Contact Guidance Effect and Prevention of Microfouling on a Beta Titanium Alloy Surface Structured by Electron-Beam Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Physico-Chemical Characterizations
2.3. Biological Characterizations
2.3.1. Cell Adhesion and Orientation
2.3.2. Bacterial Adhesion
2.3.3. Statistical Analysis of Data
3. Results
3.1. Surface Topography Investigation
3.2. Crystalline Structure Evaluation
3.3. Surface Roughness
3.4. Surface Wettability
3.5. Biological Characterizations
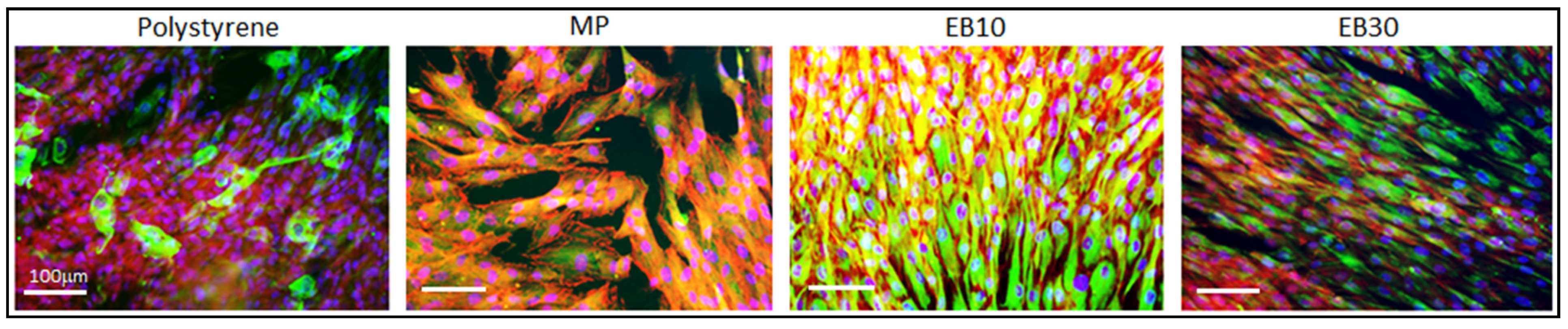
3.5.1. Cells’ Contact Guidance
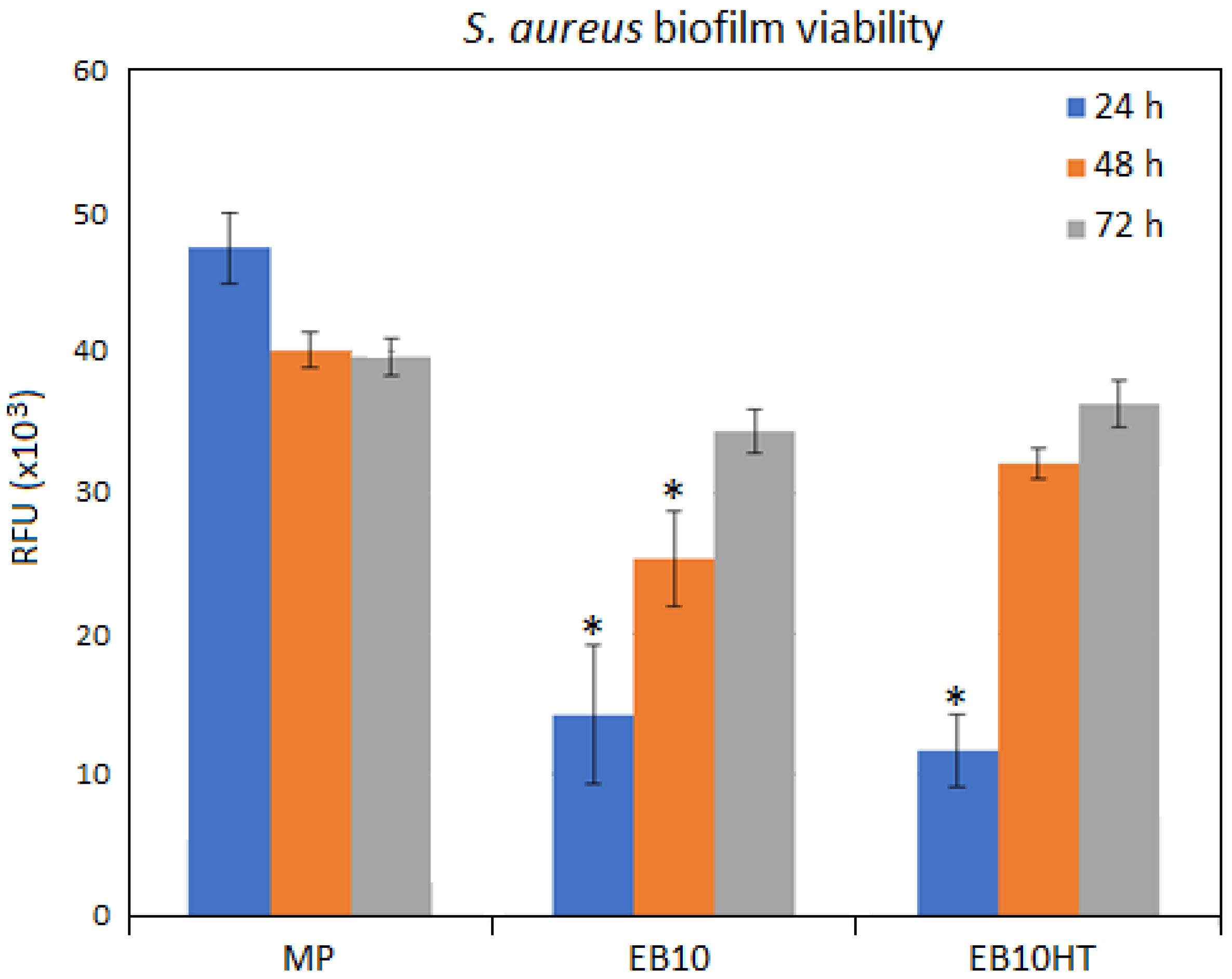
3.5.2. Antifouling Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C 2004, 24, 745–752. [Google Scholar] [CrossRef]
- Bălţatu, M.S.; Vizureanu, P.; Tierean, M.H.; Minciună, M.G.; Achitei, D.C. Ti-Mo alloys used in medical applications. Adv. Mater. Res. 2015, 1128, 105–111. [Google Scholar] [CrossRef]
- Martins, R.S.M., Jr.; Nogueira, R.A.; De Araújo, R.O.; Donato, T.A.G.; Arana-Chavez, V.E.; Claro, A.P.R.A.; Moraes, J.C.S.; Buzalaf, M.A.R.; Grandini, C.R. Preparation and characterization of Ti-15Mo alloy used as biomaterial. Mater. Res. 2011, 14, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Sandu, A.V.; Baltatu, M.S.; Nabialek, M.; Savin, A.; Vizureanu, P. Characterization and mechanical proprieties of new TiMo alloys used for medical applications. Materials 2019, 12, 2973. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.; Apaza-Bedoya, K.; Benfatti, C.; Silva, F.; Henriques, B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. Metals 2020, 10, 1272. [Google Scholar] [CrossRef]
- Bhola, R.; Chandra, C.; Alabbas, F.M.; Kundu, S.; Mishra, B.; Olson, D.L. Corrosion response of Ti6Al4V and Ti15Mo dental implant alloys in the presence of listerine oral rinse. Int. J. Corros. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Leone, S. Prosthetic joint infections: Microbiology, diagnosis, management and prevention. Int. J. Antimicrob. Agents 2008, 32, 287–293. [Google Scholar] [CrossRef]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104110. [Google Scholar] [CrossRef]
- Hall, T.J.; Villapún, V.M.; Addison, O.; Webber, M.A.; Lowther, M.; Louth SE, T.; Mountcastle, S.E.; Brunet, M.Y.; Cox, S.C. A call for action to the biomaterial community to tackle antimicrobial resistance. Biomater. Sci. 2020, 8, 4951–4974. [Google Scholar] [CrossRef] [PubMed]
- Silva Souza, J.G.; Bertolini, M.M.; Cavalcante Costa, R.; Egumi Nagay, B.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2021, 24, 102008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mat. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano- bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Attarilar, S.; Djavanroodi, F.; Ebrahimi, M.; Al-Fadhalah, K.J.; Wang, L.; Mozafari, M. Hierarchical microstructure tailoring of pure titanium for enhancing cellular response at tissue-implant interface. J. Biomed. Nanotechnol. 2021, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.-W.; Zhong, Y.; Wang, L. Multi-scale surface treatments of titanium implants for rapid osseointegration: A review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Attarilar, S.; Wang, C.; Tamaddon, M.; Yang, C.; Xie, K.; Yao, J.; Wang, L.; Liu, C.; et al. Nano-Modified Titanium Implant Materials: A Way Toward Improved Antibacterial Properties. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface modification techniques of titanium and its alloys to functionally optimize their biomedical properties: Thematic review. Front. Bioeng. Biotechnol. 2020, 8, 1261. [Google Scholar] [CrossRef]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef]
- Estrin, Y.; Lapovok, R.; Medvedev, A.E.; Kasper, C.; Ivanova, E.; Lowe, T.C. Mechanical performance and cell response of pure titanium with ultrafine-grained structure produced by severe plastic deformation. In Titanium in Medical and Dental Applications; Froes, F.H., Qian, M., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 419–454. [Google Scholar]
- Zhang, L.C.; Chen, L.Y. A review on biomedical titanium alloys: RecentProgress and prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the topographic design of commercial titanium dental implants: Towards anti-peri-implantitis surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Race to invade: Understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021, 37, 816–831. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Gulati, K.; Arora, H.; Han, P.; Fournier, B.; Ivanovski, S. Orchestrating soft tissue integration at the transmucosal region of titanium implants. Acta Biomater. 2021, 124, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Brunette, D.M. Principles of cell behavior on titanium surfaces and their application to implanted devices. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 485–512. [Google Scholar]
- Jaeger, N.A.F.; Brunette, D.M. Production of microfabricated surfaces and their effects on cell behavior. In Titanium in Medicine; Brunette, D.M., Tengvall, P., Textor, M., Thomsen, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 343–374. [Google Scholar]
- Nevins, M.; Carmelo, M.; Nevins, M.L.; Schupbach, P.; Kim, D.M. Connective tissue attachment to laser-microgrooved abutments: A human histologic case report. Int. J. Periodontics Restor. Dent. 2012, 32, 385–392. [Google Scholar]
- Ferraris, S.; Guarino, V.; Cochis, A.; Varesano, A.; Maya, I.C.; Vineis, C.; Rimondini, L.; Spriano, S. Aligned keratin submicrometric-fibers for fibroblasts guidance onto nanogrooved titanium surfaces for transmucosal implants. Mater. Lett. 2018, 229, 1–4. [Google Scholar] [CrossRef]
- Cochis, A.; Ferraris, S.; Sorrentino, R.; Azzimonti, B.; Novara, C.; Geobaldo, F.; Giachet, F.T.; Vineis, C.; Varesano, A.; Abdelgeliel, A.S.; et al. Silver-doped keratin nanofibers preserve a titanium surface from biofilm contamination and favor soft-tissue healing. J. Mater. Chem. B 2017, 5, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Giachet, F.T.; Miola, M.; Bertone, E.; Varesano, A.; Vineis, C.; Cochis, A.; Sorrentino, R.; Rimondini, L.; Spriano, S. Nanogrooves and keratin nanofibers on titanium surfaces aimed at driving gingival fibroblasts alignment and proliferation without increasing bacterial adhesion. Mater. Sci. Eng. C 2017, 76, 1–12. [Google Scholar] [CrossRef]
- Ferraris, S.; Warchomicka, F.; Ramskogler, C.; Tortello, M.; Cochis, A.; Scalia, A.; di Confiengo, G.G.; Keckes, J.; Rimondini, L.; Spriano, S. Surface structuring by Electron Beam for improved soft tissues adhesion and reduced bacterial contamination on Ti-grade 2. J. Mater. Process. Technol. 2019, 266, 518–529. [Google Scholar] [CrossRef]
- Ferraris, S.; Warchomicka, F.; Iranshahi, F.; Rimondini, L.; Cochis, A.; Spriano, S. Electron beam structuring of Ti6Al4V: New insights on the metal surface properties influencing the bacterial adhesion. Materials 2020, 13, 409. [Google Scholar] [CrossRef] [Green Version]
- Verestiuc, L.; Spataru, M.-C.; Baltatu, M.S.; Butnaru, M.; Solcan, C.; Sandu, A.V.; Voiculescu, I.; Geanta, V.; Vizureanu, P. New Ti–Mo–Si materials for bone prosthesis applications. J. Mech. Behav. Biomed. Mater. 2021, 113, 104198. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, R.-B.; Qi, G.-X.; Wang, Z.-T.; Deng, Z.-Y. Powder sintering of porous Ti–15Mo alloy from TiH2 and Mo powders. J. Alloys Compd. 2009, 485, 215–218. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Student, S.; Brzychczy-Włoch, M.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Physico-chemical and biological evaluation of doxycycline loaded into hybrid oxide-polymer layer on Ti–Mo alloy. Bioact. Mater. 2020, 5, 553–563. [Google Scholar] [CrossRef]
- Western Superdonducting. Inspection Certificate of Quality; Western Superdonducting Technologies Co., LTD: Xi’an, China, 2013. [Google Scholar]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Cochis, A.; Cazzola, M.; Tortello, M.; Scalia, A.; Spriano, S.; Rimondini, L. Cytocompatible and anti-bacterial adhesion nanotextured titanium oxide layer on titanium surfaces for dental and orthopedic implants. Front. Bioeng. Biotechnol. 2019, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef]
- Harrison, J.J.; A Stremick, C.; Turner, R.J.; Allan, N.D.; E Olson, M.; Ceri, H. Microtiter susceptibility testing of microbes growing on peg lids: A miniaturized biofilm model for high-throughput screening. Nat. Protoc. 2010, 5, 1236–1254. [Google Scholar] [CrossRef] [PubMed]
- Sreekumari, K.R.; Nandakumar, K.; Kikuchi, Y. Bacterial attachment to stainless steel welds: Significance of substratum microstructure. Biofouling 2001, 17, 303–316. [Google Scholar] [CrossRef]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. Development and validation of TiO2: Ag thin films to eradicate an-tibiotic resistant biofilms. Mater. Today 2016, 3, 1502–1509. [Google Scholar]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Loye, A.M.; Ketkaew, J.; Schroers, J.; Kyriakides, T.R. Hierarchical Micro- and Nanopatterning of Metallic Glass to Engineer Cellular Responses. ACS Appl. Bio Mater. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- Yu, H.; Das, S.; Liu, J.; Hess, J.; Hofmann, F. Surface terraces in pure tungsten formed by high temperature oxidation. Scr. Mater. 2019, 173, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Togaru, M.; Sainju, R.; Zhang, L.; Jiang, W.; Zhu, Y. Direct observation of tungsten oxidation studied by in situ environmental TEM. Mater. Charact. 2021, 174, 111016. [Google Scholar] [CrossRef]
- Yao, G.; Gao, M.; Ji, Y.; Liang, W.; Gao, L.; Zheng, S.; Wang, Y.; Pang, B.; Chen, Y.B.; Zeng, H.; et al. Surface step terrace tuned microstructures and dielectric properties of highly epitaxial CaCu3Ti4O12 thin films on vicinal LaAlO3 substrates. Sci. Rep. 2016, 6, 34683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pixner, F.; Buzolin, R.; Schönfelder, S.; Theuermann, D.; Warchomicka, F.; Enzinger, N. Contactless temperature measurement in wire-based electron beam additive manufacturing Ti-6Al-4V. Weld. World 2021, 1–16. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.-S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Fröjd, V.; Linderbäck, P.; Wennerberg, A.; de Paz, L.C.; Svensäter, G.; Davies, J.R. Effect of nanoporous TiO2 coating and anodized Ca2+ modification of titanium surfaces on early microbial biofilm formation. BMC Oral Health 2011, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; Van Steenberghe, D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: Short-term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 69–178. [Google Scholar]
- Zhou, X.; Shi, J.; Hu, J.; Chen, Y. Cells cultured on microgrooves with or without surface coating: Correlation between cell alignment, spreading and local membrane deformation. Mater. Sci. Eng. C 2013, 33, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Spriano, S.; Ferraris, S. How can topographical surface features affect the interaction of implants with soft tissue? In Recent Advances in Biology, Biomedicine and Bioengineering, Proceedings of the 3rd International Conference on Health Science and Biomedical System HSBS14, Florence, Italy, 22–24 November 2014; WSEAS Press: Athens, Greece, 2014; ISBN 9789604744015. [Google Scholar]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on LDPE with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef] [PubMed]





| Ra (µm) | Contact Angle (°) | |
|---|---|---|
| MP | 0.027 ± 0.003 | 82 ± 2 |
| EB10 | 0.179 ± 0.033 | 82 ± 6 |
| EB30 | 0.243 ± 0.009 | 95 ± 5 |
| EB10HT | 0.350 ± 0.070 | 93 ± 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraris, S.; Warchomicka, F.; Barberi, J.; Cochis, A.; Scalia, A.C.; Spriano, S. Contact Guidance Effect and Prevention of Microfouling on a Beta Titanium Alloy Surface Structured by Electron-Beam Technology. Nanomaterials 2021, 11, 1474. https://doi.org/10.3390/nano11061474
Ferraris S, Warchomicka F, Barberi J, Cochis A, Scalia AC, Spriano S. Contact Guidance Effect and Prevention of Microfouling on a Beta Titanium Alloy Surface Structured by Electron-Beam Technology. Nanomaterials. 2021; 11(6):1474. https://doi.org/10.3390/nano11061474
Chicago/Turabian StyleFerraris, Sara, Fernando Warchomicka, Jacopo Barberi, Andrea Cochis, Alessandro Calogero Scalia, and Silvia Spriano. 2021. "Contact Guidance Effect and Prevention of Microfouling on a Beta Titanium Alloy Surface Structured by Electron-Beam Technology" Nanomaterials 11, no. 6: 1474. https://doi.org/10.3390/nano11061474









