± 3.3 |
| capsCO (28 mg/L) | 0.5 ± 0.1 | 25.0 ± 1.3 | 0.9 ± 0.2 | 26.8 ± 1.6 | 28.6 ± 3.8 |
| capsDATS (17 mg/L) | 2.2 ± 0.3 * | 25.3 ± 1.5 | 0.6 ± 0.1 | 26.5 ± 1.6 | 41.2 ± 1.8 * |
| capsCO (55 mg/L) | 0.3 ± 0.1 | 22.3 ± 1.5 | 0.9 ± 0.3 | 24.1 ± 2.1 | 25.5 ± 6.2 |
| capsDADS (33 mg/L) | 1.5 ± 0.5 * | 60.0 ± 4.8 * | 2.7 ± 0.6 | 65.4 ± 6.0 * | 22.7 ± 3.7 |
| capsDATS (33 mg/L) | 1.2 ± 0.1 * | 19.7 ± 2.9 | 0.12 ± 0.01 * | 20.2 ± 4.0 | 174 ± 16 * |
| Group | Sulfane Sulfur | TST | MPST | CTH |
|---|---|---|---|---|
| [nmol/mg Protein] | [nmol/mg Protein Min] | |||
| control | 185 ± 16 | 15.2 ± 1.9 | 76 ± 26 | 0.7 ± 0.1 |
| capsCO (28 mg/L) | 180 ± 6 | 14.6 ± 2.5 | 126 ± 33 # | 0.6 ± 0.1 |
| capsDATS (17 mg/L) | 158 ± 15 * | 23.3 ± 3.6 * | 99 ± 36 * | 1.4 ± 0.5 * |
| capsCO (55 mg/L) | 187 ± 12 | 12.2 ± 1.4 # | 133 ± 51 # | 1.1 ± 0.2 # |
| capsDADS (33 mg/L) | 176 ± 17 | 16.5 ± 3.3 * | 133 ± 40 | 1.0 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik-Hazuka, M.; Kamiński, K.; Kaczor-Kamińska, M.; Szafraniec-Szczęsny, J.; Kmak, A.; Kassassir, H.; Watała, C.; Wróbel, M.; Zapotoczny, S. Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity. Nanomaterials 2021, 11, 1354. https://doi.org/10.3390/nano11051354
Janik-Hazuka M, Kamiński K, Kaczor-Kamińska M, Szafraniec-Szczęsny J, Kmak A, Kassassir H, Watała C, Wróbel M, Zapotoczny S. Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity. Nanomaterials. 2021; 11(5):1354. https://doi.org/10.3390/nano11051354
Chicago/Turabian StyleJanik-Hazuka, Małgorzata, Kamil Kamiński, Marta Kaczor-Kamińska, Joanna Szafraniec-Szczęsny, Aleksandra Kmak, Hassan Kassassir, Cezary Watała, Maria Wróbel, and Szczepan Zapotoczny. 2021. "Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity" Nanomaterials 11, no. 5: 1354. https://doi.org/10.3390/nano11051354
APA StyleJanik-Hazuka, M., Kamiński, K., Kaczor-Kamińska, M., Szafraniec-Szczęsny, J., Kmak, A., Kassassir, H., Watała, C., Wróbel, M., & Zapotoczny, S. (2021). Hyaluronic Acid-Based Nanocapsules as Efficient Delivery Systems of Garlic Oil Active Components with Anticancer Activity. Nanomaterials, 11(5), 1354. https://doi.org/10.3390/nano11051354