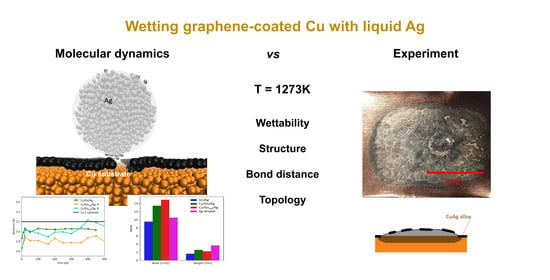
Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Dynamics Simulations (MD)
2.2. MD Simulation Set-Up
2.3. Experiment
3. Results and Discussion
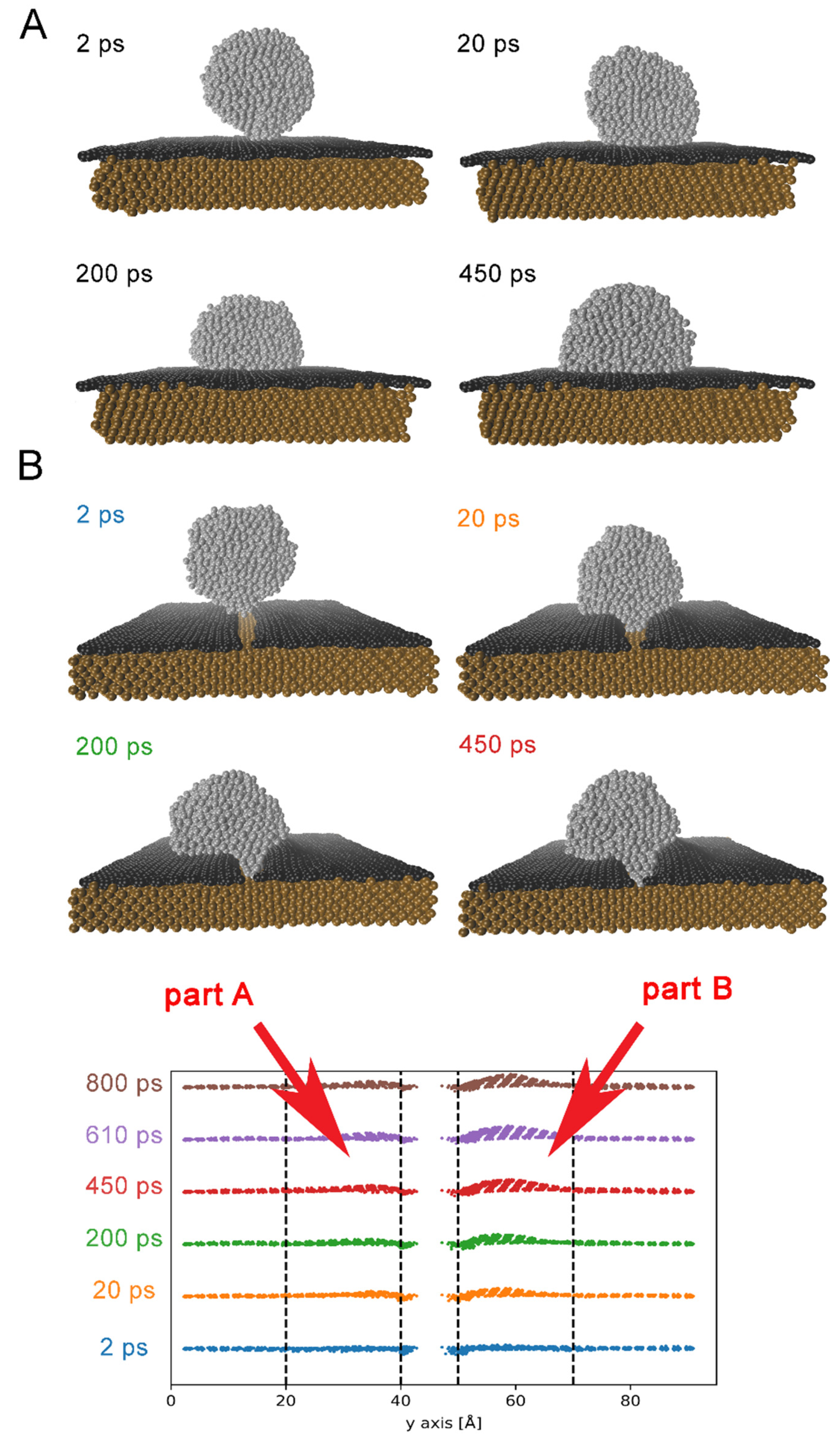
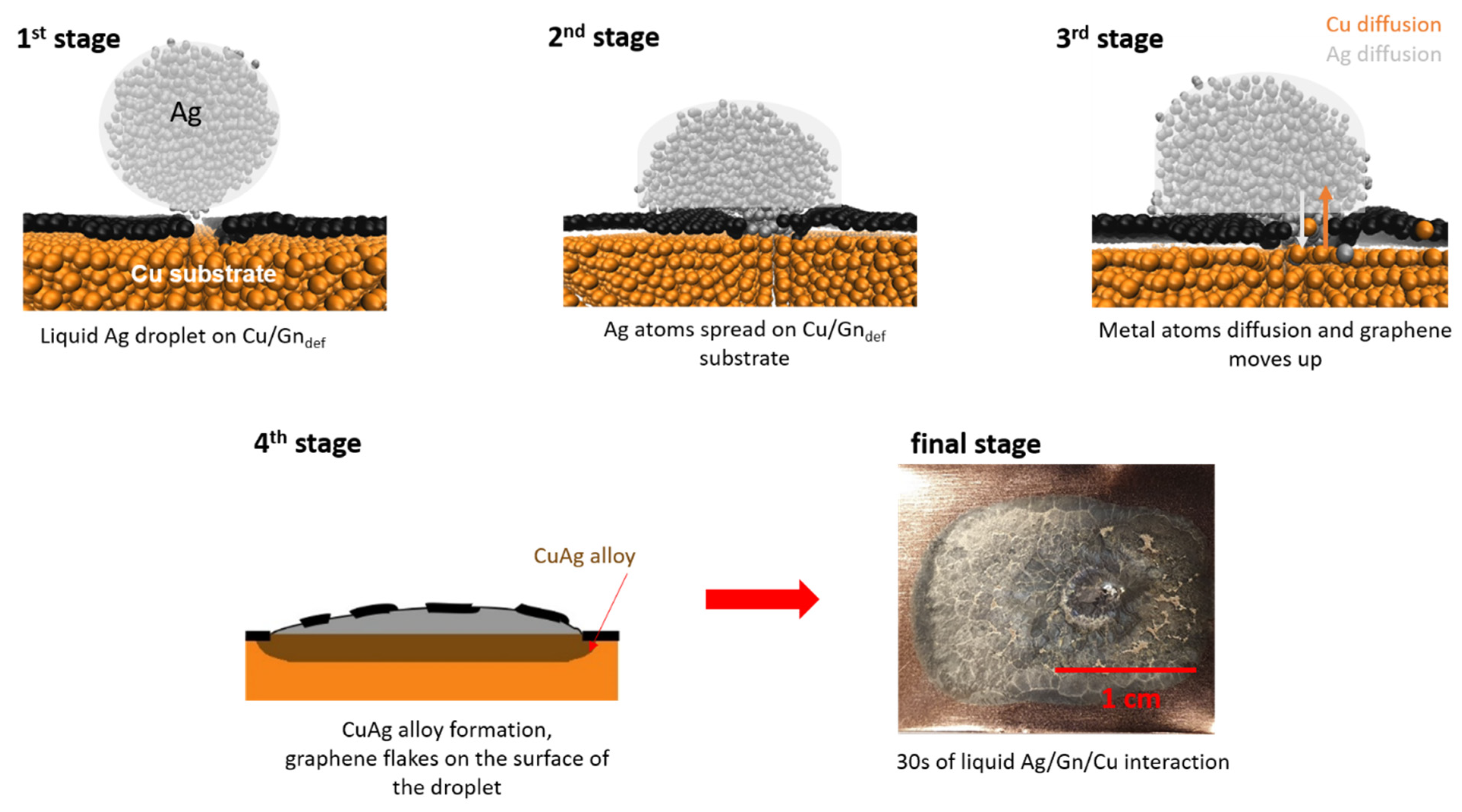
3.1. Wetting Mechanism on Cu/Gn and Cu/Gndef Substrates
3.2. Chemistry of Cu/Gn/Ag and Cu/Gndef/Ag Systems
3.2.1. Pair Distribution Function
3.2.2. Cu-C Bond Distance
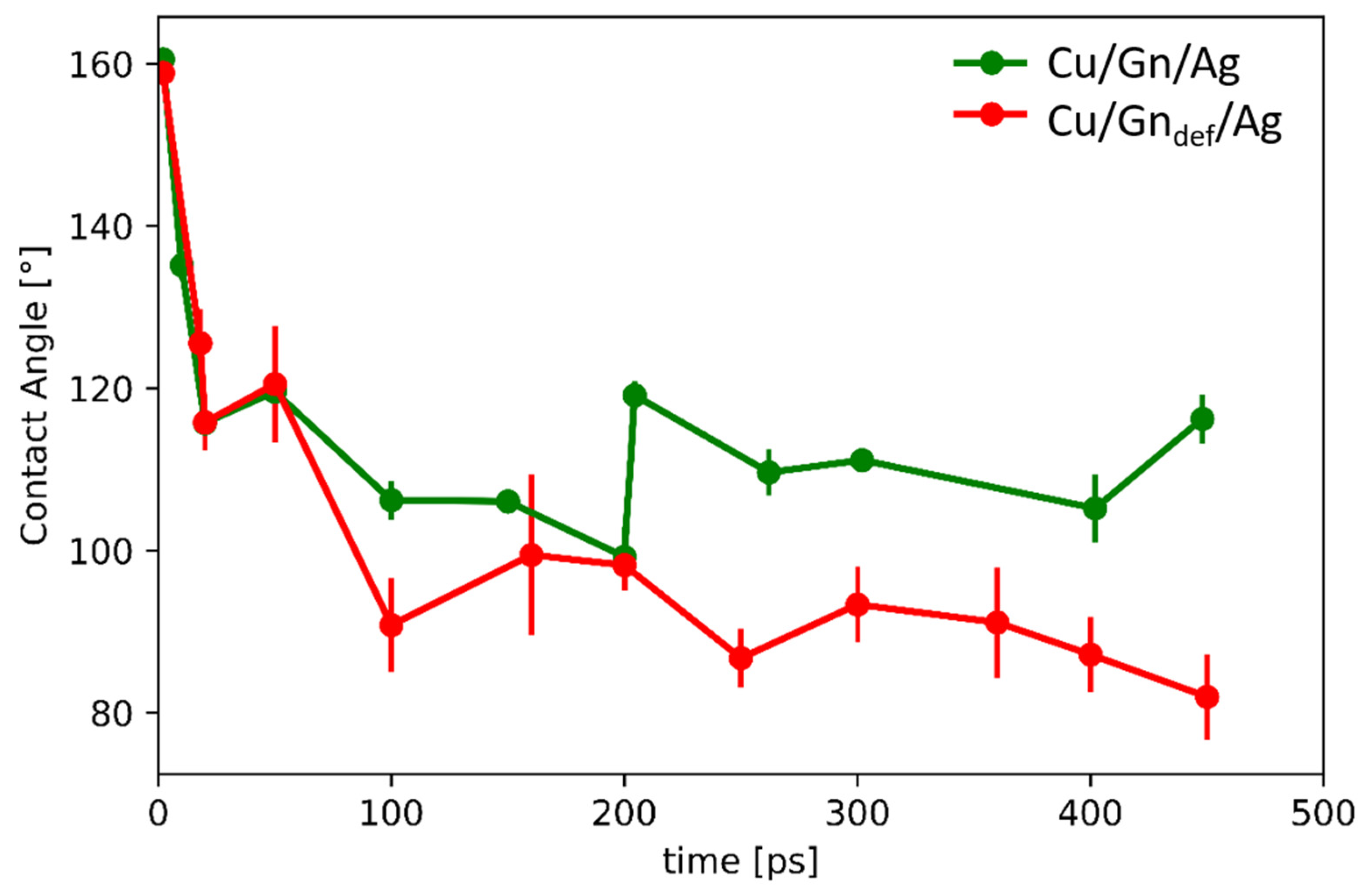
3.3. Contact Angle (CA)
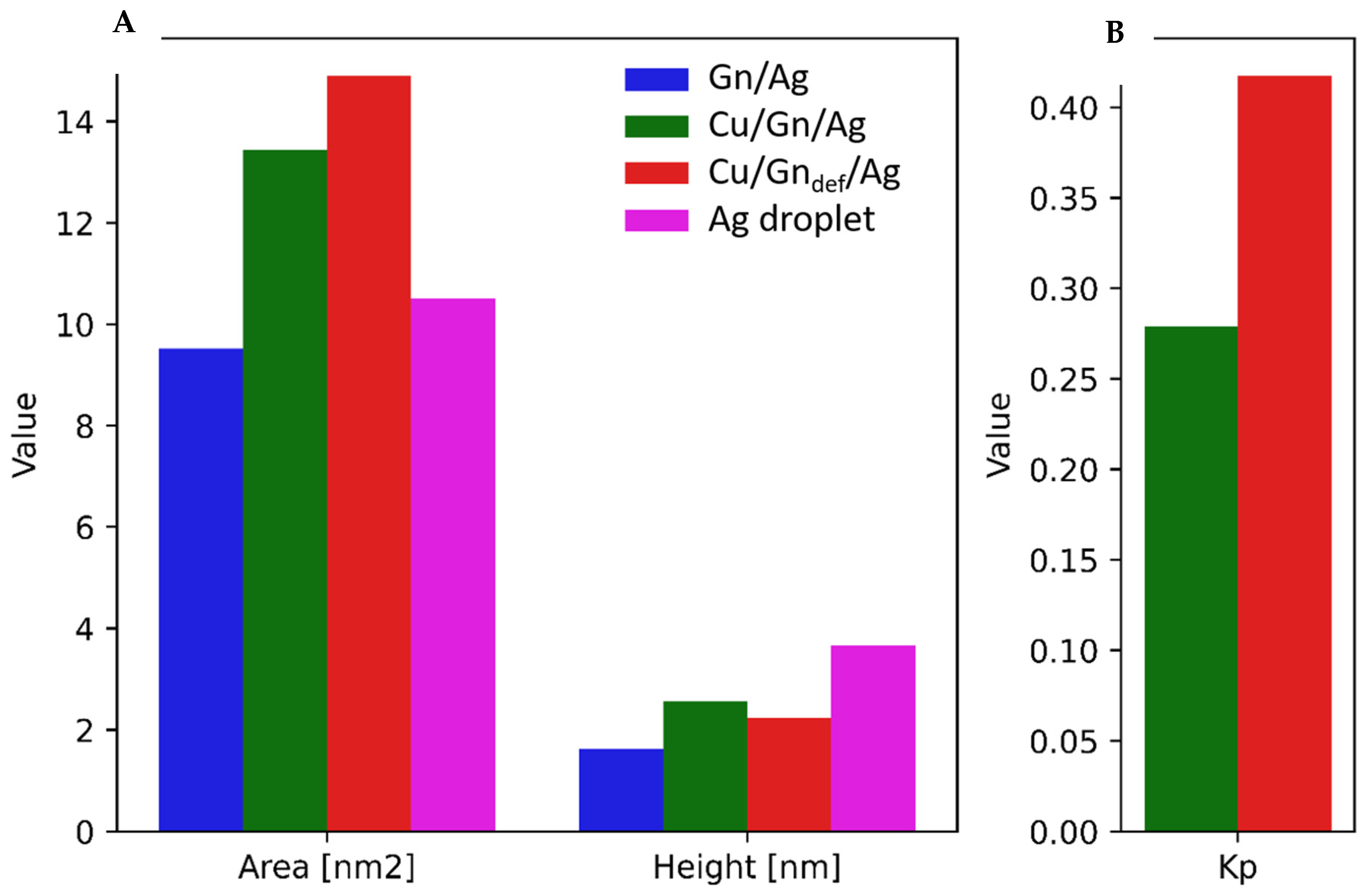
3.4. Wettability
Spreading of Ag Droplet
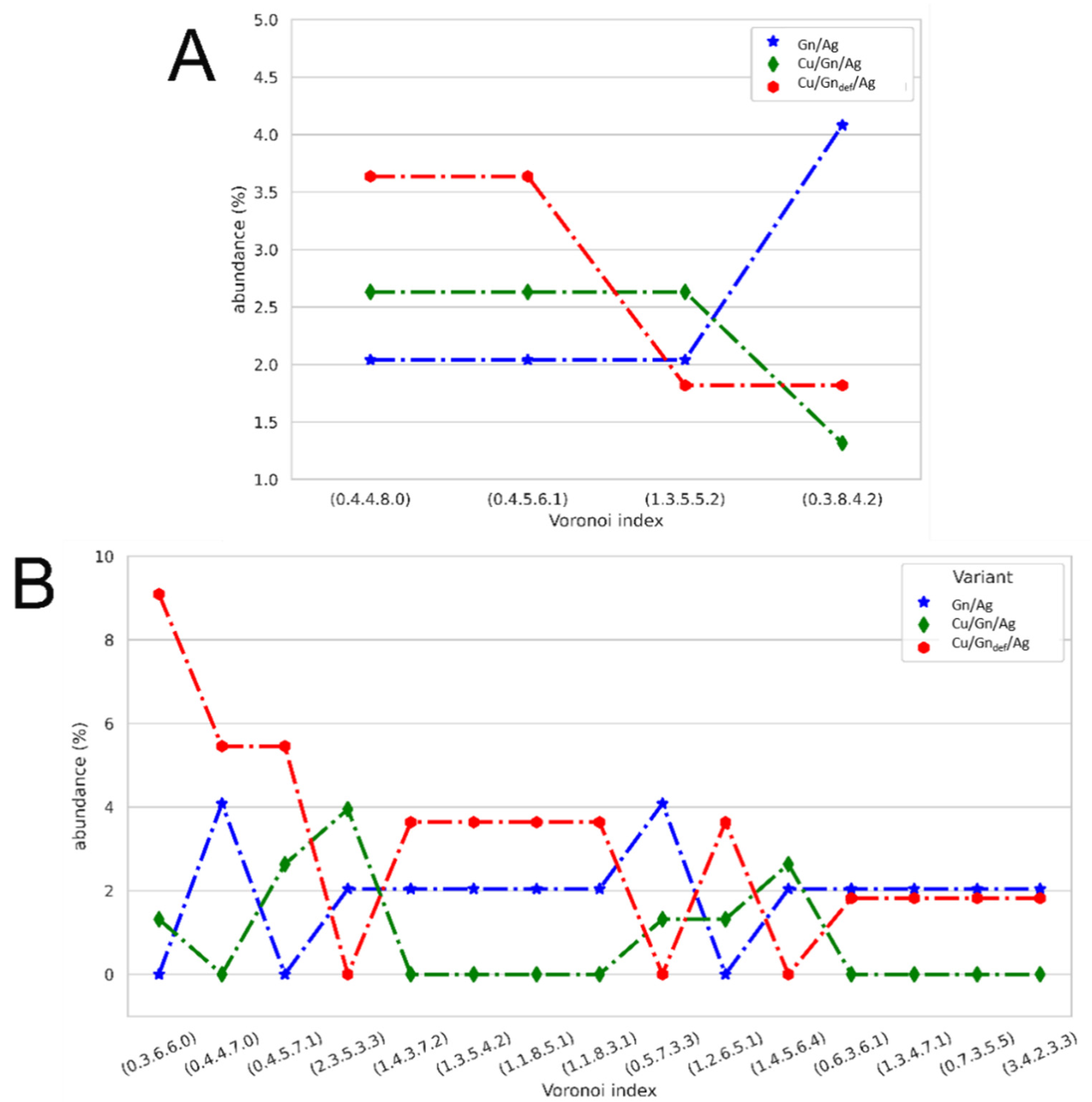
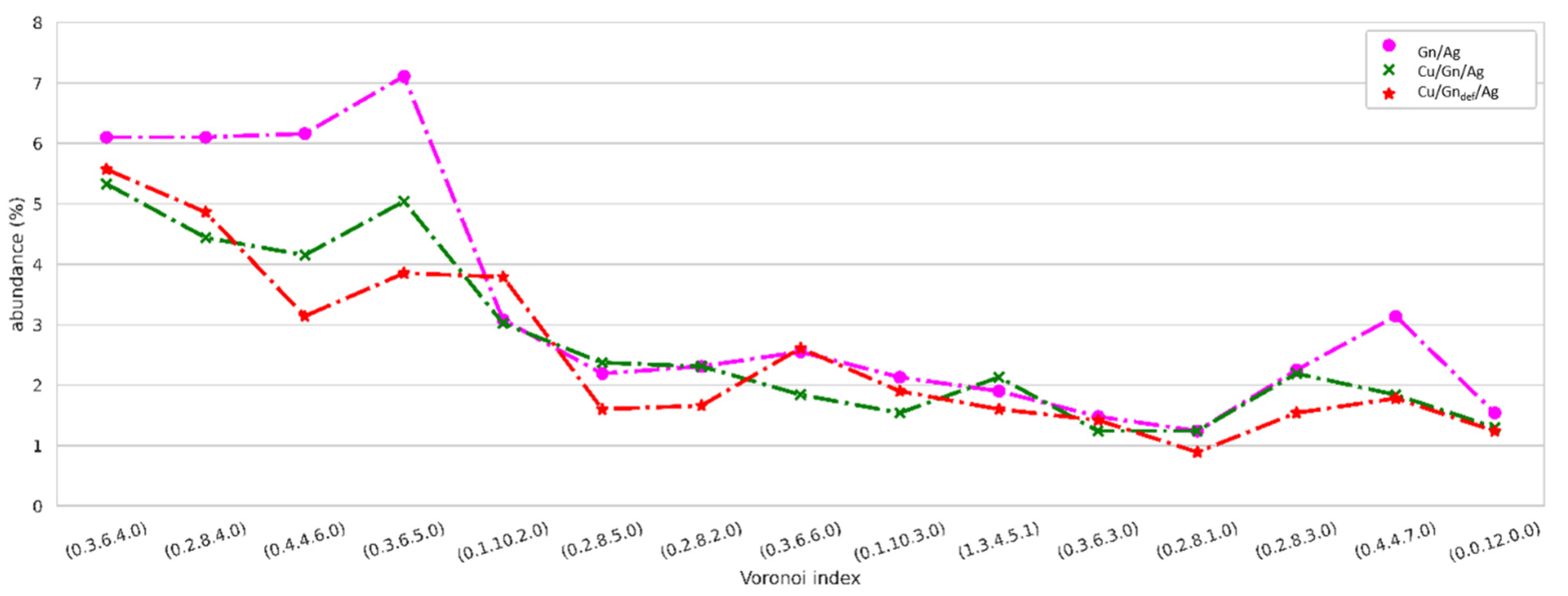
3.5. Topological Analysis
3.5.1. Ag Drop Adsorption on Cu/Gn/Ag and Cu/Gndef/Ag Systems
3.5.2. Topology of Ag Atoms in Droplet after Spreading on Cu/Gn and Cu/Gndef Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brownson, D.A.C.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhen, Z.; Zhu, H. Graphene: Fundamental research and potential applications. FlatChem 2017, 4, 20–32. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’Ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.D.; Maret, W.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. AIChE Annu. Meet. Conf. Proc. 2019, 2019. [Google Scholar] [CrossRef]
- Ahlberg, P.; Jeong, S.H.; Jiao, M.; Wu, Z.; Jansson, U.; Zhang, S.L.; Zhang, Z. Bin Graphene as a diffusion barrier in galinstan-solid metal contacts. IEEE Trans. Electron Devices 2014, 61, 2996–3000. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon N. Y. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, A.; Suenaga, K.; Gloter, A.; Urita, K.; Iijima, S. Direct evidence for atomic defects in graphene layers. Nature 2004, 430, 870–873. [Google Scholar] [CrossRef]
- Xu, T.; Sun, L. Structural defects in graphene. Defects Adv. Electron. Mater. Nov. Low Dimens. Struct. 2018, 5, 137–160. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Chen, M.H.; Jao, H.M.; Liao, T.L. Influence of interfacial intermetallic compound on fracture behavior of solder joints. Mater. Sci. Eng. A 2003, 358, 134–141. [Google Scholar] [CrossRef]
- Xu, L.; Pang, J.H.L.; Che, F. Impact of thermal cycling on Sn-Ag-Cu solder joints and board-level drop reliability. J. Electron. Mater. 2008, 37, 880–886. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, B.H. Stress and surface tension analyses of water on graphene-coated copper surfaces. Int. J. Precis. Eng. Manuf. 2016, 17, 503–510. [Google Scholar] [CrossRef]
- Hung, S.W.; Shiomi, J. Dynamic Wetting of Nanodroplets on Smooth and Patterned Graphene-Coated Surface. J. Phys. Chem. C 2018, 122, 8423–8429. [Google Scholar] [CrossRef]
- Andrews, J.E.; Sinha, S.; Chung, P.W.; Das, S. Wetting dynamics of a water nanodrop on graphene. Phys. Chem. Chem. Phys. 2016, 18, 23482–23493. [Google Scholar] [CrossRef]
- Lai, C.Y.; Tang, T.C.; Amadei, C.A.; Marsden, A.J.; Verdaguer, A.; Wilson, N.; Chiesa, M. A nanoscopic approach to studying evolution in graphene wettability. Carbon N. Y. 2014, 80, 784–792. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Song, W.; Wang, X.; Zhang, H.; Sun, F. Interfacial reaction, microstructure and mechanical properties of Sn58Bi solder joints on graphene-coated Cu substrate. Results Phys. 2019, 13, 102256. [Google Scholar] [CrossRef]
- Ko, Y.H.; Lee, J.D.; Yoon, T.; Lee, C.W.; Kim, T.S. Controlling Interfacial Reactions and Intermetallic Compound Growth at the Interface of a Lead-free Solder Joint with Layer-by-Layer Transferred Graphene. ACS Appl. Mater. Interfaces 2016, 8, 5679–5686. [Google Scholar] [CrossRef]
- Pstruś, J.; Ozga, P.; Gancarz, T.; Berent, K. Effect of Graphene Layers on Phenomena Occurring at Interface of Sn-Zn-Cu Solder and Cu Substrate. J. Electron. Mater. 2017, 46, 5248–5258. [Google Scholar] [CrossRef]
- Sobczak, N.; Sobczak, J.; Kudyba, A.; Homa, M.; Bruzda, G.; Kudyba, A.; Grobelny, M.; Kalisz, M.; Strobl, K.; Singhal, R.; et al. Wetting transparency of graphene deposited on copper in contact with liquid tin. Wetting Transpar. Graphene Depos. Copp. Contact Liq. Tin 2014, 54, 3–11. [Google Scholar] [CrossRef]
- Homa, M.; Sobczak, N.; Sobczak, J.J.; Kudyba, A.; Bruzda, G.; Nowak, R.; Pietrzak, K.; Chmielewski, M.; Strupiński, W. Interaction Between Graphene-Coated SiC Single Crystal and Liquid Copper. J. Mater. Eng. Perform. 2018, 27, 2317–2329. [Google Scholar] [CrossRef]
- Homa, M.; Sobczak, N.; Sobczak, J.J.; Kudyba, A.; Bruzda, G.; Nowak, R.; Giuranno, D.; Pietrzak, K.; Chmielewski, M. Interaction Between Liquid Silver and Graphene-Coated SiC Substrate. J. Mater. Eng. Perform. 2018, 27, 4140–4149. [Google Scholar] [CrossRef]
- Caccia, M.; Giuranno, D.; Molina-Jorda, J.M.; Moral, M.; Nowak, R.; Ricci, E.; Sobczak, N.; Narciso, J.; Sanz, J.F. Graphene Translucency and Interfacial Interactions in the Gold/Graphene/SiC System. J. Phys. Chem. Lett. 2018, 9, 3850–3855. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Spreading and orientation of silver nano-drop over a flat graphene substrate: An atomistic investigation. Carbon N. Y. 2018, 138, 26–41. [Google Scholar] [CrossRef]
- Slapikas, R.; Dabo, I.; Sinnott, S.B. Optimized utilization of COMB3 reactive potentials in LAMMPS. J. Chem. Phys. 2020, 152, 224702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fonseca, A.F.; Liang, T.; Phillpot, S.R.; Sinnott, S.B. Dynamics of Graphene/Al Interfaces using COMB3 Potentials. Phys. Rev. Mater. 2019, 3, 114002. [Google Scholar] [CrossRef]
- Fonseca, A.F.; Dantas, S.O.; Galvão, D.S.; Zhang, D.; Sinnott, S.B. The structure of graphene on graphene/C60/Cu interfaces: A molecular dynamics study. Nanotechnology 2019, 30, 505707. [Google Scholar] [CrossRef]
- Liang, T.; Devine, B.; Phillpot, S.R.; Sinnott, S.B. Variable Charge Reactive Potential for Hydrocarbons to Simulate Organic-Copper Interactions. J. Phys. Chem. A 2012, 116, 7976–7991. [Google Scholar] [CrossRef]
- Klaver, T.P.C.; Zhu, S.; Sluiter, M.H.F.; Janssen, G.C.A.M. Molecular dynamics simulation of graphene on Cu (1 0 0) and (1 1 1) surfaces. Carbon N. Y. 2014, 82, 538–547. [Google Scholar] [CrossRef]
- Martinez, J.; Liang, T.; Sinnott, S.B.; Phillpot, S.R. A third-generation charge optimized many body (COMB3) potential for nitrogen-containing organic molecules. Comput. Mater. Sci. 2017, 139, 153–161. [Google Scholar] [CrossRef]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF reactive force-field: Development, applications and future directions. NPJ Comput. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Trybula, M.E.; Korzhavyi, P.A. Temperature dependency of structure and order evolution in 2D confined oxide films grown on Al substrates using reactive molecular dynamics. Vacuum 2021, 110243. [Google Scholar] [CrossRef]
- Trybula, M.E.; Korzhavyi, P.A. Atomistic Simulations of Al(100) and Al(111) Surface Oxidation: Chemical and Topological Aspects of the Oxide Structure. J. Phys. Chem. C 2019, 123, 334–346. [Google Scholar] [CrossRef]
- Van Duin, A.C.T.; Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A.; Rahaman, O.; Doren, D.J.; Raymand, D.; Hermansson, K. Development and validation of a ReaxFF reactive force field for Cu cation/water interactions and copper metal/metal oxide/metal hydroxide condensed phases. J. Phys. Chem. A 2010, 114, 9507–9514. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Van Duin, A.C.T. Molecular Dynamics Simulations of the Oxidation of Aluminum Nanoparticles using the ReaxFF Reactive Force Field. J. Phys. Chem. C 2015, 119, 17876–17886. [Google Scholar] [CrossRef]
- Song, L.; Zhao, F.Q.; Xu, S.Y.; Ju, X.H. Atomic origin of the morphological evolution of aluminum hydride (AlH3) nanoparticles during oxidation using reactive force field simulations. Appl. Surf. Sci. 2020, 519, 1–10. [Google Scholar] [CrossRef]
- Foiles, S.M.; Baskes, M.I.; Daw, M.S. Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys. Rev. B 1986, 33, 7983–7991. [Google Scholar] [CrossRef]
- Williams, P.M.; Mishin, Y.; Hamilton, J.C. An embedded-atom potential for the Cu–Ag system. Model. Simul. Mater. Sci. Eng. 2006, 14. [Google Scholar] [CrossRef]
- Baskes, M.I.; Nelson, J.S.; Wright, A.F. Semiempirical modified embedded-atom potentials for silicon and germanium. Phys. Rev. B 1989, 40, 6085–6100. [Google Scholar] [CrossRef]
- Daw, M.S.; Baskes, M.I. Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals. Phys. Rev. B 1984, 29, 6443–6453. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Stukowski, A.; Albe, K. Extracting dislocations and non-dislocation crystal defects from atomistic simulation data. Model. Simul. Mater. Sci. Eng. 2010, 18. [Google Scholar] [CrossRef]
- Stukowski, A.; Albe, K. Dislocation detection algorithm for atomistic simulations. Model. Simul. Mater. Sci. Eng. 2010, 18. [Google Scholar] [CrossRef]
- Lazar, E.A.; Han, J.; Srolovitz, D.J. Topological framework for local structure analysis in condensed matter. Proc. Natl. Acad. Sci. USA 2015, 112, E5769–E5776. [Google Scholar] [CrossRef]
- Pstruś, J.; Fima, P.; Gancarz, T. Wetting of Cu and Al by Sn-Zn and Zn-Al eutectic alloys. J. Mater. Eng. Perform. 2012, 21, 606–613. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with image. J. Biophotonics Int. 2004, 11, 36–41. [Google Scholar] [CrossRef]
- Protsenko, P.; Kozlova, O.; Voytovych, R.; Eustathopoulos, N. Dissolutive wetting of Si by molten Cu. J. Mater. Sci. 2008, 43, 5669–5671. [Google Scholar] [CrossRef]
- Webb, E.B.; Grest, G.S. Molecular dynamics simulations of reactive wetting. Scr. Mater. 2002, 47, 393–398. [Google Scholar] [CrossRef]
- Karakaya, İ.; Thompson, W.T. The Ag C Silver Carbon System. Bull. Alloy Phase Diagr. 1988, 9, 226. [Google Scholar] [CrossRef]
- Webb, E.B.; Grest, G.S.; Heine, D.R.; Hoyt, J.J. Dissolutive wetting of Ag on Cu: A molecular dynamics simulation study. Acta Mater. 2005, 53, 3163–3177. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Trybula, M.E. Structure and chemistry of liquid Al–Cu alloys: Molecular dynamics study versus thermodynamics-based modelling. J. Mater. Sci. 2018, 8285–8301. [Google Scholar] [CrossRef]
- Trybula, M.E. Structure and transport properties of the liquid Al 80 Cu 20 alloy—A molecular dynamics study. Comput. Mater. Sci. 2016, 122, 341–352. [Google Scholar] [CrossRef]
- Xiong, L.H.; Guo, F.M.; Wang, X.D.; Cao, Q.P.; Zhang, D.X.; Ren, Y.; Jiang, J.Z. Structural evolution and dynamical properties of Al2Ag and Al2Cu liquids studied by experiments and ab initio molecular dynamics simulations. J. Non. Cryst. Solids 2017, 459, 160–168. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drewienkiewicz, A.; Żydek, A.; Trybula, M.E.; Pstruś, J. Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment. Nanomaterials 2021, 11, 1465. https://doi.org/10.3390/nano11061465
Drewienkiewicz A, Żydek A, Trybula ME, Pstruś J. Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment. Nanomaterials. 2021; 11(6):1465. https://doi.org/10.3390/nano11061465
Chicago/Turabian StyleDrewienkiewicz, Aleksandra, Arkadiusz Żydek, Marcela E. Trybula, and Janusz Pstruś. 2021. "Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment" Nanomaterials 11, no. 6: 1465. https://doi.org/10.3390/nano11061465
APA StyleDrewienkiewicz, A., Żydek, A., Trybula, M. E., & Pstruś, J. (2021). Atomic Level Insight into Wetting and Structure of Ag Droplet on Graphene Coated Copper Substrate—Molecular Dynamics versus Experiment. Nanomaterials, 11(6), 1465. https://doi.org/10.3390/nano11061465