Computational Study of Quenching Effects on Growth Processes and Size Distributions of Silicon Nanoparticles at a Thermal Plasma Tail
Abstract
:1. Introduction
2. Experiment Setup
3. Numerical Model Description
3.1. Model Outline and Assumptions
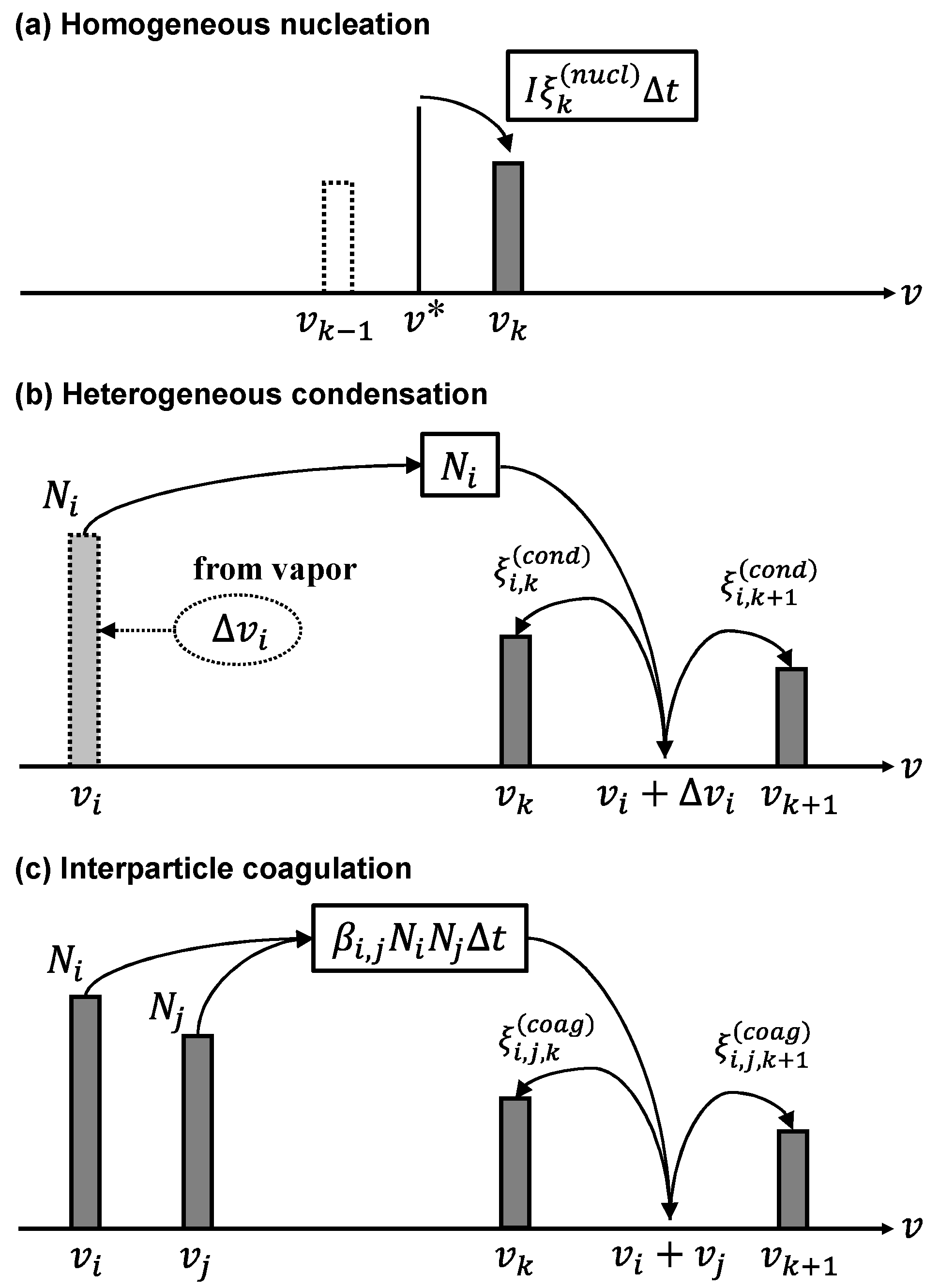
3.2. Homogeneous Nucleation
3.3. Heterogeneous Condensation
3.4. Interparticle Coagulation
3.5. Vapor Consumption
3.6. Melting Point Depression
3.7. Computational Conditions
4. Results and Discussion
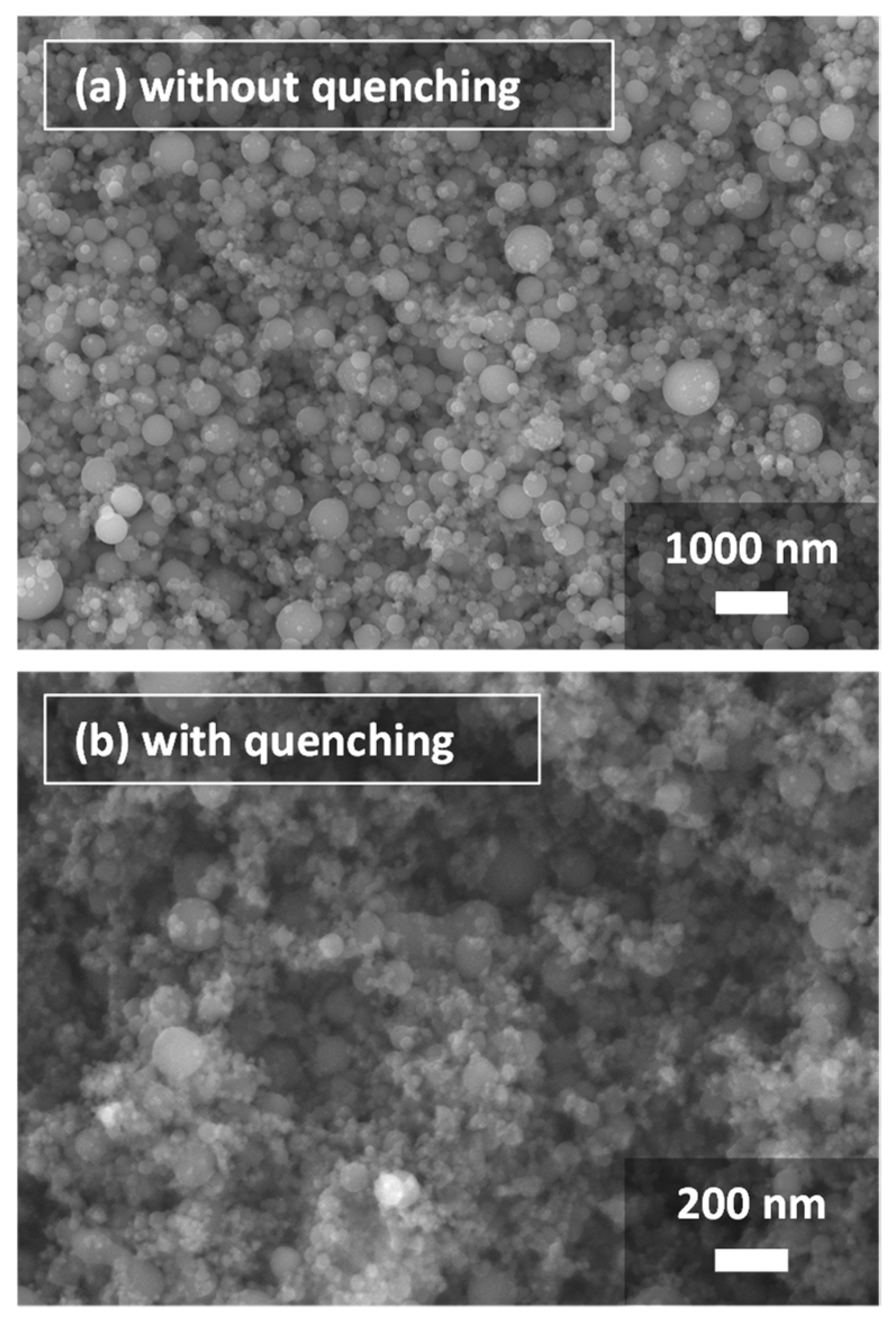
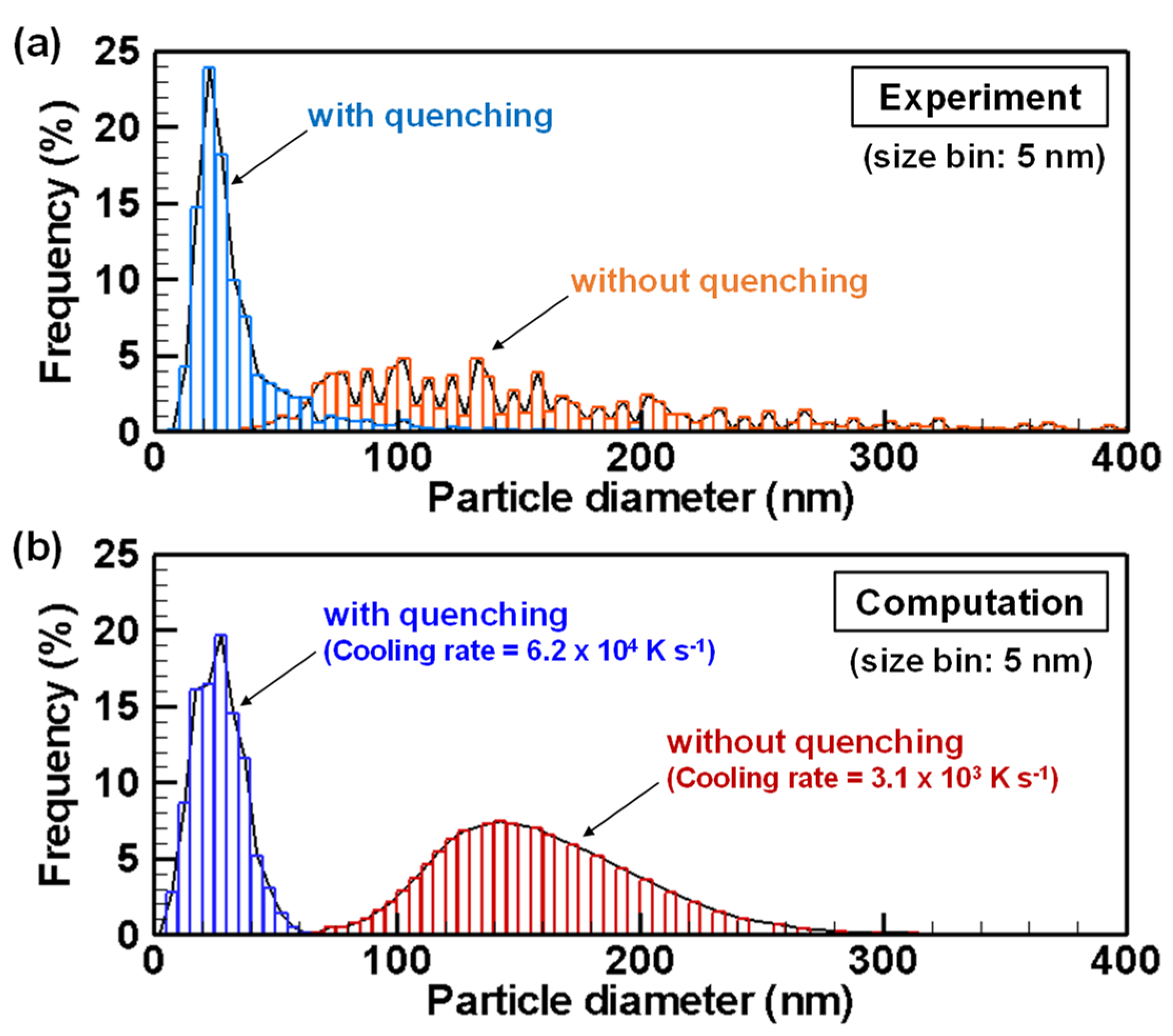
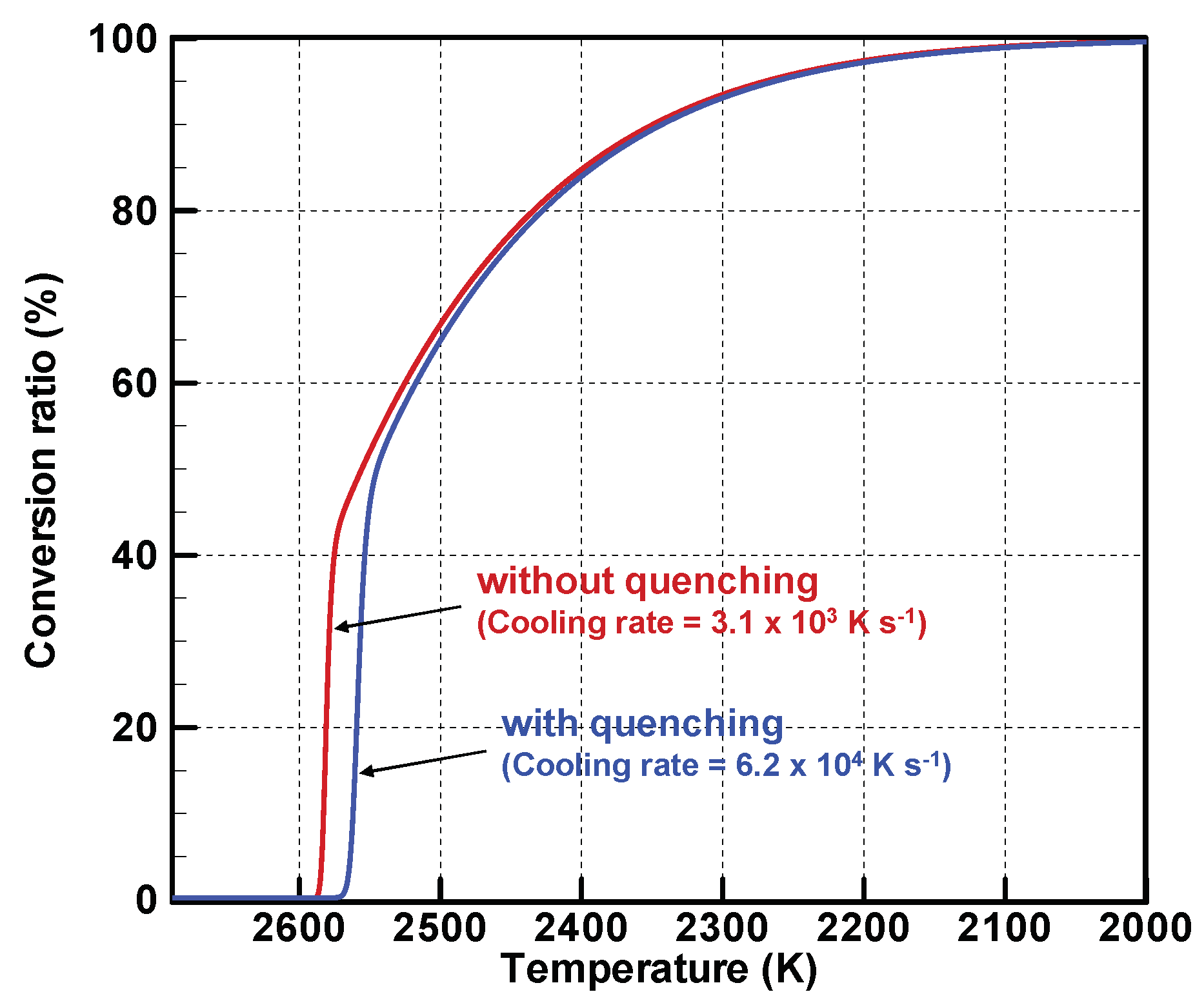
4.1. Experiment Results and Model Validation
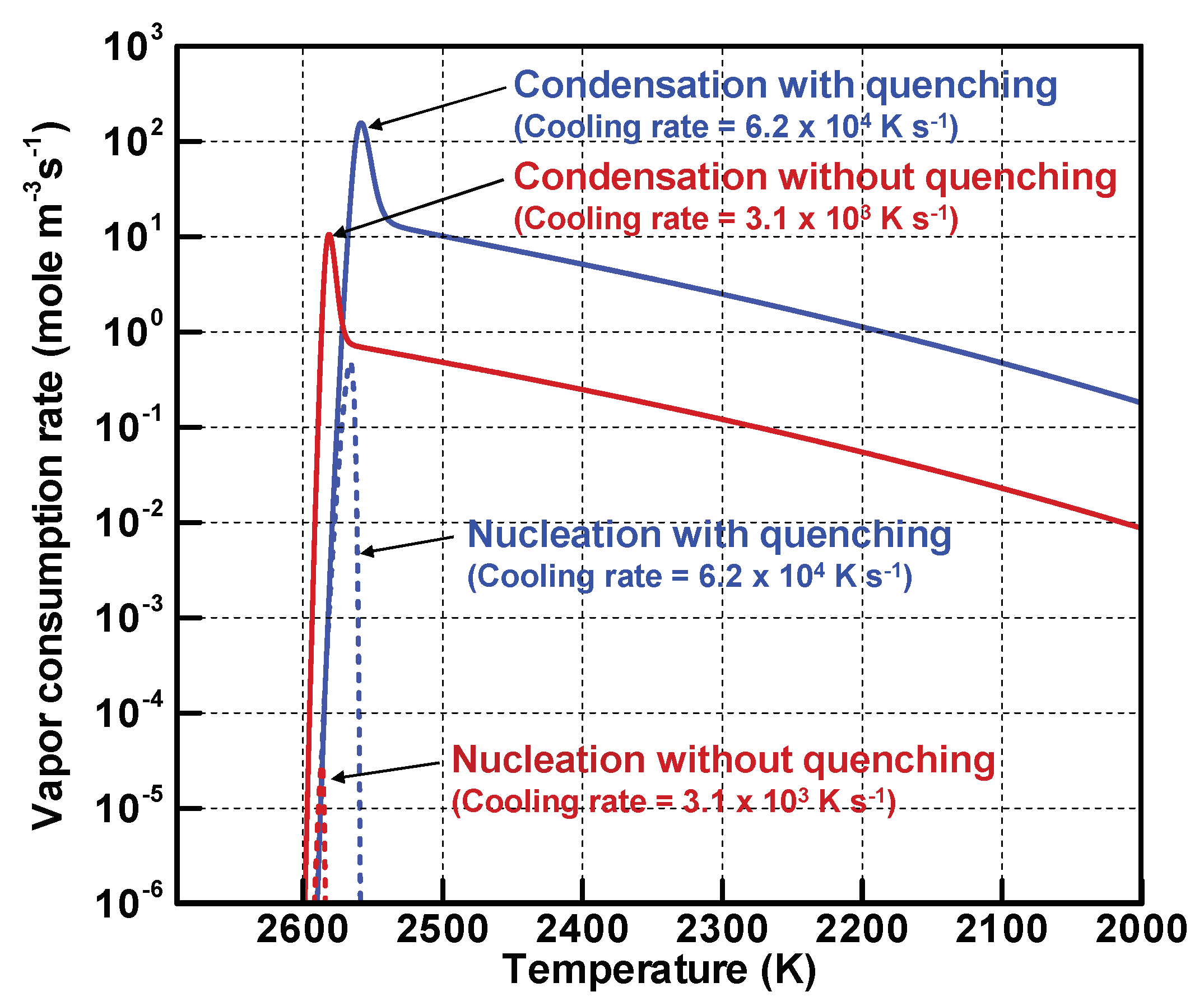
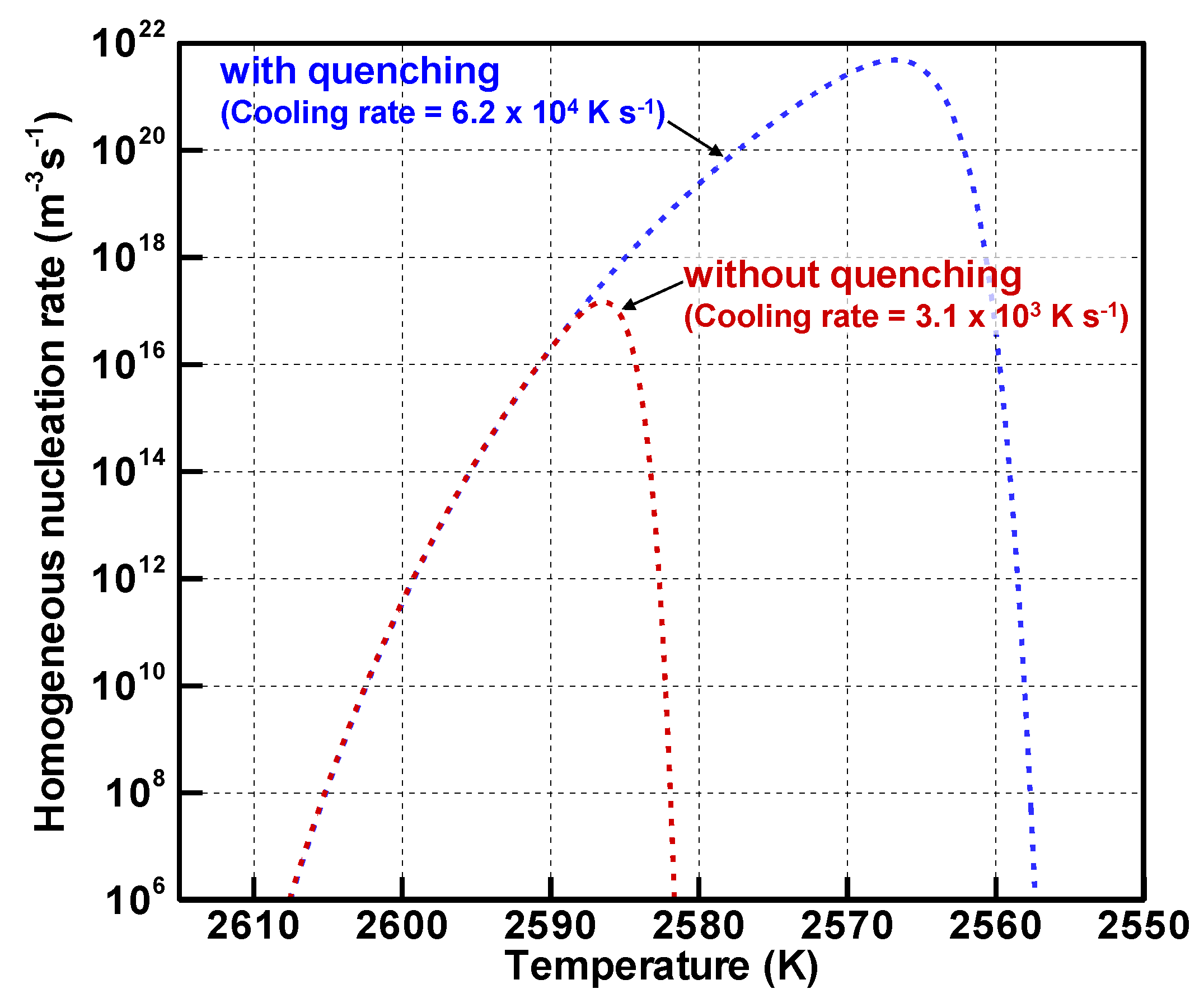
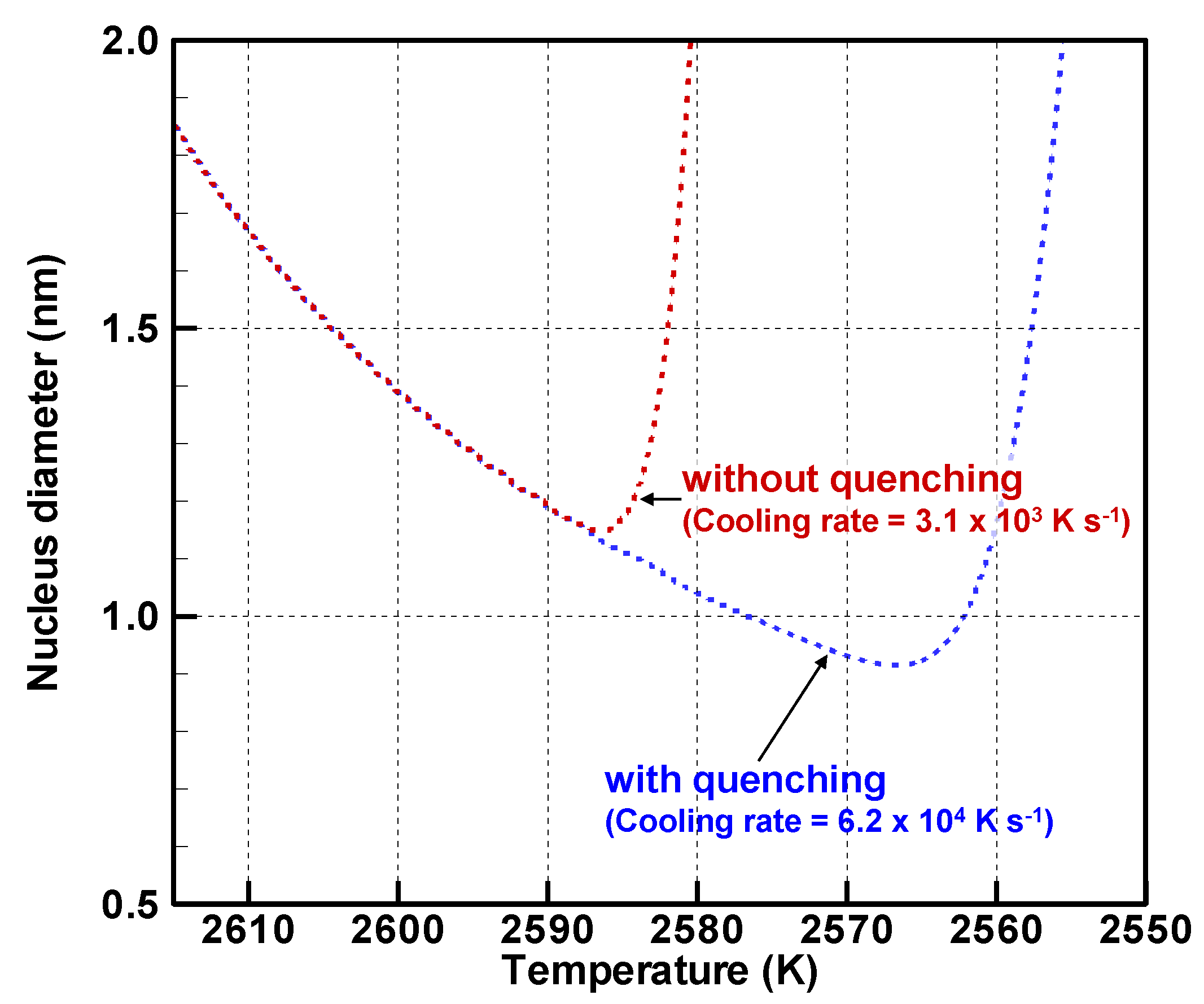
4.2. Implicit Mechanism of Collective Nanoparticle Growth
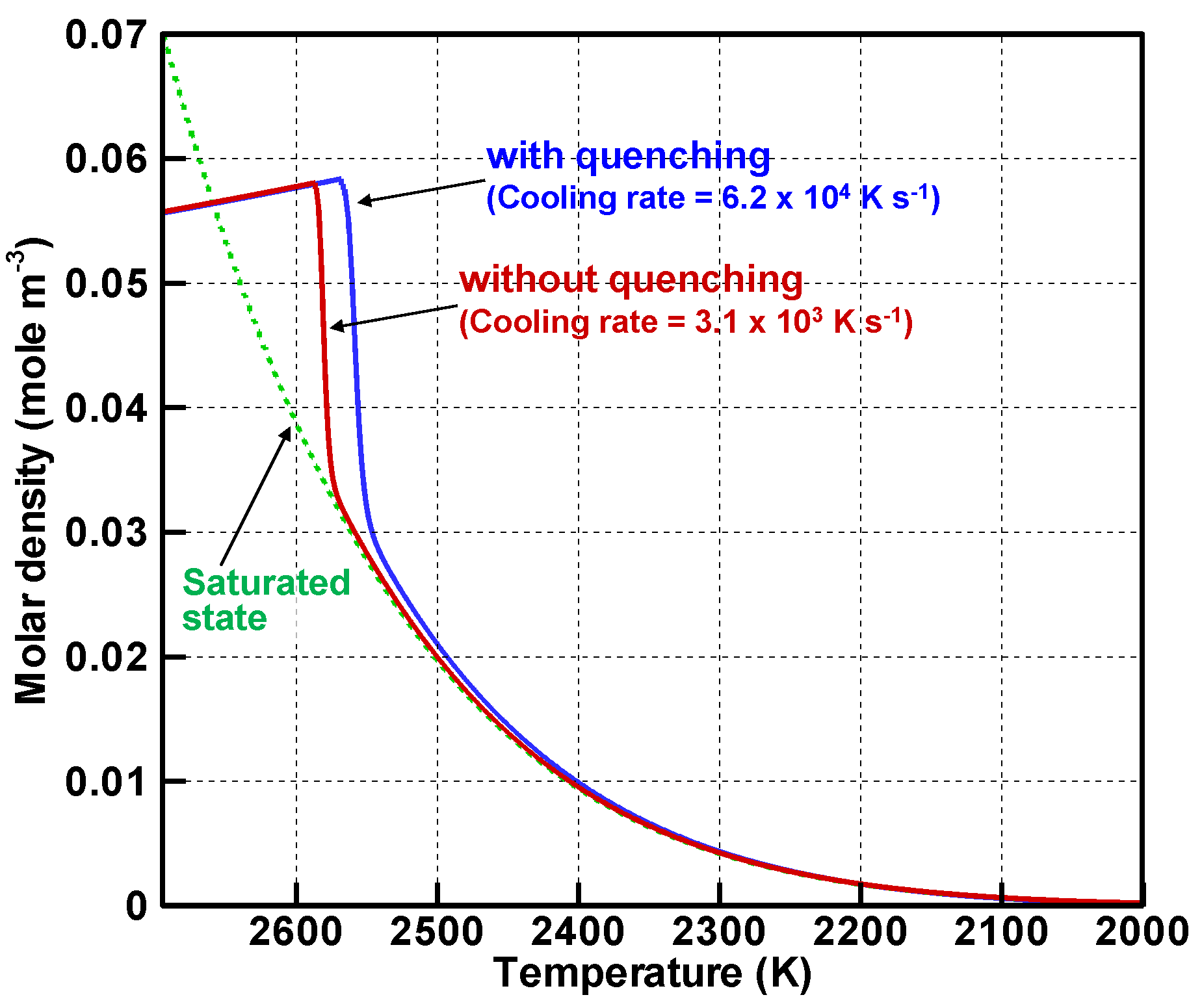
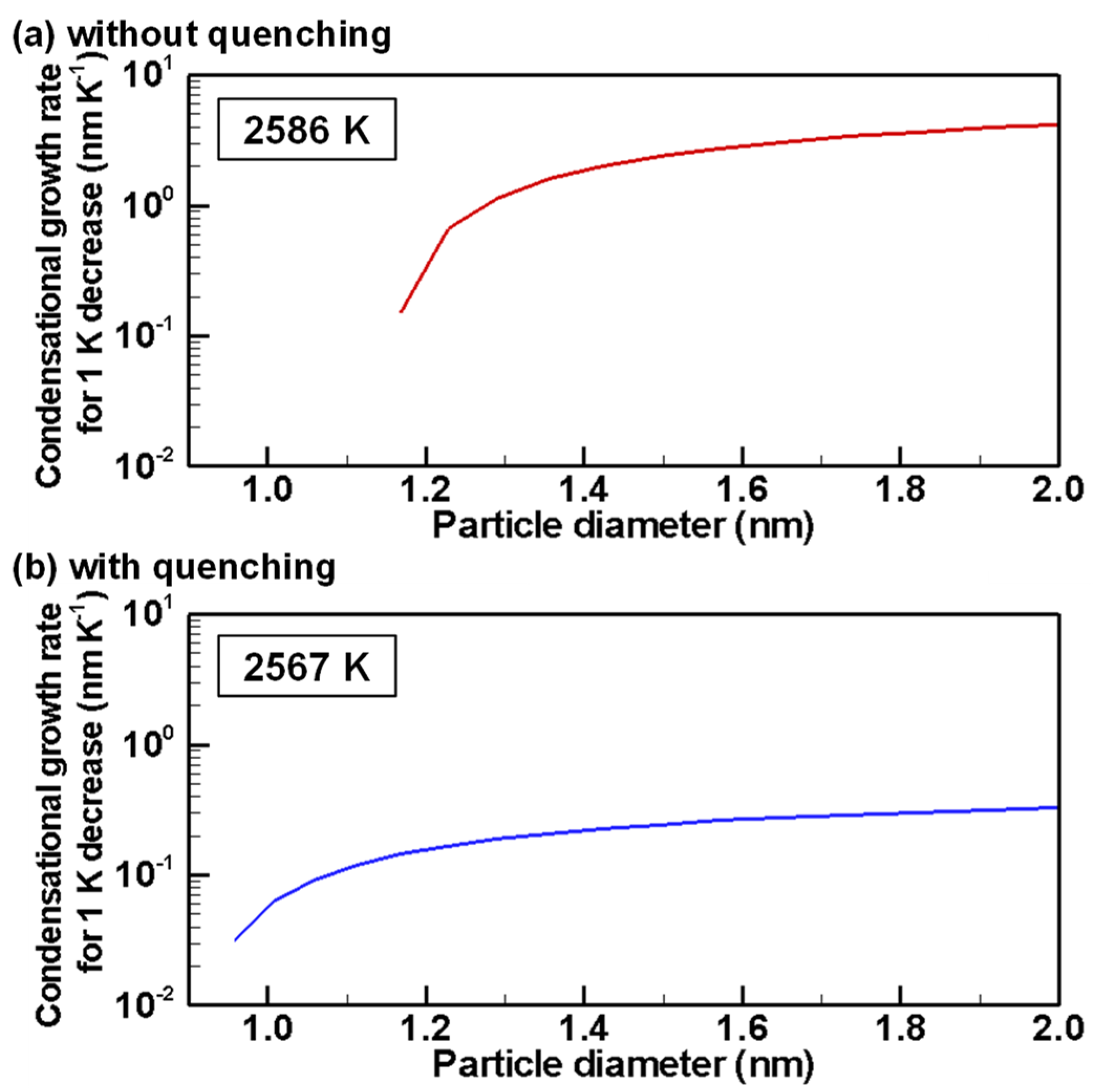
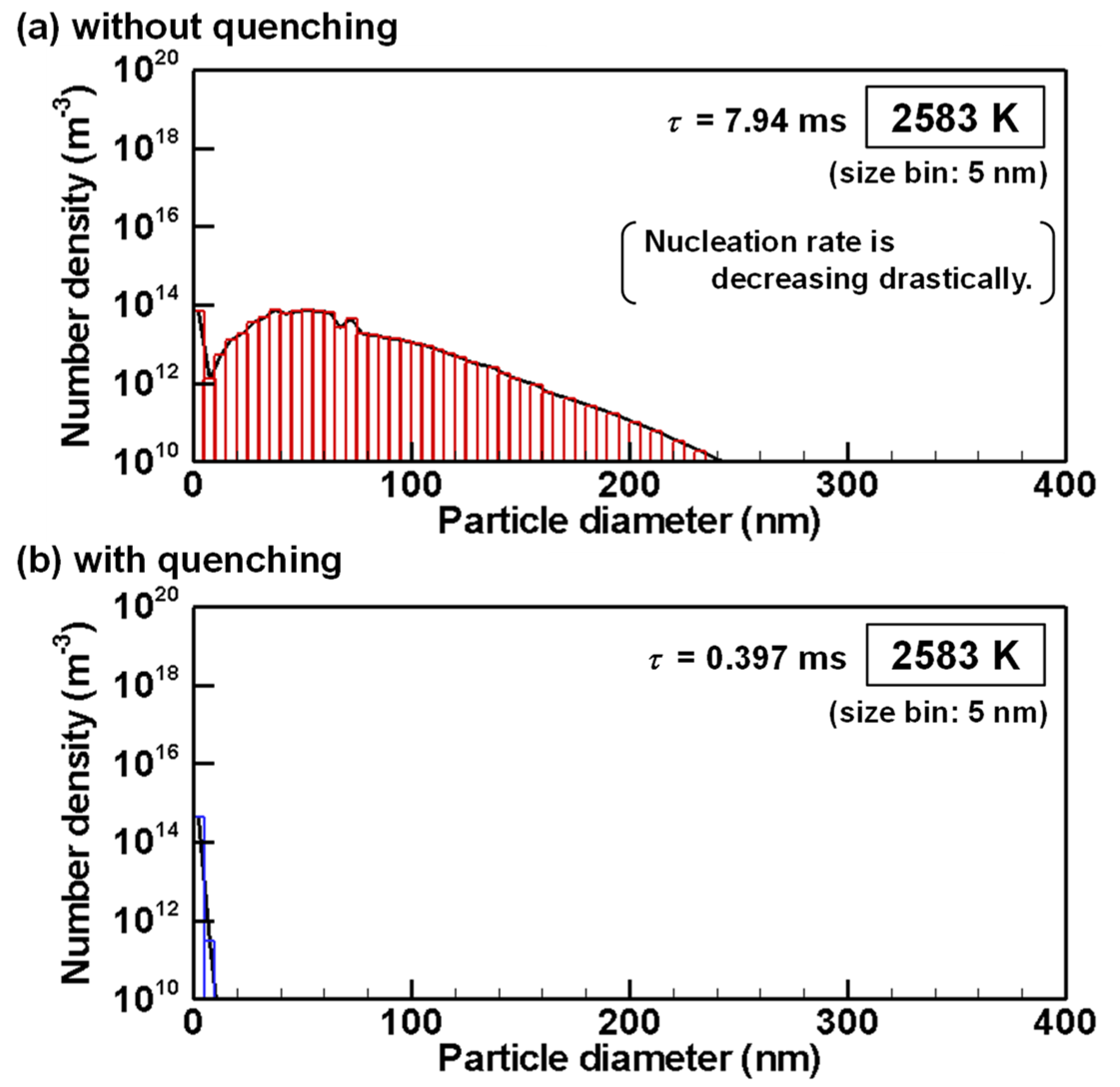
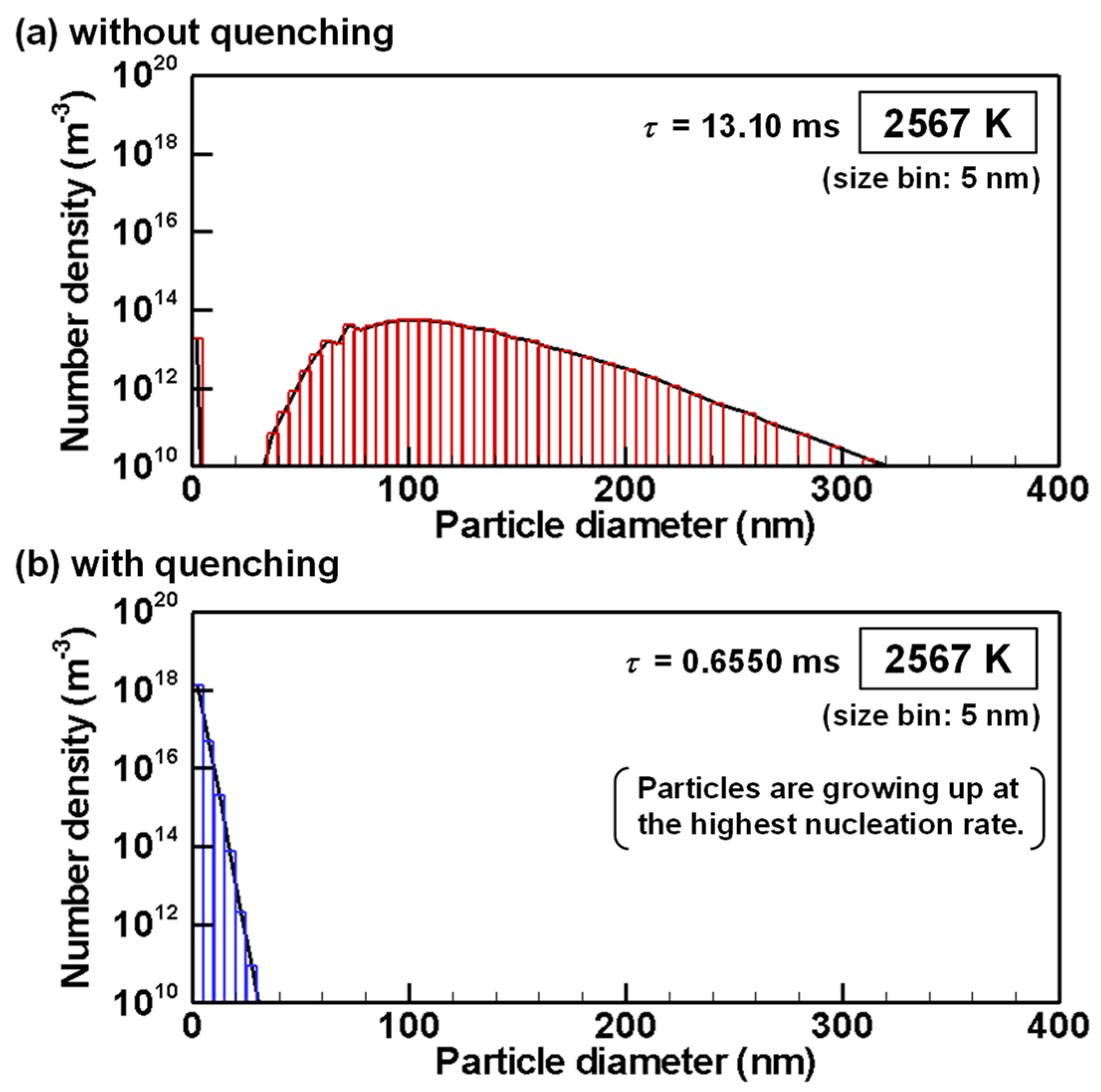
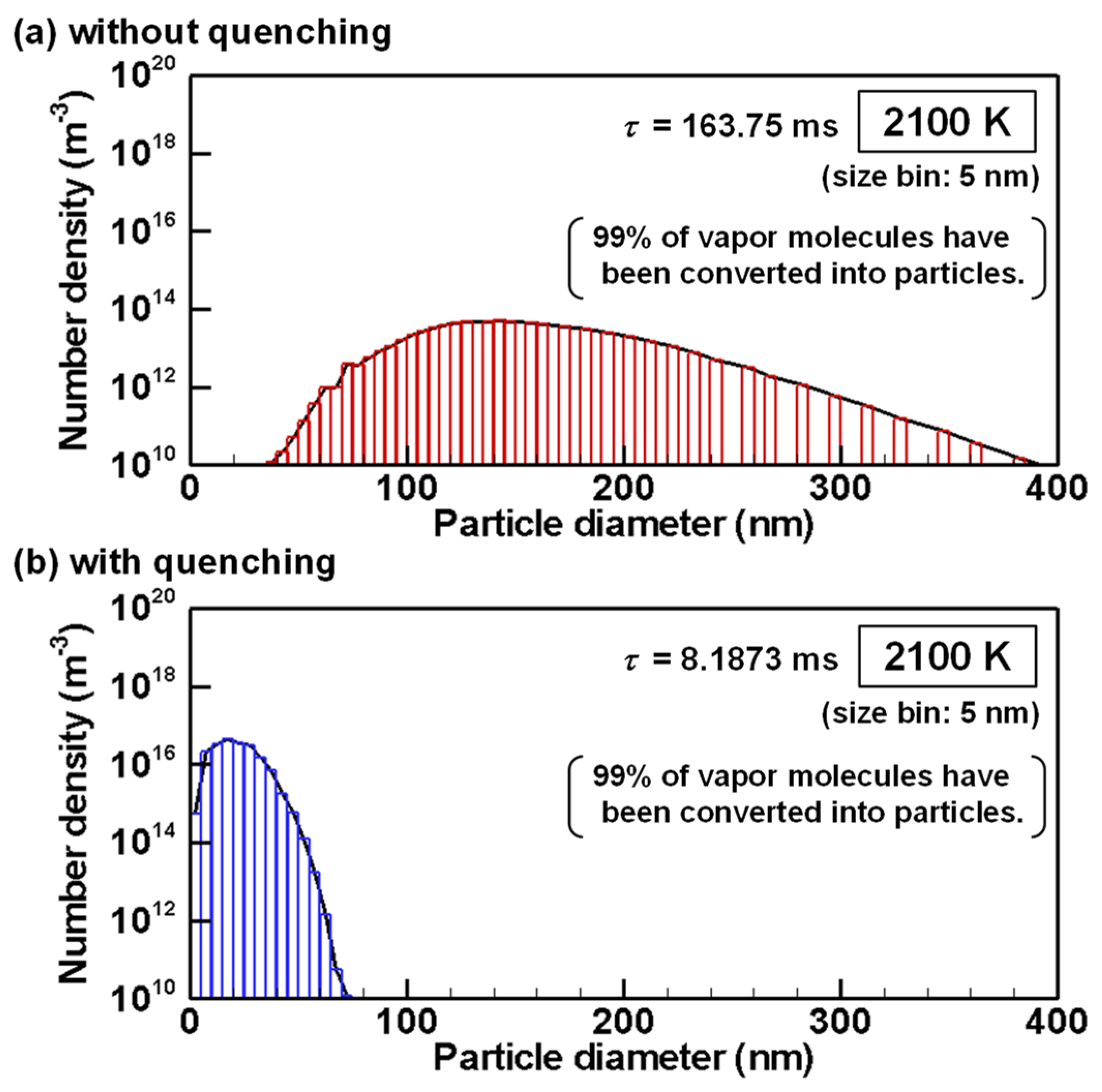
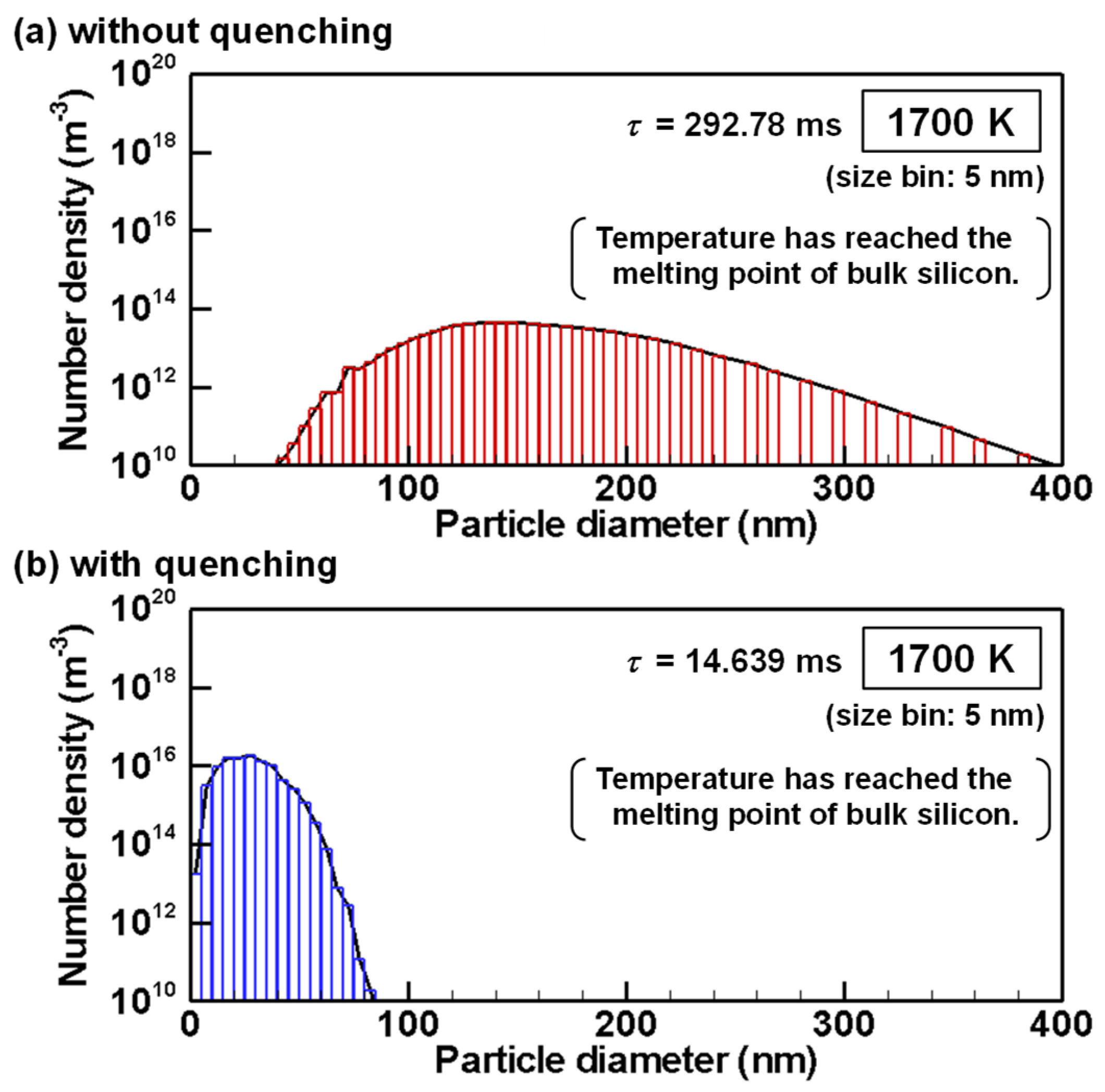
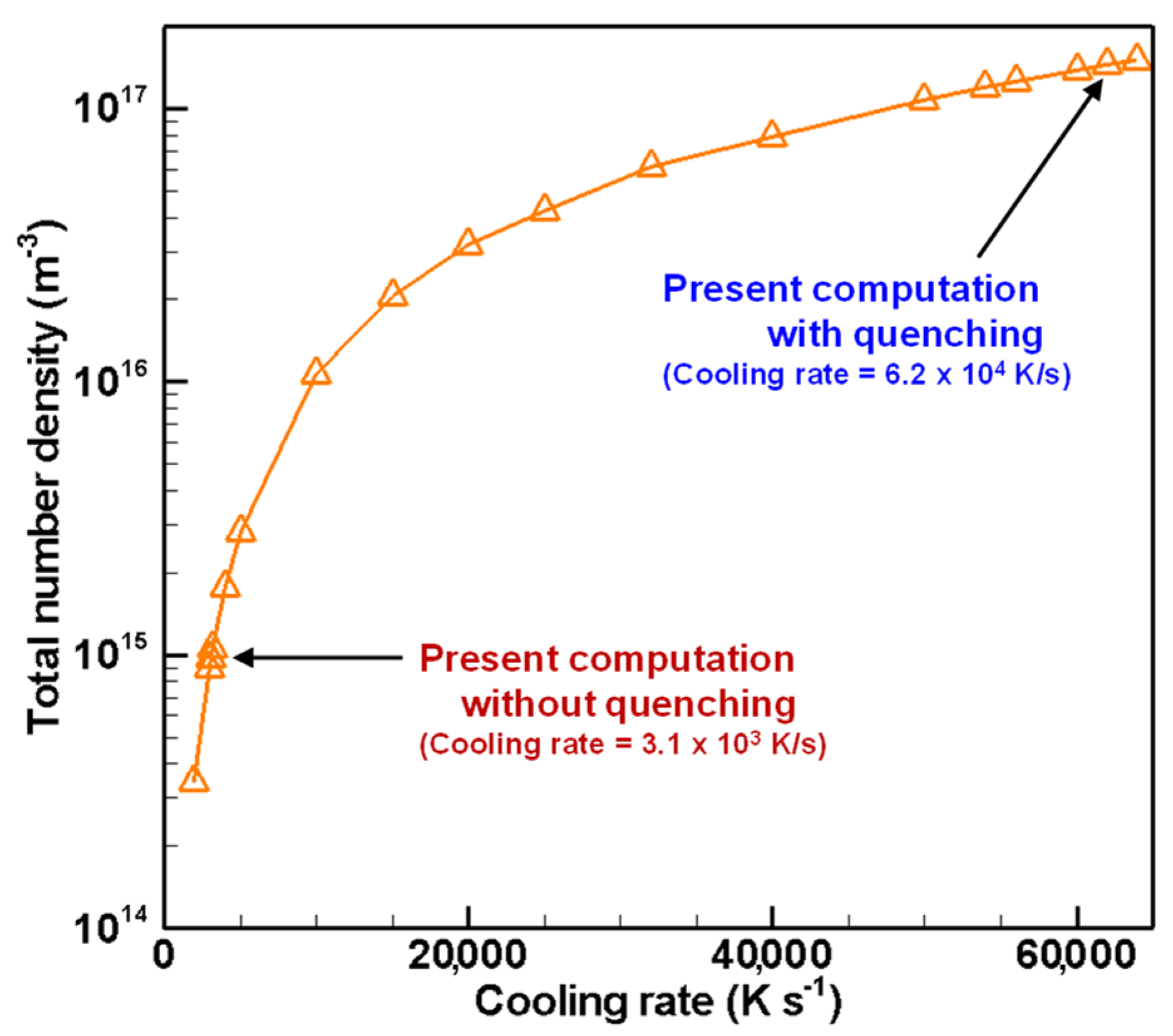
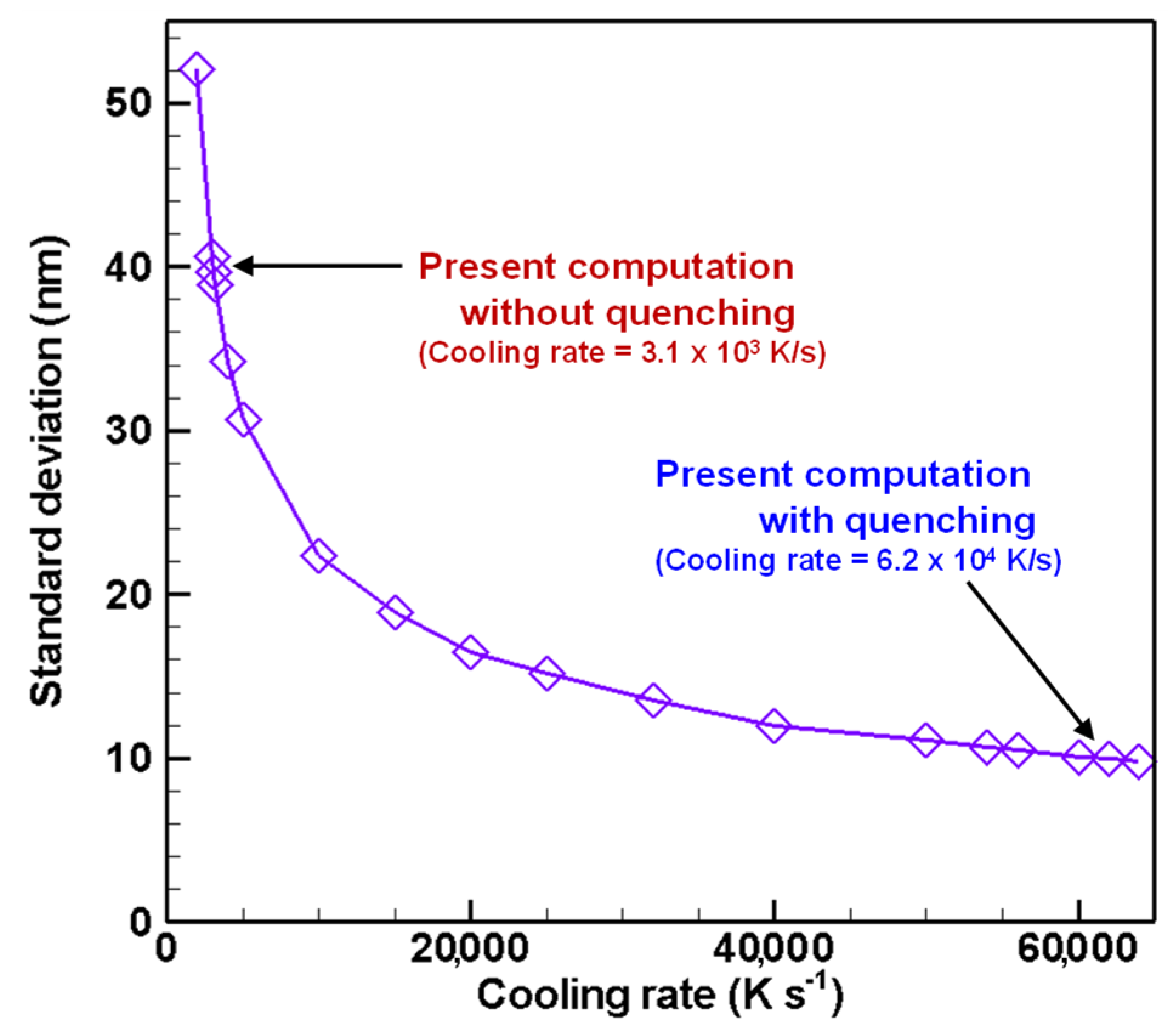
4.3. Evolution of Particle Size Distribution and Cooling Rate Dependency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.W. Synthesis and properties of nanophase materials. Mater. Sci. Eng. A 1993, 168, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Bapat, A.; Perrey, C.R.; Campbell, S.A.; Carter, C.B.; Kortshagen, U. Synthesis of highly oriented, single-crystal silicon nanoparticles in a low-pressure, inductively coupled plasma. J. Appl. Phys. 2003, 94, 1969–1974. [Google Scholar] [CrossRef]
- Bapat, A.; Anderson, C.; Perrey, C.R.; Carter, C.B.; Campbell, S.A.; Kortshagen, U. Plasma synthesis of single-crystal silicon nanoparticles for novel electronic device applications. Plasma Phys. Control. Fusion 2004, 46, B97–B109. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Dutta, A.; Willander, M.; Oda, S. Carrier conduction in a Si-nanocrystal-based single-electron transistor-I. Effect of gate bias. Superlattices Microstruct. 2000, 28, 177–187. [Google Scholar] [CrossRef]
- Nishiguchi, K.; Oda, S. Electron transport in a single silicon quantum structure using a vertical silicon probe. J. Appl. Phys. 2000, 88, 4186–4190. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Huang, S.; Yamanaka, T.; Oda, S. Evidence of storing and erasing of electrons in a nanocrystalline-Si based memory device at 77 K. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2002, 20, 1135–1138. [Google Scholar] [CrossRef]
- Ostraat, M.L.; De Blauwe, J.W.; Green, M.L.; Bell, L.D.; Brongersma, M.L.; Casperson, J.; Flagan, R.C.; Atwater, H.A. Synthesis and characterization of aerosol silicon nanocrystal nonvolatile floating-gate memory devices. Appl. Phys. Lett. 2001, 79, 433–435. [Google Scholar] [CrossRef]
- Tiwari, S.; Rana, F.; Hanafi, H.; Hartstein, A.; Crabb’e, E.F.; Chan, K. A silicon nanocrystals based memory. Appl. Phys. Lett. 1996, 68, 1377–1379. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Rana, F.; Chan, K.; Shi, L.; Hanafi, H. Single charge and confinement effects in nano-crystal memories. Appl. Phys. Lett. 1996, 69, 1232–1234. [Google Scholar] [CrossRef]
- Botas, A.M.P.; Brites, C.D.S.; Wu, J.; Kortshagen, U.; Pereira, R.N.; Carlos, L.D.; Ferreira, R.A.S. A new generation of primary luminescent thermometers based on silicon nanoparticles and operating in different media. Part. Part. Syst. Charact. 2016, 33, 740–748. [Google Scholar] [CrossRef]
- Larcher, D.; Beattie, S.; Morcrette, M.; Edström, K.; Jumasc, J.-C.; Tarascon, J.-M. Recent findings and prospects in the field of pure metals as negative electrodes for Li-ion batteries. J. Mater. Chem. 2007, 17, 3759–3772. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Kambara, M.; Kitayama, A.; Homma, K.; Hideshima, T.; Kaga, M.; Sheem, K.-Y.; Ishida, S.; Yoshida, T. Nano-composite Si particle formation by plasma spraying for negative electrode of Li ion batteries. J. Appl. Phys. 2014, 115, 143302. [Google Scholar] [CrossRef]
- Ohta, R.; Fukada, K.; Tashiro, T.; Dougakiuchi, M.; Kambara, M. Effect of PS-PVD production throughput on Si nanoparticles for negative electrode of lithium ion batteries. J. Phys. D Appl. Phys. 2018, 51, 105501. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shimizu, K.; Akashi, K.; Onda, K.; Uesugi, Y.; Ishijima, T.; Watanabe, S.; Sueyasu, S.; Nakamura, K. High rate synthesis of graphene-encapsulated silicon nanoparticles using pulse-modulated induction thermal plasmas with intermittent feedstock feeding. Jpn. J. Appl. Phys. 2020, 59, SHHE07. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hayashida, R.; Tanaka, M.; Watanabe, T. Synthesis of carbon-coated silicon nanoparticles by induction thermal plasma for lithium ion battery. Powder Technol. 2020, 371, 26–36. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Tanaka, M.; Watanabe, T. Formation mechanism of amorphous silicon nanoparticles with additional counter-flow quenching gas by induction thermal plasma. Chem. Eng. Sci. 2020, 230, 116217. [Google Scholar] [CrossRef]
- Boulos, M.I.; Fauchais, P.; Pfender, E. Thermal Plasmas Fundamentals and Applications; Plenum Press: New York, NY, USA, 1994; Volume 1, Available online: https://www.springer.com/gp/book/9780306446078 (accessed on 20 May 2021).
- Sato, T.; Shigeta, M.; Kato, D.; Nishiyama, H. Mixing and magnetic effects on a nonequilibrium argon plasma jet. Int. J. Therm. Sci. 2001, 40, 273–278. [Google Scholar] [CrossRef]
- Shigeta, M. Numerical study of axial magnetic effects on a turbulent thermal plasma jet for nanopowder production using 3D time-dependent simulation. J. Flow Control Meas. Vis. 2018, 6, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Shigeta, M.; Murphy, A.B. Thermal plasmas for nanofabrication. J. Phys. D Appl. Phys. 2011, 44, 174025. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuke, T.; Guo, W.; Uesugi, Y.; Ishijima, T.; Watanabe, S.; Nakamura, K. A large amount synthesis of nanopowder using modulated induction thermal plasmas synchronized with intermittent feeding of raw materials. J. Phys. Conf. Ser. 2012, 406, 012001. [Google Scholar] [CrossRef] [Green Version]
- Kodama, N.; Tanaka, Y.; Kita, K.; Uesugi, Y.; Ishijima, T.; Watanabe, S.; Nakamura, K. A method for large-scale synthesis of Al-doped TiO2 nanopowder using pulse-modulated induction thermal plasmas with time-controlled feedstock feeding. J. Phys. D Appl. Phys. 2014, 47, 195304. [Google Scholar] [CrossRef] [Green Version]
- Shigeta, M.; Nishiyama, H. Numerical analysis of metallic nanoparticle synthesis using RF inductively coupled plasma flows. Trans. ASME J. Heat Transf. 2005, 127, 1222–1230. [Google Scholar] [CrossRef]
- Shigeta, M.; Tanaka, M.; Ghedini, E. Numerical analysis of correlation between arc plasma fluctuation and nanoparticle growth-transport under atmospheric pressure. Nanomaterials 2019, 9, 1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, S.; Tanaka, M.; Yamane, S.; Miyasaka, F.; Shigeta, M.; Nomura, K.; Ogino, Y. Recent progresses of welding and joining engineering. J. Jpn. Weld. Soc. 2020, 89, 322–335. (In Japanese) [Google Scholar] [CrossRef]
- Shigeta, M. Modeling and simulation of a turbulent-like thermal plasma jet for nanopowder production. IEEJ Trans. Electr. Electron. Eng. 2019, 14, 16–28. [Google Scholar] [CrossRef] [Green Version]
- Shigeta, M. Simulating turbulent thermal plasma flows for nanopowder fabrication. Plasma Chem. Plasma Process. 2020, 40, 775–794. [Google Scholar] [CrossRef]
- Shigeta, M. Turbulence modelling of thermal plasma flows. J. Phys. D Appl. Phys. 2016, 49, 493001. [Google Scholar] [CrossRef]
- Watanabe, T.; Okumiya, H. Formation mechanism of silicide nanoparticles by induction thermal plasmas. Sci. Technol. Adv. Mater. 2004, 5, 639–646. [Google Scholar] [CrossRef]
- Kambara, M.; Hideshima, T.; Kaga, M.; Yoshida, T. Powders: Plasma Spray PVD for High-Throughput Production, Encyclopedia of Plasma Technology, 1st ed.; Shohet, J.L., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2017; pp. 1176–1190. Available online: https://www.taylorfrancis.com/books/mono/10.1081/E-EPLT/encyclopedia-plasma-technology-leon-shohet (accessed on 20 May 2021).
- Ishisaka, Y.; Kodama, N.; Kita, K.; Tanaka, T.; Uesugi, Y.; Ishijima, T.; Sueyasu, S.; Watanabe, S.; Nakamura, K. High-rate synthesis of Si nanowires using modulated induction thermal plasmas. Appl. Phys. Express 2017, 10, 096201. [Google Scholar] [CrossRef]
- Kong, P.C.; Lau, Y.C. Plasma synthesis of ceramic powders. Pure Appl. Chem. 1990, 62, 1809–1816. [Google Scholar] [CrossRef]
- Leparoux, M.; Schreuders, C.; Fauchais, P. Improved plasma synthesis of Si-nanopowders by quenching. Adv. Eng. Mater. 2008, 10, 1147–1150. [Google Scholar] [CrossRef]
- Li, J.G.; Wang, X.; Tang, C.; Ishigaki, T.; Tanaka, S. Energy transfer enables 1.53 μm photoluminescence from erbium-doped TiO2 semiconductor nanocrystals synthesized by Ar/O2 radio-frequency thermal plasma. J. Am. Ceram. Soc. 2008, 91, 2032–2035. [Google Scholar] [CrossRef]
- Proulx, P.; Bilodeau, J.-F. A model for ultrafine powders production in a thermal plasma reactor. Plasma Chem. Plasma Process. 1991, 11, 371–386. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.-P.; Muno, R.; Wu, C.Y.; Yang, L.; Singh, S.K.; McMurry, P.H. Thermal plasma synthesis of ultrafine iron particles. J. Aerosol Sci. 1993, 24, 367–382. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Proulx, P. A mathematical model for ultrafine iron powder growth in thermal plasma. Aerosol Sci. Technol. 1996, 24, 175–189. [Google Scholar] [CrossRef]
- Désilets, M.; Bilodeau, J.F.; Proulx, P. Modelling of the reactive synthesis of ultra-fine powders in a thermal plasma reactor. J. Phys. D Appl. Phys. 1997, 30, 1951–1960. [Google Scholar] [CrossRef]
- Cruz, A.C.D.; Munz, R.J. Vapor phase synthesis of fine particles. IEEE Trans. Plasma Sci. 1997, 25, 1008–1016. [Google Scholar] [CrossRef]
- Aristizabal, F.; Munz, R.J.; Berk, D. Modeling of the production of ultra fine aluminium particles in rapid quenching turbulent flow. J. Aerosol Sci. 2006, 37, 162–186. [Google Scholar] [CrossRef]
- Mendoza-Gonzalez, N.Y.; Goortani, B.M.; Proulx, P. Numerical simulation of silica nanoparticles production in an RF plasma reactor: Effect of quench. Mater. Sci. Eng. C 2007, 27, 1265–1269. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Numerical investigation of cooling effect on platinum nanoparticle formation in inductively coupled thermal plasmas. J. Appl. Phys. 2008, 103, 074903. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Two-dimensional analysis of nanoparticle formation in induction thermal plasmas with counterflow cooling. Thin Solid Films 2008, 516, 4415–4422. [Google Scholar] [CrossRef]
- Mendoza-Gonzalez, N.Y.; Morsli, M.E.; Proulx, P. Production of nanoparticles in thermal plasmas: A model including evaporation, nucleation, condensation, and fractal aggregation. J. Therm. Spray Technol. 2008, 17, 533–550. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Model integration for metal nanoparticle synthesis by an rf thermal plasma flow with counterflow cooling. Trans. Jpn. Soc. Mech. Eng. Part B 2009, 75, 2019–2028. (In Japanese) [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.-P.; McMurry, P.H. Modelling particle formation and growth in a plasma synthesis reactor. Plasma Chem. Plasma Process. 1988, 8, 145–157. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, C.-P. Numerical study of MgO powder synthesis by thermal plasma. J. Aerosol Sci. 1990, 21, 641–650. [Google Scholar] [CrossRef]
- Joshi, S.V.; Liang, Q.; Park, J.Y.; Batdorf, J.A. Effect of quenching conditions on particle formation and growth in thermal plasma synthesis of fine powders. Plasma Chem. Plasma Process. 1990, 10, 339–358. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T.; Nishiyama, H. Numerical investigation for nano-particle synthesis in an RF inductively coupled plasma. Thin Solid Films 2004, 457, 192–200. [Google Scholar] [CrossRef]
- Hirayama, Y.; Suzuki, K.; Yamaguchi, W.; Takagi, K. Cold welding behavior of fine bare aluminum powders prepared by new low oxygen induction thermal plasma system. J. Alloys Compd. 2018, 768, 608–612. [Google Scholar] [CrossRef]
- Hirayama, Y.; Shigeta, M.; Liu, Z.; Yodoshi, N.; Hosokawa, A.; Takagi, K. Anisotropic Nd-Fe ultrafine particles with stable and metastable phases prepared by induction thermal plasma. J. Alloys Compd. 2021, 873, 159724. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Numerical analysis for preparation of silicon-based intermetallic nano-particles in induction thermal plasma flow systems. JSME Int. J. Ser. B Fluids Therm. Eng. 2005, 48, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Shigeta, M.; Watanabe, T. Numerical analysis for co-condensation processes in silicide nanoparticle synthesis using induction thermal plasma at atmospheric pressure conditions. J. Mater. Res. 2005, 20, 2801–2811. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Multi-component co-condensation model of Ti-based boride/silicide nanoparticle growth in induction thermal plasmas. Thin Solid Films 2007, 515, 4217–4227. [Google Scholar] [CrossRef]
- Nemchinsky, V.A.; Shigeta, M. Simple equations to describe aerosol growth. Model. Simul. Mater. Sci. Eng. 2012, 20, 045017. [Google Scholar] [CrossRef]
- Shigeta, M. Simple nonequilibrium model of collective growth and transport of metal nanomist in a thermal plasma process. Theor. Appl. Mech. Jpn. 2015, 63, 147–154. [Google Scholar] [CrossRef]
- Murphy, A.B. Formation of titanium nanoparticles from a titanium tetrachloride plasma. J. Phys. D Appl. Phys. 2004, 37, 2841–2847. [Google Scholar] [CrossRef]
- Watanabe, T.; Shigeta, M. Nanoparticle synthesis by thermal plasmas. In Nanoparticles: Properties, Classification, Characterization, and Fabrication; Kestell, A.E., DeLorey, G.T., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 149–212. Available online: http://www.novapublishers.org/catalog/product_info.php?products_id=18755 (accessed on 20 May 2021).
- Watanabe, T.; Shigeta, M. Nanoparticle synthesis by thermal plasmas. In Nanomaterials: Properties, Preparation and Processes; Cabral, V., Silva, R., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 115–176. Available online: http://www.novapublishers.org/catalog/product_info.php?products_id=11125 (accessed on 20 May 2021).
- Tanaka, Y.; Onda, K.; Akashi, K.; Furukawa, R.; Nakano, Y.; Ishijima, T.; Uesugi, Y.; Sueyasu, S.; Watanabe, S.; Nakamura, K. A numerical study on nanoparticle synthesis in pulse-modulated induction thermal plasmas with intermittent feedstock powder feeding by method of moment. In Proceedings of the Gaseous Electronics Conference, Online, 6 October 2020. GT3.00006. [Google Scholar]
- Rao, N.; Girshick, S.L.; Heberlein, J.; McMurry, P.; Jones, S.; Hansen, D.; Micheel, B. Nanoparticle formation using a plasma expansion process. Plasma Chem. Plasma Process. 1995, 15, 581–606. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Growth mechanism of silicon-based functional nanoparticles fabricated by inductively coupled thermal plasmas. J. Phys. D Appl. Phys. 2007, 40, 2407–2419. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Two-directional nodal model for co-condensation growth of multi-component nanoparticles in thermal plasma processing. J. Therm. Spray Technol. 2009, 18, 1022–1037. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Growth model of binary alloy nanopowders for thermal plasma synthesis. J. Appl. Phys. 2010, 108, 043306. [Google Scholar] [CrossRef]
- Cheng, Y.; Shigeta, M.; Choi, S.; Watanabe, T. Formation mechanism of titanium boride nanoparticles by RF induction thermal plasma. Chem. Eng. J. 2012, 183, 483–491. [Google Scholar] [CrossRef]
- Shigeta, M.; Miyake, M.; Tanaka, M. Numerical analysis of collective growth of primary nanoparticles in arc welding. Q. J. Jpn. Weld. Soc. 2015, 33, 365–375. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Shigeta, M.; Watanabe, T. Effect of precursor fraction on silicide nanopowder growth under thermal plasma conditions: A computational study. Powder Technol. 2016, 288, 191–201. [Google Scholar] [CrossRef]
- Shigeta, M.; Watanabe, T. Effect of saturation pressure difference on metal-silicide nanopowder formation in thermal plasma fabrication. Nanomaterials 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, V.; Ghedini, E.; Gherardi, M.; Sanibondi, P.; Shigeta, M. A two-dimensional nodal model with turbulent effects for the synthesis of Si nano-particles by inductively coupled thermal plasmas. Plasma Sources Sci. Technol. 2012, 21, 025001. [Google Scholar] [CrossRef]
- Bora, B.; Saikia, B.J.; Borgohain, C.; Kakati, M.; Das, A.K. Numerical investigation of nanoparticle synthesis in supersonic thermal plasma expansion. Vacuum 2010, 85, 283–289. [Google Scholar] [CrossRef]
- Shigeta, M.; Minami, S.; Tanaka, M. Modeling for collective growth of fume primary particles with charge effect in arc welding. Weld. World 2018, 62, 203–213. [Google Scholar] [CrossRef]
- Girshick, S.L.; Chiu, P.C.; McMurry, P.H. Time-dependent aerosol models and homogeneous nucleation rates. Aerosol Sci. Technol. 1990, 13, 465–477. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. Atmospheric Chemistry and Physics, From Air Pollution to Climate Change; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B. Molecular Theory of Gases and Liquids; Wiley: New York, NY, USA, 1954. [Google Scholar]
- Friedlander, S.K. Smoke, Dust and Haze: Fundamentals of Aerosol Dynamics, 2nd ed.; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Guisbiers, G.; Kazan, M.; Overschelde, O.V.; Wautelet, M.; Pereira, S. Mechanical and thermal properties of metallic and semiconductive nanostructures. J. Phys. Chem. 2008, 112, 4097–4103. [Google Scholar] [CrossRef]
- The Japan Institute of Metals and Materials. Metal Data Book; Maruzen: Tokyo, Japan, 1993. (In Japanese) [Google Scholar]
- Kawajiri, K.; Sato, T.; Nishiyama, H. Experimental analysis of a DC–RF hybrid plasma flow. Surf. Coat. Technol. 2003, 171, 134–139. [Google Scholar] [CrossRef]
- Takana, H.; Jang, J.; Igawa, J.; Nakajima, T.; Solonenko, O.P.; Nishiyama, H. Improvement of in-flight alumina spheroidization process using a small power argon DC-RF hybrid plasma flow system by helium mixture. J. Therm. Spray Technol. 2011, 20, 432–439. [Google Scholar] [CrossRef]
- Shigeta, M. Time-dependent 3-D simulation of an argon RF inductively coupled thermal plasma. Plasma Sources Sci. Technol. 2021, 21, 055029. [Google Scholar] [CrossRef]
- Shigeta, M. Numerical investigation of nano-material processing by thermal plasma flows. In Sustained Simulation Performance 2012; Resch, M.M., Wang, X., Bez, W., Focht, E., Kobayashi, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 169–182. [Google Scholar] [CrossRef]
- Shigeta, M. Three-dimensional flow dynamics of an argon RF plasma with dc jet assistance: A numerical study. J. Phys. D Appl. Phys. 2013, 46, 015401. [Google Scholar] [CrossRef]
- Shigeta, M.; Sato, T.; Nishiyama, H. Numerical simulation of a potassium-seeded turbulent RF inductively coupled plasma with particles. Thin Solid Films 2003, 435, 5–12. [Google Scholar] [CrossRef]
- Shigeta, M.; Sato, T.; Nishiyama, H. Computational simulation of a particle-laden RF inductively coupled plasma with seeded potassium. Int. J. Heat Mass Transf. 2004, 47, 707–716. [Google Scholar] [CrossRef]
- Xiang, J.; Park, H.; Tanaka, K.; Shigeta, M.; Tanaka, M.; Murphy, A.B. Numerical study of the effects and transport mechanisms of iron vapour in tungsten inert-gas welding in argon. J. Phys. D Appl. Phys. 2020, 53, 044004. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, F.F.; Park, H.; Tanaka, K.; Shigeta, M.; Tanaka, M.; Murphy, A.B. Numerical study of the metal vapour transport in tungsten inert-gas welding in argon for stainless steel. Appl. Math. Model. 2020, 79, 713–728. [Google Scholar] [CrossRef]
- Tanaka, K.; Shigeta, M.; Tanaka, M.; Murphy, A.B. Imaging spectroscopy for transient transport of chromium vapor during helium TIG welding. Q. J. Jpn. Weld. Soc. 2020, 38, 21s–24s. [Google Scholar] [CrossRef]
- Tanaka, K.; Shigeta, M.; Tanaka, M.; Murphy, A.B. Investigation of transient metal vapour transport processes in helium arc welding by imaging spectroscopy. J. Phys. D Appl. Phys. 2020, 53, 425202. [Google Scholar] [CrossRef]




| Model Type A [51,54,55,56] | Model Type B [26,27,28,29,57,58] | Model Type C [39,40,42,43,44,45,46,47,59,60,61,62] | Model Type D [37,48,49,53,63,64,65,66,67,68,69,70,71,72,73] | |
|---|---|---|---|---|
| Nucleation | considered | considered | considered | considered |
| Condensation | considered | considered | considered | considered |
| Coagulation | not considered | considered | considered | considered |
| Size distribution | any | mono-disperse | lognormal | any |
| Mathematical description | simpler | simpler | more complex | more complex |
| Computational costs | higher | lower | lower | higher |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeta, M.; Hirayama, Y.; Ghedini, E. Computational Study of Quenching Effects on Growth Processes and Size Distributions of Silicon Nanoparticles at a Thermal Plasma Tail. Nanomaterials 2021, 11, 1370. https://doi.org/10.3390/nano11061370
Shigeta M, Hirayama Y, Ghedini E. Computational Study of Quenching Effects on Growth Processes and Size Distributions of Silicon Nanoparticles at a Thermal Plasma Tail. Nanomaterials. 2021; 11(6):1370. https://doi.org/10.3390/nano11061370
Chicago/Turabian StyleShigeta, Masaya, Yusuke Hirayama, and Emanuele Ghedini. 2021. "Computational Study of Quenching Effects on Growth Processes and Size Distributions of Silicon Nanoparticles at a Thermal Plasma Tail" Nanomaterials 11, no. 6: 1370. https://doi.org/10.3390/nano11061370






