Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Bulk g-C3N4Photocatalysts
2.3. Synthesis of Nonmetal Group-Doped g-C3N4Photocatalyst
2.4. Characterization
2.5. Photocatalytic Test
3. Results and Discussion
3.1. Composition and Structure of the as-Prepared Photocatalysts
3.2. Chemical Composition and Surface States of the as-Prepared Photocatalysts
3.3. Optical Property of the as-Prepared Photocatalysts
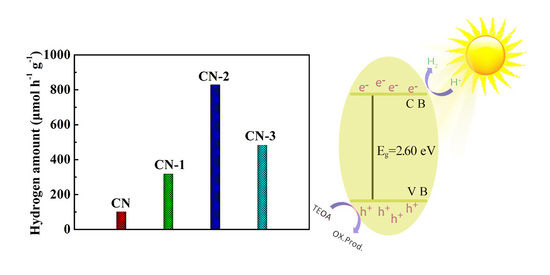
3.4. Photocatalytic Activity Investigation of the as-Prepared Photocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2008, 8, 76–80. [Google Scholar] [CrossRef]
- Lv, C.; Qian, Y.M.; Yan, C.S.; Ding, Y.; Liu, Y.Y.; Chen, G.; Yu, G.H. Defect Engineering Metal-Free Polymeric Carbon Nitride Electrocatalyst for Effective Nitrogen Fixation under Ambient Conditions. Angew. Chem. Int. Ed. 2018, 57, 10246–10250. [Google Scholar] [CrossRef]
- Tang, H.; Wang, R.; Zhao, C.X.; Chen, Z.P.; Yang, X.F.; Bukhvalov, D.; Lin, Z.X.; Liu, Q.Q. Oxamide-modified g-C3N4 nanostructures: Tailoring surface topography for high-performance visible light photocatalysis. Chem. Eng. J. 2019, 374, 1064–1075. [Google Scholar] [CrossRef]
- Chen, Y.L.; Qu, Y.; Zhou, X.; Li, D.Z.; Xu, P.; Sun, J.M. Phenyl-Bridged Graphitic Carbon Nitride with a Porous and Hollow Sphere Structure to Enhance Dissociation of Photogenerated Charge Carriers and Visible-Light-Driven H2 Generation. ACS Appl. Mater. Interfaces 2020, 12, 41527–41537. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Feng, Y.; Chen, S.L.; Ding, M.L.; Yao, J.F. Carbon nitride nanotube-based materials for energy and environmental applications: A review of recent progresses. J. Mater. Chem. A 2020, 8, 25626–25648. [Google Scholar] [CrossRef]
- Xiong, S.S.; Lin, M.X.; Wang, L.D.; Liu, S.; Weng, S.T.; Jiang, S.Y.; Xu, Y.C.; Jiao, Y.; Chen, J.R. Defects-type three-dimensional Co3O4nanomaterials for energy conversion and low temperature energy storage. Appl. Surf. Sci. 2021, 546, 149064. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y.H. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 1–15. [Google Scholar] [CrossRef]
- Xing, W.N.; Tu, W.G.; Han, Z.H.; Hu, Y.D.; Meng, Q.Q.; Chen, G. Template-Induced High-Crystalline g-C3N4Nanosheets for Enhanced Photocatalytic H2 Evolution. ACS Energy Lett. 2018, 3, 514–519. [Google Scholar] [CrossRef]
- Wu, H.H.; Yu, S.Y.; Wang, Y.; Han, J.; Wang, L.; Song, N.; Dong, H.J.; Li, C.M. A facile one-step strategy to construct 0D/2D SnO2/g-C3N4heterojunctionphotocatalyst for high-efficiency hydrogen production performance from water splitting. Int. J. Hydrog. Energy 2020, 45, 30142–30152. [Google Scholar] [CrossRef]
- Yang, X.F.; Tian, L.; Zhao, X.L.; Tang, H.; Liu, Q.Q.; Li, G.S. Interfacial optimization of g-C3N4-based Z-scheme heterojunction toward synergistic enhancement of solar-driven photocatalytic oxygen evolution. Appl. Catal. B Environ. 2019, 244, 240–249. [Google Scholar] [CrossRef]
- Rokesh, K.; Sakar, M.; Do, T.-O. Emerging Hybrid Nanocomposite Photocatalysts for the Degradation of Antibiotics: Insights into Their Designs and Mechanisms. Nanomaterials 2021, 11, 572. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.; Kang, M. Strategy for improving the visible photocatalytic H2 evolution activity of 2D graphitic carbon nitride nanosheets through the modification with metal and metal oxide nanocomponents. Appl. Catal. B Environ. 2019, 248, 538–551. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Pan, Y.; Liang, J.; Zeng, G.M.; Wu, Z.B.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Starukh, H.; Praus, P. Doping of Graphitic Carbon Nitride with Non-Metal Elements and Its Applications in Photocatalysis. Catalysts 2020, 10, 1119. [Google Scholar] [CrossRef]
- Tu, W.G.; Xu, Y.; Wang, J.J.; Zhang, B.W.; Zhou, T.H.; Yin, S.M.; Wu, S.Y.; Li, C.M.; Huang, Y.Z.; Zhou, Y.; et al. Investigating the Role of Tunable Nitrogen Vacancies in Graphitic Carbon Nitride Nanosheets for Efficient Visible-Light-Driven H2 Evolution and CO2 Reduction. ACS Sustain. Chem. Eng. 2017, 5, 7260–7268. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Ma, C.C.; Yu, K.S.; Lu, Z.Y.; Liu, Z.; Huo, P.W.; Tang, X.; Yan, Y.S. Synthesis Ce-doped biomass carbon-based g-C3N4 via plant growing guide and temperature-programmed technique for degrading 2-Mercaptobenzothiazole. Appl. Catal. B Environ. 2020, 268, 118432. [Google Scholar] [CrossRef]
- Song, X.H.; Li, X.; Zhang, X.Y.; Wu, Y.F.; Ma, C.C.; Huo, P.W.; Yan, Y.S. Fabricating C and O co-doped carbon nitride with intramolecular donor-acceptor systems for efficient photoreduction of CO2 to CO. Appl. Catal. B Environ. 2020, 268, 118736. [Google Scholar] [CrossRef]
- Ou, M.; Tu, W.G.; Yin, S.M.; Xing, W.N.; Wu, S.Y.; Wang, H.J.; Wan, S.P.; Zhong, Q.; Xu, R. Amino-Assisted Anchoring of CsPbBr3Perovskite Quantum Dots on Porous g-C3 N4 for Enhanced Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 13570–13574. [Google Scholar] [CrossRef]
- Zhu, J.J.; Xiao, P.; Li, H.L.; Carabineiro, S.A.C. Graphitic Carbon Nitride: Synthesis, Properties, and Applications in Catalysis. ACS Appl. Mater. Interfaces 2014, 6, 16449–16465. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, A.D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Xing, W.N.; Li, C.M.; Chen, G.; Han, Z.H.; Zhou, Y.S.; Hu, Y.D.; Meng, Q.Q. Incorporating a novel metal-free interlayer into g-C3N4 framework for efficiency enhanced photocatalytic H2 evolution activity. Appl. Catal. B Environ. 2017, 203, 65–71. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zheng, X.L.; Lu, Y.Q.; Yang, X.X.; Kheradmand, A.; Jiang, Y.J. Efficient upconverting carbon nitride nanotubes for near-infrared-driven photocatalytic hydrogen production. Nanoscale 2019, 11, 20274–20283. [Google Scholar] [CrossRef]
- Che, H.N.; Che, G.B.; Zhou, P.J.; Song, N.; Li, C.X.; Li, C.M.; Liu, C.B.; Liu, X.T.; Dong, H.J. Precursor-reforming strategy induced g-C3N4microtubes with spatial anisotropic charge separation established by conquering hydrogen bond for enhanced photocatalytic H2-production performance. J. Colloid Interface Sci. 2019, 547, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhang, L.X.; Huang, W.M.; Kong, Q.L.; Fan, X.Q.; Wang, M.; Shi, J.L. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light. Carbon 2016, 99, 111–117. [Google Scholar] [CrossRef]
- Che, H.N.; Liu, C.B.; Che, G.B.; Liao, G.F.; Dong, H.J.; Li, C.X.; Song, N.; Li, C.M. Facile construction of porous intramolecular g-C3N4-based donor-acceptor conjugated copolymers as highly efficient photocatalysts for superior H2 evolution. Nano Energy 2020, 67, 104273. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Cheng, K.; Zhang, Y.; Ran, J.; Wu, G. Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials 2021, 11, 1480. https://doi.org/10.3390/nano11061480
Xing W, Cheng K, Zhang Y, Ran J, Wu G. Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials. 2021; 11(6):1480. https://doi.org/10.3390/nano11061480
Chicago/Turabian StyleXing, Weinan, Ke Cheng, Yichi Zhang, Jie Ran, and Guangyu Wu. 2021. "Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production" Nanomaterials 11, no. 6: 1480. https://doi.org/10.3390/nano11061480
APA StyleXing, W., Cheng, K., Zhang, Y., Ran, J., & Wu, G. (2021). Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials, 11(6), 1480. https://doi.org/10.3390/nano11061480