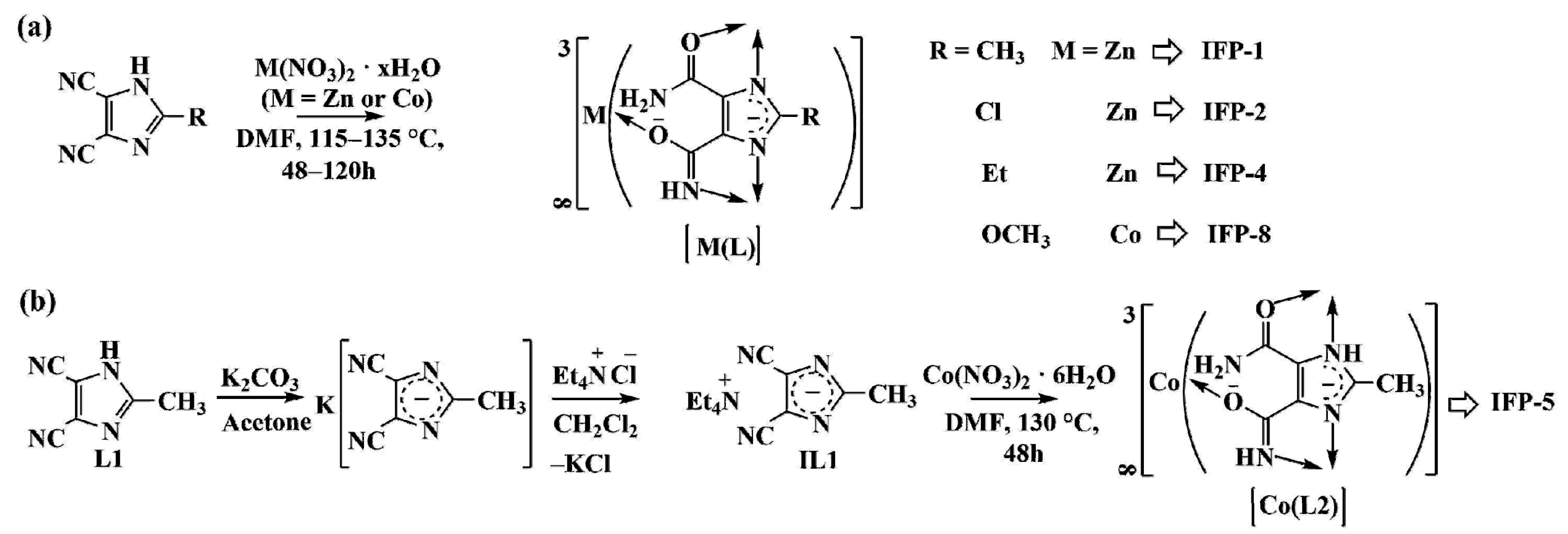
3. Results and Discussion
Although N
2 isotherms at 77.4 K (see
Figure 5) are the common method for the characterization of porous materials, it is limited with respect to resolution in the ultramicroporous range as well as macropores bigger than 350 nm in diameter [
45,
46]. For this purpose, CO
2 isotherms at 273.15 K (see
Figure 6) were also used to resolve the micropores. CO
2 not only has a smaller kinetic diameter (
) in principle (see
Table 1), but is also kinetically more active at an experimental temperature of 273.15 K compared to N
2 at 77.4 K and therefore penetrates micropores more easily. Another reason for the better resolution of the micropore range is given due to a temperature-related flexibility of the adsorbents used, as it has often been reported for MOFs [
47,
48,
49,
50,
51] as well as for IFP compounds [
52,
53]. However, isotherms derived from water vapor, which exhibits the smallest kinetic diameter of the selected adsorptives, allow a better insight in local phenomena.
Figure 7 shows the experimental adsorption and desorption equilibria of IFP-1, -2, -4, -5 and -8 at 303.15 K approximated by the model equation introduced above (see Equation (3)). What all isotherms have in common is a distinct hysteresis loop between the adsorption and desorption branch over the entire range of moisture. The phenomenon of low pressure hysteresis down to the lowest attainable pressures, which is most significant for IFP-4 and -8 is a strong indicator for highly microporous (
2 nm) up to ultramicroporous (
0.7 nm) systems [
19,
32]. Fully sample regeneration is only possible if the adsorbent is outgassed at higher temperatures. This characteristic feature may occur due to the swelling of non-rigid pore structures, which is known for several microporous MOF species [
48,
54], or due to irreversible uptake by chemisorption [
19,
32]. However, a fundamental difference can be observed regarding the shape of the isotherms. While IFP-1, IFP-2 and IFP-5 exhibit an H3-type behavior (see
Appendix A,
Figure A1) according to the IUPAC classification [
32], IFP-4 and IFP-8 are attributable to the H4-type (see
Appendix A,
Figure A1), but without showing the trend towards unlimited adsorption capacities at high relative humidity (
1).
Both types, H3 and H4, have in common that they are primarily observed on aggregate or agglomerate forming plate-like particles, which are giving rise to the assumption of the presence of slit-shaped pores, which was already concluded from previous investigations [
40,
52,
53]. Comparison of the particle size analysis of the untreated as synthesized samples and the dispersed samples are validating this postulate. Thus, the particle size distributions—volume density distribution,
(see
Appendix A,
Figure A2, right column), and volume sum distribution,
(see
Appendix A,
Figure A2, left column)—of each sample were significantly shifted towards smaller particle sizes after breaking the agglomerates and aggregates by employing the above mentioned dispersion methods (see
Section 2.2). A quantitative evaluation of the results is possible by comparison of the median diameters (see
Appendix A,
Table A1). For IFP-1, -4, -5 and -8, the median diameters were reduced by the factor of about 3, for IFP-2 even by the factor of almost 6. This becomes even more comprehensible in view of the volume density distribution of IFP-2, since here a completely new, very small particle fraction with a size between 50 and 100 nm is detected after dispersion.
The H3 type with three significant inflection points, which are particularly pronounced in the desorption branch, indicates the coexistence of two dominant pore sizes in the ultramicropore and supermicro-/mesopore range, which are emptying at
0 and
0.3–0.4, respectively. This is in good accordance to the pore size distributions derived from isotherm measurements taken with the adsorptives CO
2 and N
2. For IFP-1, -2 and -5, significant amounts of N
2 as well as CO
2 were adsorbed at 77.4 K and 273.15 K, respectively (see
Figure 5;
Figure 6). The pore size distributions (see
Appendix A,
Figure A3) derived from these measurements by means of NLDFT yield a cumulative pore volume that is shared between micro- (
2 nm) and mesopores (2 nm
50 nm), with a clear predominance of micropores for all three adsorbents under consideration (see
Table 2). This significant deviation of the pore size distributions compared to simulated accessible pore diameters derived from the crystallographic data (see
Figure 2) can only be attributed to the formation of defects in the crystal structure during material synthesis, which were already known for various MOFs [
55] and also for IFPs themselves [
56,
57].
The H4 type, which is equal to the shape of a type I isotherm according to IUPAC, is characteristic for adsorbents that contain micropores solely and shows again good agreement to the conclusions to be drawn from N
2 and CO
2 measurements. Since the measurements with N
2 show no uptake capacity for both samples—IFP-4 and -8—(see
Figure 5), but merely for CO
2 (see
Figure 6), the presence of macro- and especially mesopores can be excluded. Therefore, water vapor isotherms are exhibiting no inflection points in this case. Although only micropores are detected for IFP-4 and -8, it must also be stated that the experimentally determined pore diameters exceed the theoretical values derived from the crystallographic data, since the kinetic diameter of all used adsorptives is larger. Again, only flexibility and gate opening effects or defects in crystal structure of the IFP samples can be considered as cause for this deviation. In fact, previous publications have already referred to the flexible properties of ethyl- and methoxy-substituted linkers of IFP-4 [
53] and -8 [
52], respectively.
The reason why the H3 type isotherms (IFP-1, -2 and -5), in contrast to the H4 type isotherms (IFP-4 and -8), appear to tend towards unlimited adsorption capacities at high relative humidity (
) is attributed to the presence of either intra- or interparticle macro-/mesopores [
19]. As already seen from the N
2 measurements for IFP-1, -2 and -5, defects in the metal framework might lead to the formation of intraparticle meso- and macropores. In addition, interparticle macropores can also be present by a special crystal morphology due to aggregate formation or properties of the particle bed itself. The latter, however, seems to be most probable and can be explained by the median values of the particle size distributions, which are lowest for IFP-1, -2 and -5 (see
Appendix A,
Table A1), allowing for the highest bulk densities and lowest interparticle voids, respectively.
These observations are also in good agreement with the conclusions of Sircar’s theory for capillary condensation of vapors on porous materials [
31]. According to his theory isotherms taken on adsorbents with high surface area to pore volume ratios (
) are appoaching type I (or H4) shapes, while in case of lower surface area to pore volume ratios type IV (or H3) is approached. As shown in
Table 2 the surface area to pore volume ratio for IFP-4 and -8 (H4) is significantly higher compared to IFP-1, -2 and -5, which is convincing as the pore volume decreases exponentially stronger than the surface area with decreasing pore diameter.
The fact that CO
2 isotherms are, in contrast to N
2, exhibiting a hysteresis behavior can either be explained by stronger adsorbent-adsorbate interactions due to the higher polarizability (
= 2.34 Å
3,
= 1.05 Å
3, [
58]) and quadrupole momentum (
= −16.94·10
−20 C Å
2,
= −4.47·10
−20 C Å
2, [
58]) of CO
2 or related to network effects in the micropore range of the IPF-samples.
Table 2 also lists the maximum water loads of the examined IFP samples in order to draw conclusions about the influence of the different moieties of the linkers and transition metals employed. For IFP-1, which has Zn as transition metal center and a methyl-group as moiety connected to the C2 position of the imidazolate linker, the maximum load at 273.15 K is approximately 2.81 mmol g
−1. By substituting the methyl group with chloride (IFP-2), the maximum load increases by over 100% to 5.75 mmol g
−1. Thus, the adsorption capacity of water vapor can be significantly increased by the introduction of a polar C-Cl bond, but with only a slightly lower surface area. However, if the methyl residue of IFP-1 is extended by methylene unit (IFP-4) the adsorption capacity collapses almost by the factor of 6 to 0.48 mmol g
−1. This can be explained by the reduction of the pore diameter and the specific surface area as well as the increase of the non-polar character of the channel walls (see
Table 2 and
Appendix A,
Figure A3). If—again starting from IFP-1—the transition metal center Zn is replaced by Co (IFP-5), the adsorption capacity can even be further increased by 150% to 6.99 mmol g
−1. Apparently, the substitution of Zn by Co causes an increase in hydrophilicity and an increase in the cluster density of water molecules, compensating for the lower specific surface area and pore volume compared to IFP-1. This claim is also supported by the isotherm model, in which the cluster size of water molecules,
, increases from
6 for IFP-1 also by 150% to
15 for IFP-5 (see
Appendix A,
Table A2). Under consideration of the electron configuration of both transition metals it seems reasonable since Co
2+ ([Ar] 3d
7) in contrast to Zn
2+ ([Ar] 3d
10) exhibits an incompletely electron-filled d-shell, which polarize the channel walls and is also displayed by electronegativity on the Pauling scale (
= 1.88 >
= 1.65, [
59]). Substitution of the methyl group by the sterically more demanding methoxy group (IFP-8) leads in turn to a reduction in capacity by the factor of 4 to 1.6 mmol g
−1 as the pore volume and the specific surface decrease drastically. The cluster size of water molecules, however, appears to increase further since the methoxy group raises the polarity of the channel walls, forming an ether unit.
As mentioned before, the pore volumes cannot be determined reliably from the measurements with N
2 and CO
2 in their entirety, since on the one hand a large part of the pore volume is inaccessible to the N
2 molecule because it is blocked by micropores and on the other hand because CO
2 at 273.15 K is only measured up to 0.1 MPa—which is far below its saturation vapor pressure of 3.49 MPa—due to plant limitations. Thus, only the micropore range could be resolved with the CO
2 measurements accordingly. Based on the maximum loads achieved from experiments with water vapor, the pore volumes (see
Table 2) were estimated assuming complete filling of the pore system of the IFP samples and an adsorbate density corresponding to the density of water at liquid state at the same temperature. With the exception of IFP-4, the pore volumes calculated in this way are significantly larger than those estimated from the measurements with N
2 and CO
2. In particular, the apparent pore volumes of the most polar adsorbents IFP-2, -5 and -8 determined from adsorption with water vapor exceed those determined by N
2 and CO
2 by a factor of three to five. In addition to the strong polarity—due to the C-Cl bond of IFP-2 and the polarizable Co centers of IFP-5 and -8—this can also be attributed to a better resolution of the micropore range by employing the adsorptive H
2O, which is quite smaller than CO
2 in terms of its kinetic diameter (see
Table 2). The latter also explains the 25% increase in pore volume for IFP-1 with its non-polar methyl group. Only the apparent pore volume of IFP-4 is reduced by half. This may be due to a bridging effect of the water molecules around the strongly non-polar and sterically demanding ethyl group at its imidazolate linker [
18,
60].
The shown water uptakes for the IFP samples are comparable to those reported for zeolitic imidazolate frameworks, ZIF-8 (0.5 mmol g
−1) and ZIF-90 (18 mmol g
−1) at 308 K [
61,
62]. The imidazolate linkers used in ZIF-8 and ZIF-90 are 2-methylimidazolate and imidazolate-2-carboxyaldehyde. ZIF-8 exhibits low water uptake demonstrating its framework hydrophobicity. Due to the presence of the hydrophilic carbonyl group in the linker of ZIF-90 a large enhancement in water sorption capacity occurs. The water vapor uptake of the IFPs can be influenced by the substituent R in the position 2 of the amide-imidate-imidazolate linker too. Moreover it is also shown that the node—Zn or Co(II)—has an impact on the water vapor uptake, however, in comparison to a lot of other MOFs the IFPs have to be classified as hydrophobic porous coordination polymers too [
10,
11].
All IFP samples show reproducible water uptake during the three isotherm cycle runs. However, the PXRD analyses performed after the isothermal cycles indicate that IFP-8 loses its crystalline structure and is degraded to an amorphous substance (see
Figure 8), while IFP-1, -2, -4 and -5 maintain their structure. This can be related to the −I-effect induced by the methoxy group of IFP-8 and the polarizable effect of the Co center which enhance the tendency to a nucleophilic attack on the C2 atom of the imidazolate linker, leading to destabilization of the crystal structure. The BET surface area of IFP-8 also collapses by 80%, while it remains approximately constant for the other IFPs. Therefore, the material degradation must have occurred either during the first cycle or after the last one. In the latter case, recontamination by atmospheric oxygen or long-term effects could be decisive.
4. Conclusions
An isostructural series of IFPs with different functional properties but identical topology was investigated for its characteristic sorption properties with water vapor (at 303.15 K), N2 (at 77.4 K) and CO2 (at 273.15 K). In general, it could be shown that the qualitative analysis of water vapor sorption measurements has good agreement with the evaluations drawn from the established measuring methods with N2 and CO2. All sorption measurements with water vapor show a strong hysteresis, which indicates a dominance of microporous networks on the one hand and is especially documented for powders forming agglomerates or aggregates on the other hand. The latter was confirmed after dispersion of the samples by determining particle size distributions.
The maximum water uptake is in the range of 0.48 mmol g−1 (IFP-4) to 6.99 mmol g−1 (IFP-5) and similar to those reported for ZIFs. All IFPs showed reproducible results for three isotherm cycles. However, PXRD measurements and BET analysis revealed that none of the samples are degraded by water vapor sorption, with the exception of IFP-8, which degraded to an amorphous substance, losing 80% of its BET surface area. Thus, material decomposition must have started after the isotherm cycles with water vapor due to recontamination with atmospheric oxygen or long-term effects.
The water isotherms are approximated with a combined model, by extending the GAB model with the capillary condensation term of the Do and Do model. Comparison of IFPs with the same transition metal Zn among themselves shows that the highest water vapor adsorption capacities were achieved with the polar linker substituent chloride (IFP-2), followed by the less polar methyl (IFP-1) and ethyl (IFP-4), consecutively. The substitution of the transition metal Zn (IFP-1) by Co (IFP-5) with the same linker moiety (methyl) also leads to a significant increase in uptake capacity due to the higher channel wall polarizability induced by the Co entre. However, the substitution of the methyl group by the sterically more demanding methoxy group leads to a collapse of the adsorption capacity in analogy to IFP-4 with its ethyl group. Although the theoretically accessible pore diameters for IFP-4 and -8 are significantly smaller than the kinetic diameters of all adsorptives employed, significant adsorption capacities were found at least for water vapor and CO2, which is an indicator for the flexibility of the substituent R. Both species are exhibiting H4 (type I like) behavior for adsorption of water vapor, which clearly indicates the exclusive presence of micropores and is confirmed by the CO2 and N2 measurements. IFP-1, -2 and -5 are exhibiting an H3 (type IV like) behavior with three distinct inflection points, which is indicative for the presence of all pore sizes (micro-, meso- and macropores).
In a nutshell, it can be stated that the information derived from the water vapor sorption measurements exceeds the collective information content of the CO2 and N2 measurements on a qualitative level.