Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Conductive Superhydrophobic Composite Coating
2.3. Durability
2.4. Adhesiveness
2.5. Wearability
2.6. Characterization
3. Results and Discussion
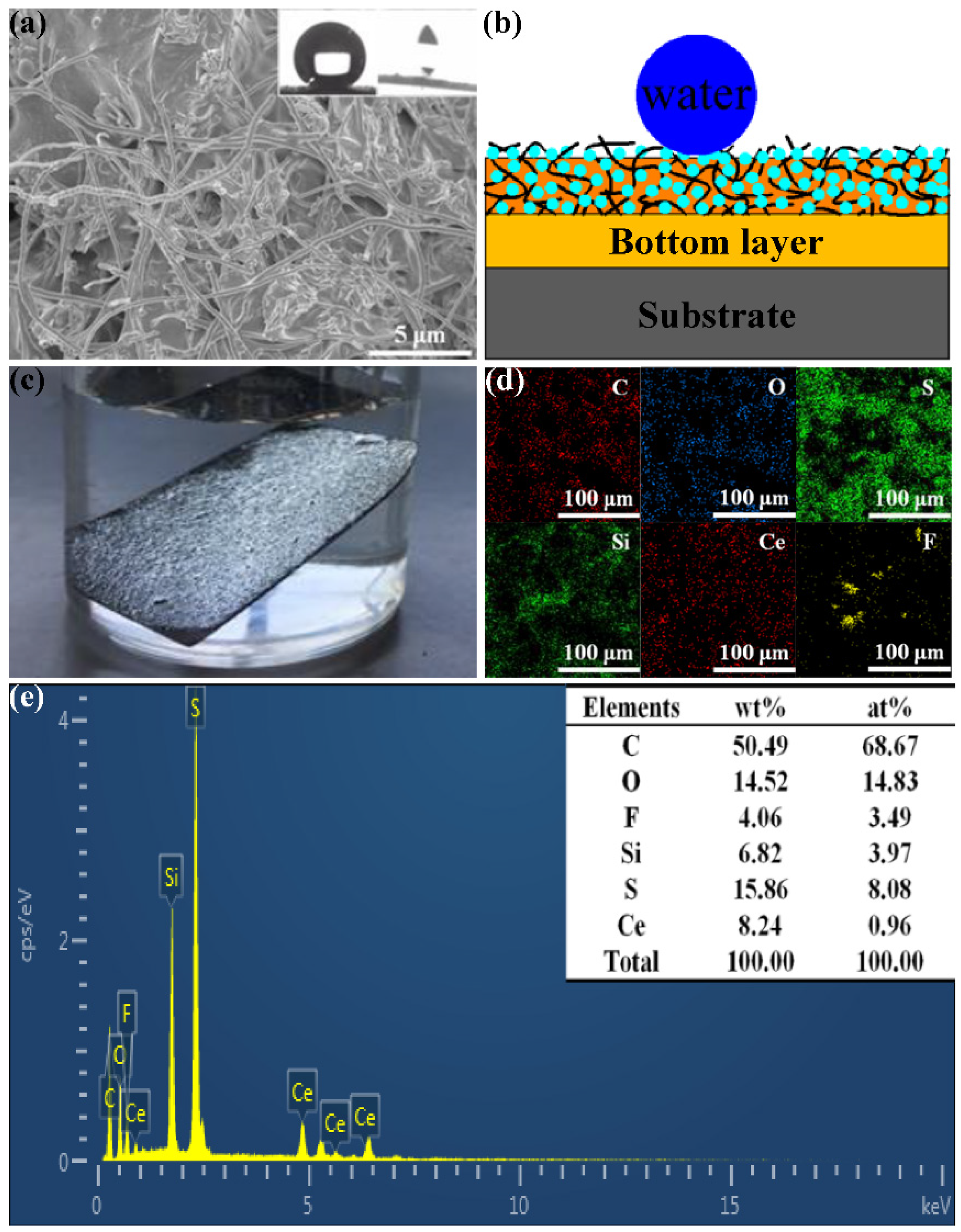
3.1. Fabrication of the CSC-Coating
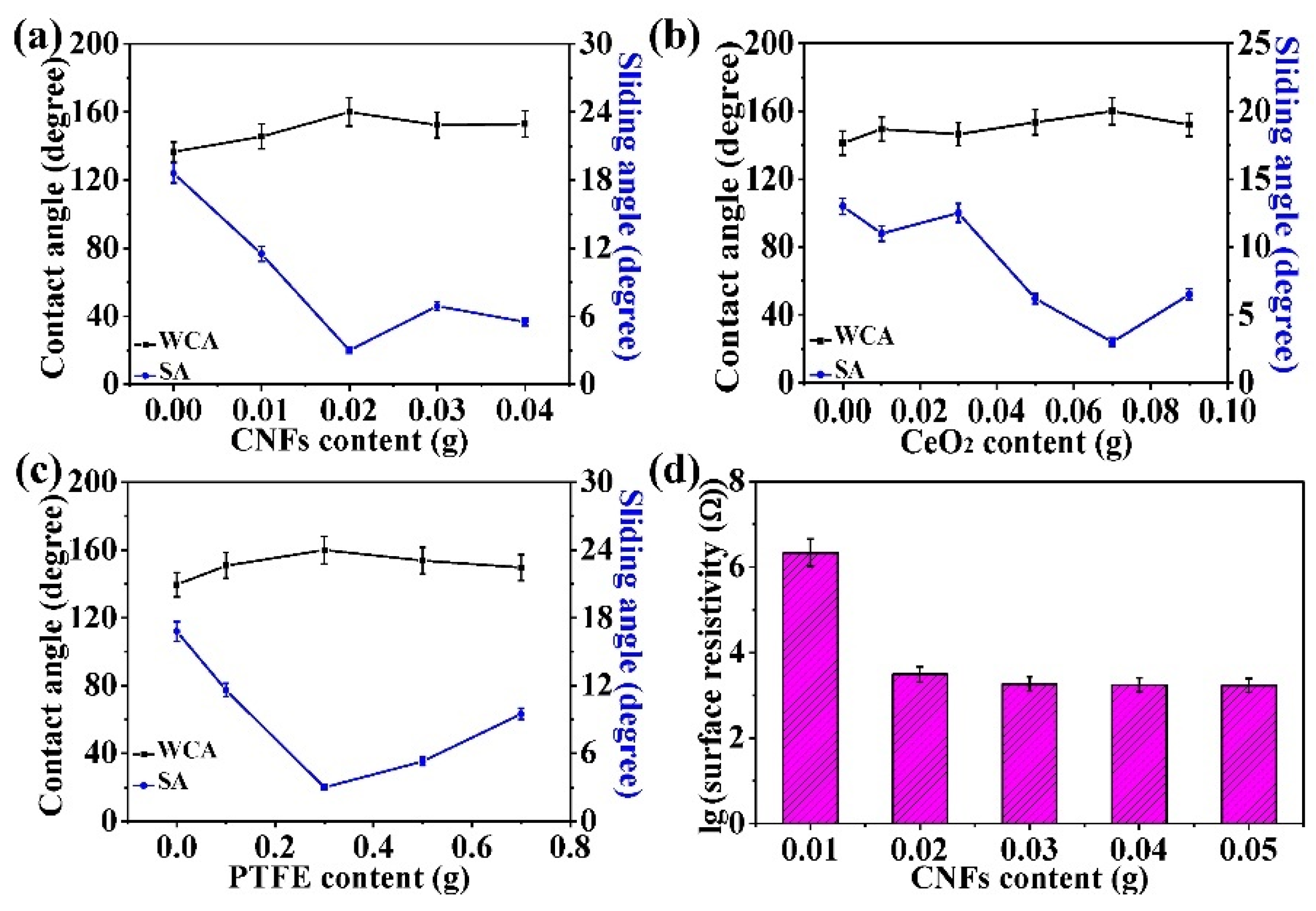
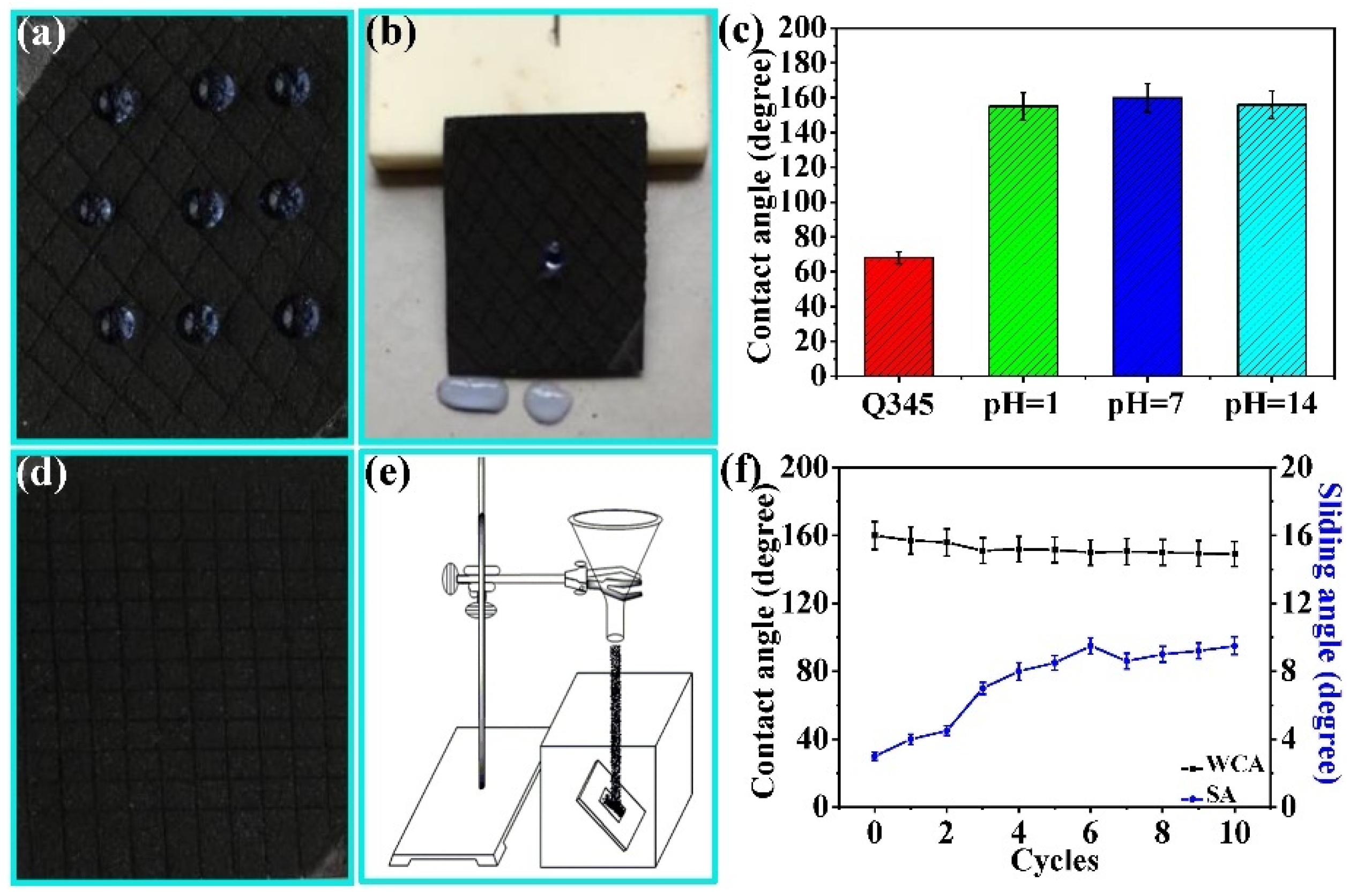
3.2. Surface Morphology and Wettability
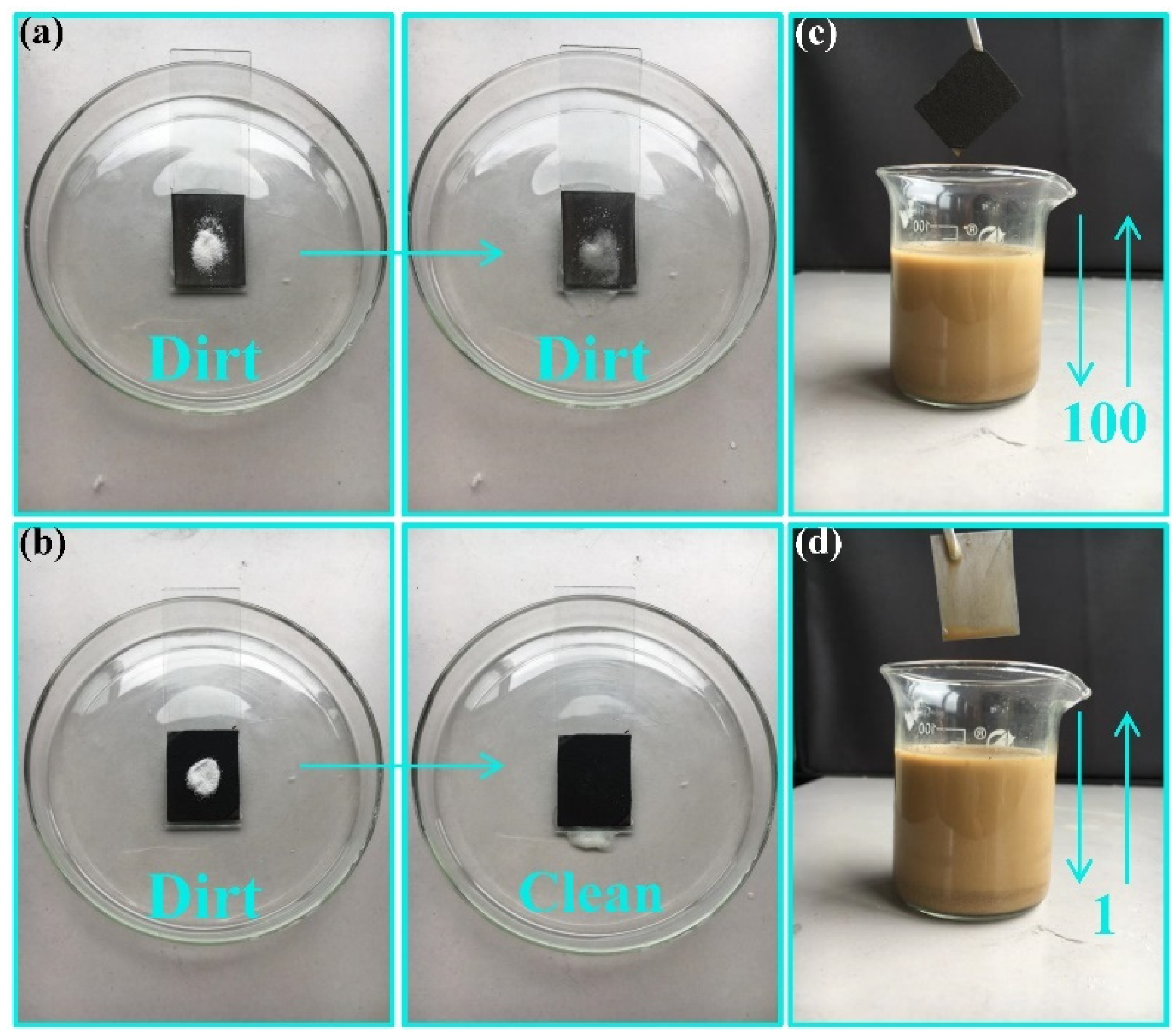
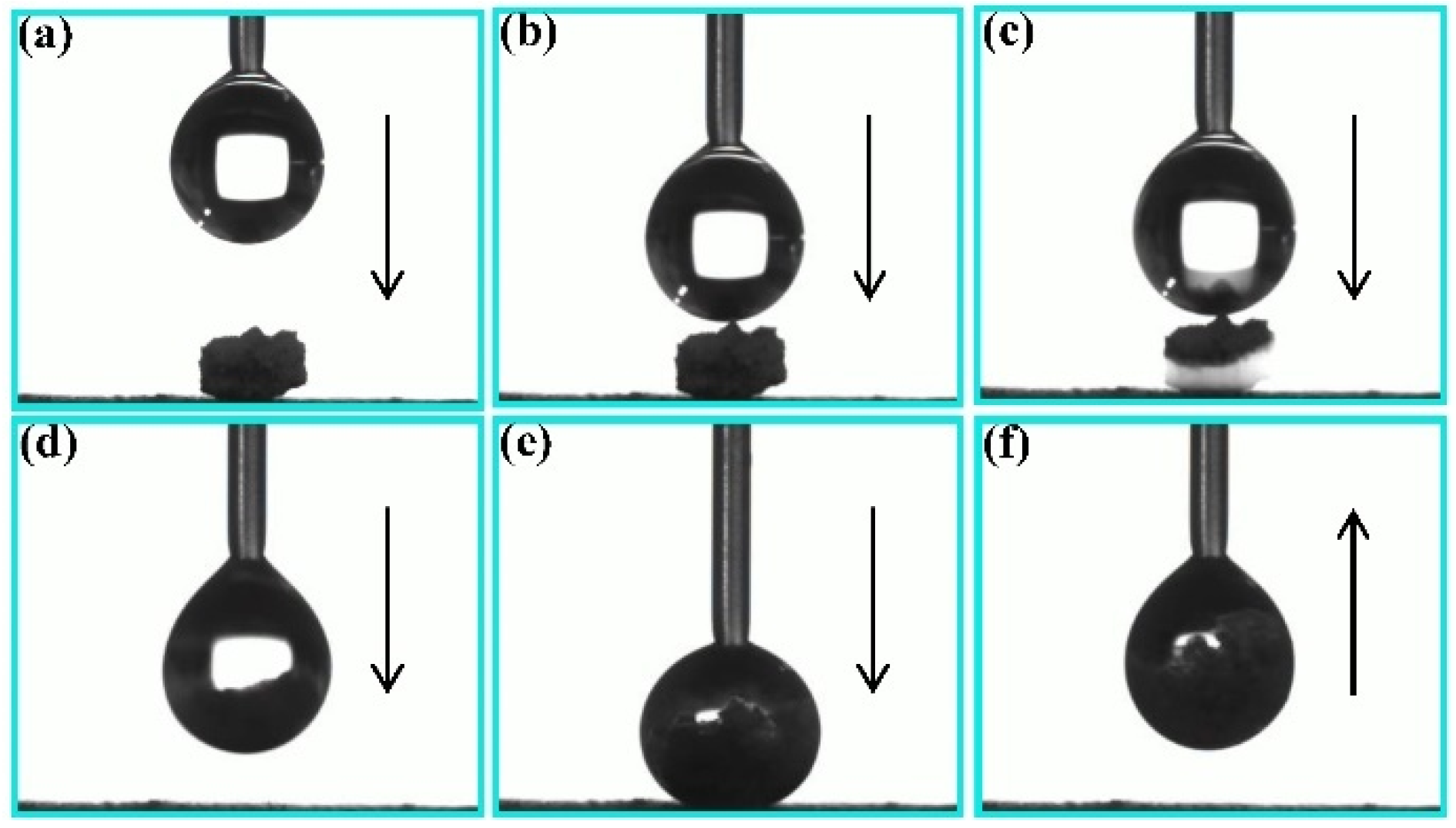
3.3. Self-Cleaning and Anti-Fouling Properties
3.4. Durability Analysis
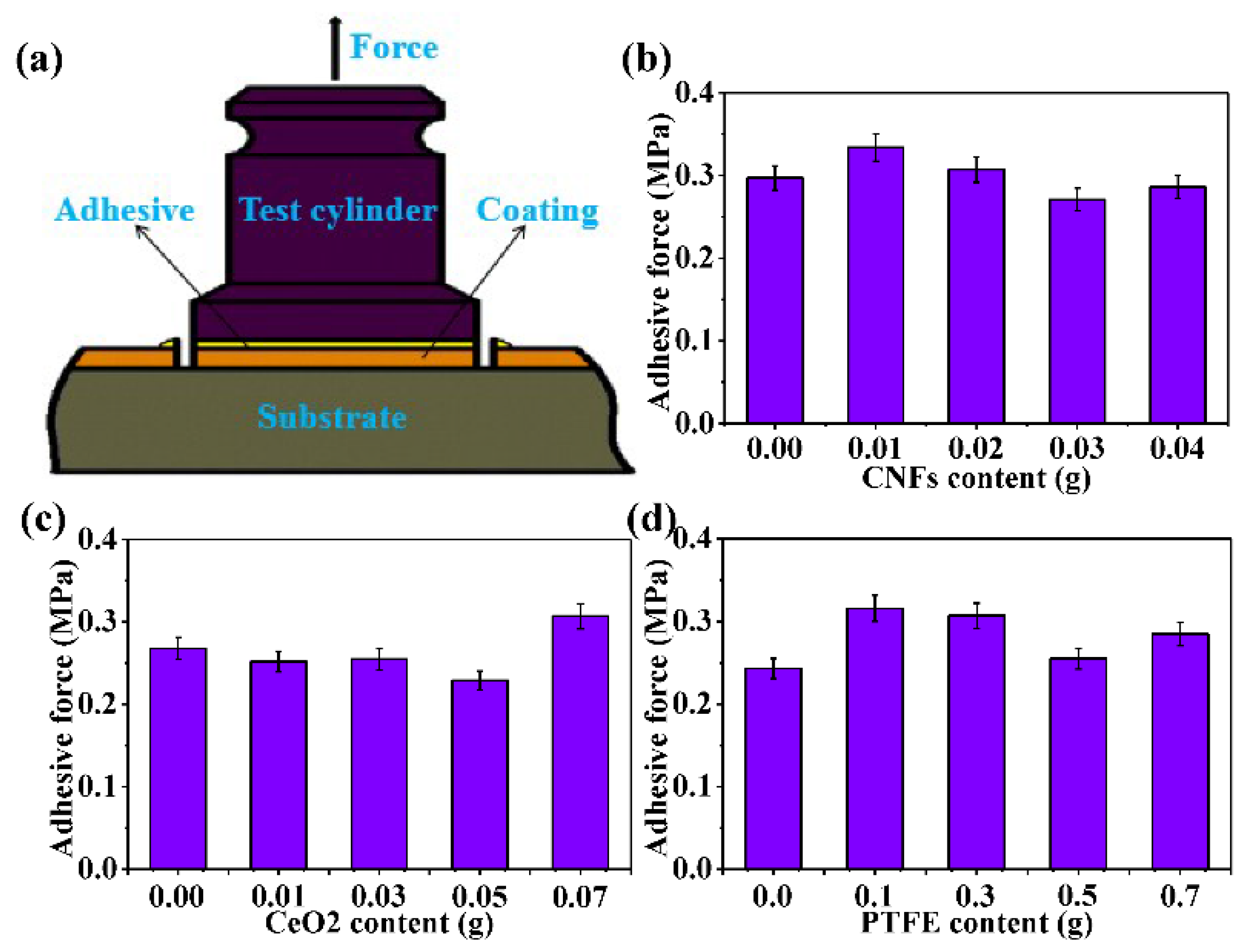
3.5. Adhesiveness Analysis
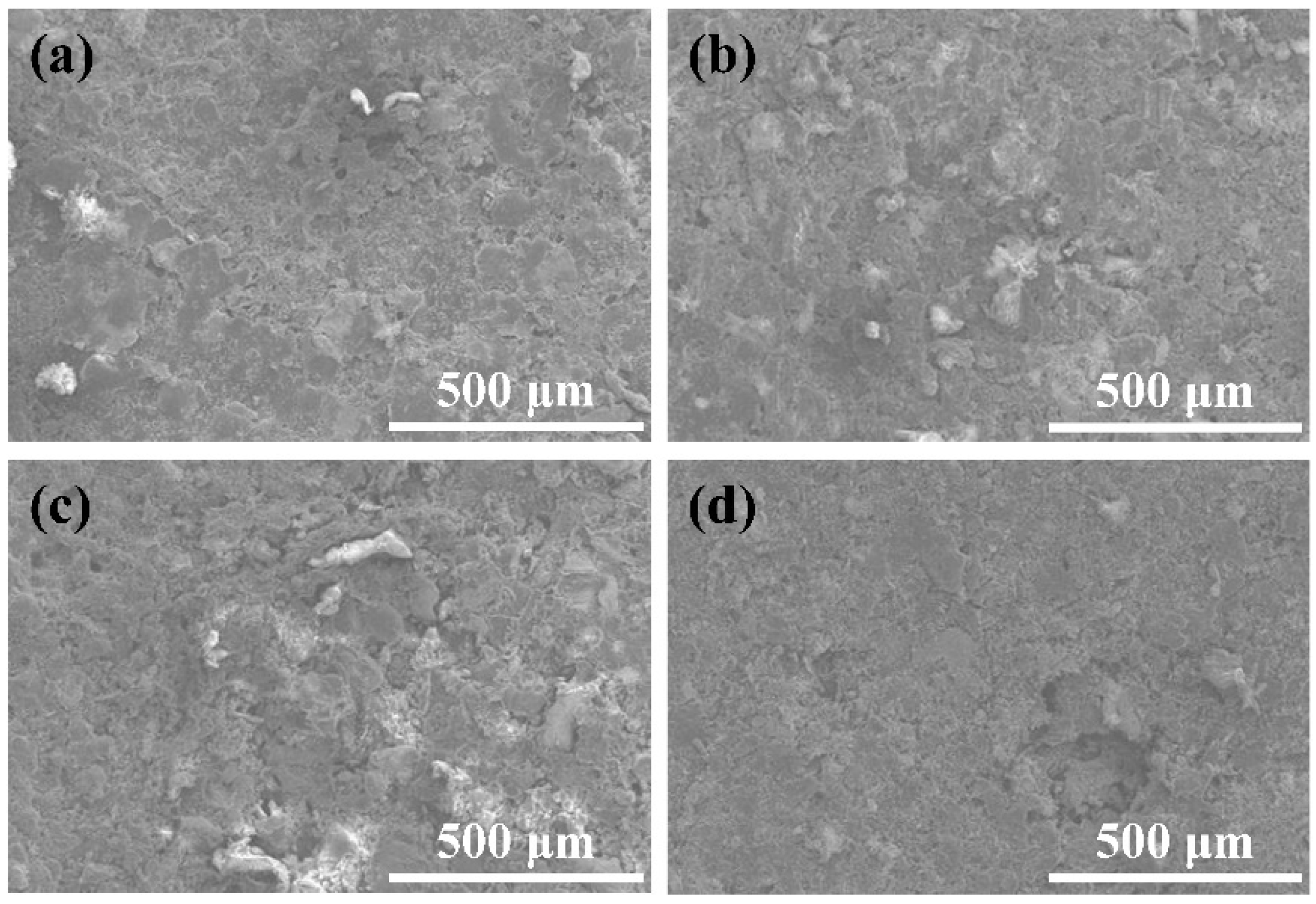
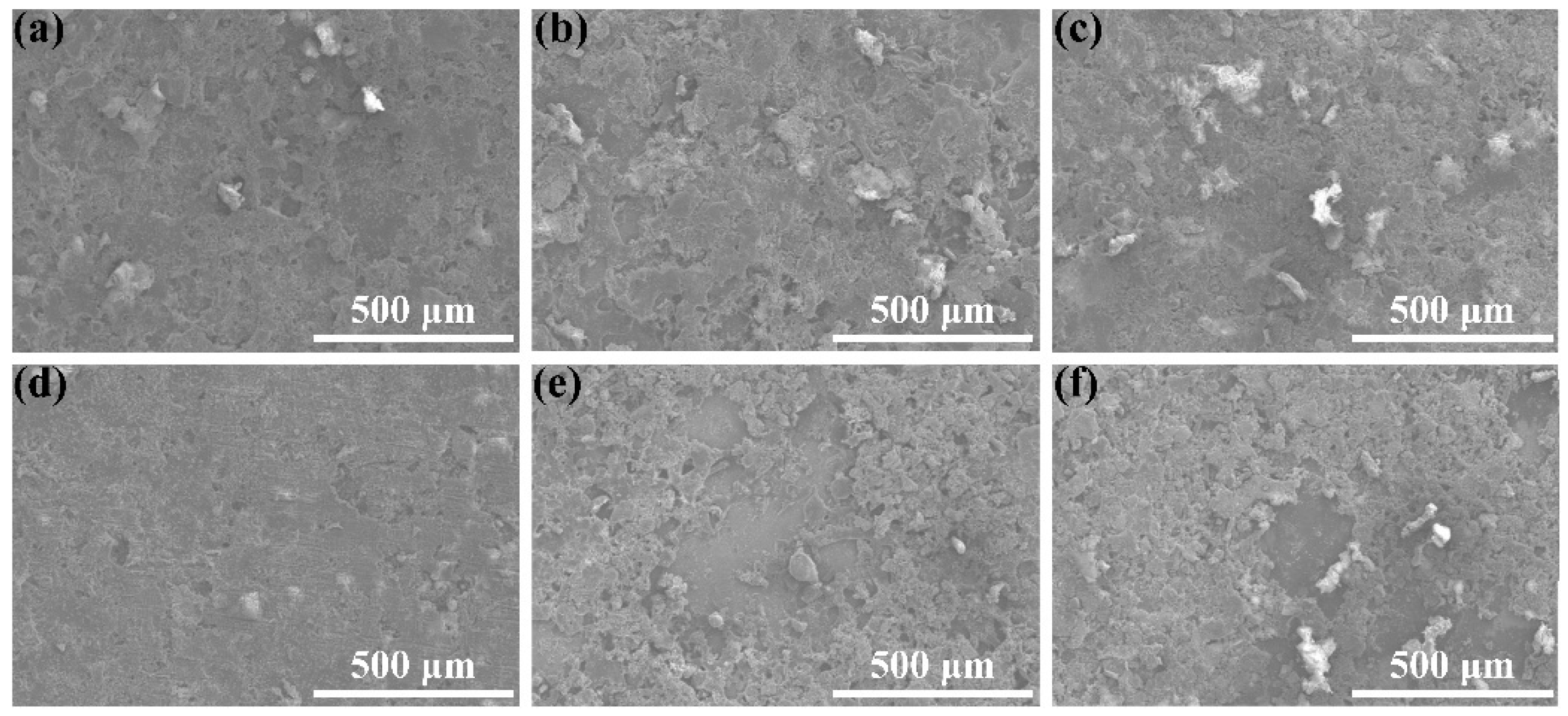
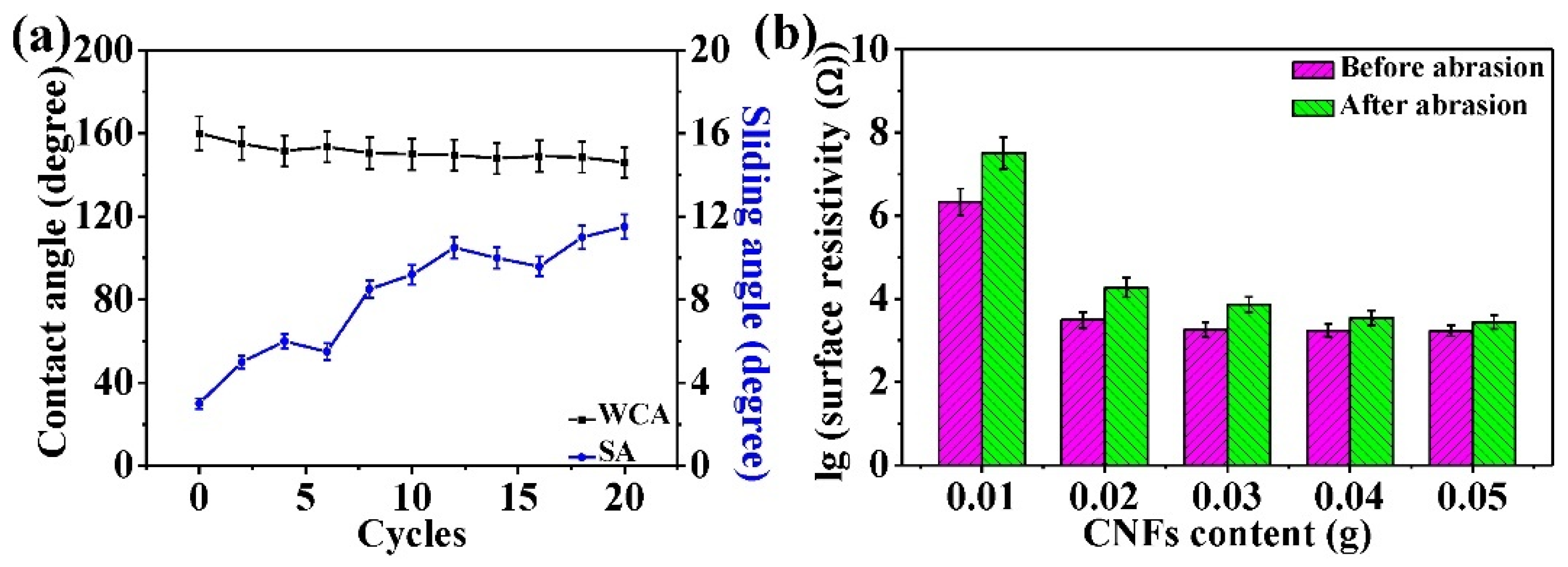
3.6. Wearability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulz, C.; Schlfer, T.; Charrault, E.; Hall, C. Erosive wear testing of laser clad and HVOF coatings for drilling in mining. J. Therm. Spray Technol. 2020, 29, 520–529. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S. Corrosion behavior of WC-Co tool bits in simulated (concrete, soil, and mine) solutions with and without chloride additions. Int. J. Refract. Met. Hard Mater. 2019, 85, 105062. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Fang, Y. Cause-chain analysis of coal-mine gas explosion accident based on Bayesian network model. Cluster Comput. 2019, 22, 1549–1557. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Cao, W.; Sang, S.; Wu, Q.; Liu, H.; Liu, K. Conductive and high anticorrosive rGO-modified copper foil prepared by electrocoagulation and chemical reduction. Ionics 2018, 25, 2935–2944. [Google Scholar] [CrossRef]
- Xiao, F.; Qian, C.; Guo, M.; Wang, J.; Yan, X.; Li, H.; Yue, L. Anticorrosive durability of zinc-based waterborne coatings enhanced by highly dispersed and conductive polyaniline/graphene oxide composite. Prog. Org. Coat. 2015, 125, 79–88. [Google Scholar] [CrossRef]
- Singh, H.; Sodhi, G.P.S.; Singh, M.; Chelliah, N.M.; Singh, H. Study: Wear and superhydrophobic behaviour of PTFE-ceria composite. Sur Eng. 2018, 35, 550–556. [Google Scholar] [CrossRef]
- Dai, H.; Gao, C.; Sun, J.; Li, C.; Li, N.; Wu, L.; Dong, Z.; Jiang, L. Controllable high-speed electrostatic manipulation of water droplets on a superhydrophobic surface. Adv. Mater. 2019, 31, 1905449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, W.; Sun, F.; Zhang, P.; He, Y.; Wang, X.; Luo, D.; Ma, W. Construction of a superhydrophobic coating using triethoxyvinylsilane-modified silica nanoparticles. Surf. Eng. 2018, 35, 418–425. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Yan, Q.; Wang, S. Corrosion resistance and self-cleaning behaviour of Ni/a-C:H superhydrophobic films. Sur. Eng. 2018, 34, 611–619. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Salim, A.; Seeger, S. Hierarchical structured multifunctional self-cleaning material with durable superhydrophobicity and photocatalytic functionalities. Small 2019, 15, 1901822. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, Y.; Tao, J.; He, Z.; Xie, Y.; Chen, H.; Jin, M.; Hou, W. Facile spraying fabrication of highly flexible and mechanically robust superhydrophobic F-SiO2@PDMS coatings for self-cleaning and drag-reduction applications. New J. Chem. 2018, 42, 18208–18216. [Google Scholar] [CrossRef]
- Li, D.; Gou, X.; Wu, D.; Guo, Z. A robust and stretchable superhydrophobic PDMS/PVDF@KNFs membrane for oil/water separation and flame retardancy. Nanoscale 2018, 10, 6695–6703. [Google Scholar] [CrossRef] [PubMed]
- Emelyanenko, K.A.; Nikita, S.; Elizaveta, C.; Ganne, A.A.; Emelyanenko, A.M.; Boinovich, L. Superhydrophobic corrosion resistant coatings for copper via IR nanosecond laser processing. Mater. Res. Express 2018, 5, 115001. [Google Scholar] [CrossRef]
- Dong, X.; Meng, J.; Hu, Y.; Wei, X.; Luan, X.; Zhou, H. Fabrication of self-cleaning superhydrophobic surfaces with improved corrosion resistance on 6061 aluminum alloys. Micromachines 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Q.; Wang, N.; Zheng, X. Fabrication of superhydrophobic green surfaces with good self-cleaning, chemical stability and anti-corrosion properties. J. Mater. Sci. 2019, 54, 13006–13016. [Google Scholar] [CrossRef]
- Jo, S.; Ahn, S.; Lee, H.; Jung, C.; Sing, S.; Kim, D.R. Water-repellent hybrid nanowire and micro-scale denticle structures on flexible substrates of effective air retention. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Tafreshi, H.V. Role of particles spatial distribution in drag reduction performance of superhydrophobic granular coatings. Int. J. Multiphas. Flow 2018, 98, 128–138. [Google Scholar] [CrossRef]
- Sarshar, M.A.; Song, D.; Swarctz, C.; Lee, J.; Choi, C. Anti-icing or deicing: Icephobicities of superhydrophobic surfaces with hierarchical structures. Langmuir 2018, 34, 13821–13827. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-step fabrication of robust superhydrophobic steel surfaces with mechanical durability, thermal stability, and anti-icing function. Acs Appl. Mater. Inter. 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Jin, H.; Li, Z.; Wei, S.; Nie, S.; Pan, Z.; Zhang, T.; Gao, N. Corrosion resistance and dynamic anti-icing of superhydrophobic surface on ASW. Surf. Eng. 2018, 34, 603–610. [Google Scholar] [CrossRef]
- Chen, X.; Wang, P.; Zhang, D. Designing a superhydrophobic surface for enhanced atmospheric corrosion resistance based on coalescence-induced droplet jumping behavior. ACS Appl. Mater. Interfaces 2019, 11, 38276–38284. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, W.; Zhao, S.; Zhang, X.; Pei, L.; Zhao, G.; Wang, Z. Flexible and thermally stable superhydrophobic surface with excellent anti-corrosion behavior. J. Mater. Sci. 2020, 55, 2215–2225. [Google Scholar] [CrossRef]
- Yin, K.; Yang, Y.; Cheng, Y. Permeability of coal tar enamel coating to cathodic protection current on pipelines. Constr. Build. Mater. 2018, 192, 20–27. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C.; et al. Superhydrophobic electrically conductive paper for ultrasensitive strain sensor with excellent anti-corrosion and self-cleaning property. ACS Appl. Mater. Inter. 2019, 11, 21904–21914. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.; Chen, Y.; Lin, L.; Huang, X.; Xue, H.; Gao, J. Fluorine-free superhydrophobic and conductive rubber composite with outstanding deicing performance for highly sensitive and stretchable strain sensors. ACS Appl. Mater. Inter. 2019, 11, 17774–17783. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; An, N.; Sun, J. Applied voltage and near-infrared light enable healing of superhydrophobicity loss caused by severe scratches in conductive superhydrophobic films. Adv. Funct. Mater. 2016, 26, 6777–6784. [Google Scholar] [CrossRef]
- Cho, Y.; Nam, S.; Jeong, S.; Kim, Y. Low-cost fabrication of flexible water-repellent film by spray coating of a hydrophobic nanoparticle dispersion. J. Disper. Sci. Technol. 2019, 41, 1526–1539. [Google Scholar] [CrossRef]
- Huang, G.; Fu, W.; Ma, L.; Li, X.; Wang, H. Cold spraying B4C particles reinforced aluminium coatings. Sur. Eng. 2018, 35, 772–783. [Google Scholar] [CrossRef]
- Arminger, B.; Gindl-Altmutter, W.; Keckes, J.; Hansmann, C. Facile preparation of superhydrophobic wood surfaces via spraying of aqueous alkyl ketene dimer dispersions. RSC Adv. 2019, 9, 24357–24367. [Google Scholar] [CrossRef]
- Tao, C.; Yang, K.; Zou, X.; Yan, H.; Yuan, X.; Zhang, L.; Jiang, B. Double-layer tri-wavelength hydrophobic antireflective coatings derived from methylated silica nanoparticles and hybrid silica nanoparticles. J. Sol-gel Sci. Technol. 2018, 86, 285–292. [Google Scholar] [CrossRef]
- Shen, F.; Wang, K.; Yin, Y.; Shi, L.; Zeng, D.; Han, X. PAN/PI functional double-layer coating for dendrite-free lithium metal anodes. J. Mater. Chem. A 2020, 8, 6183–6189. [Google Scholar] [CrossRef]
- Khan, Z.; Rasheed, H.U.; Alharbi, S.O.; Khan, I.; Abbas, T.; Chin, D. Manufacturing of double layer optical fiber coating using phan-thien-tanner fluid as coating material. Coatings 2019, 9, 147. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Guo, Z. A facile modifier-free approach to fabricate antistatic superhydrophobic composite coatings with remarkable thermal stability and corrosion resistance. J. Bionic Eng. 2020, 17, 421–435. [Google Scholar] [CrossRef]
- Dong, H.; Li, X.; Dong, Y.; Guo, S.; Zhao, L. A novel preparation method of electrically conductive adhesives by powder spraying process. Materials 2019, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.; Zhang, Y.; Jarad, N.A.; Soleymani, L.; Didar, T.F. Liquid-infused surfaces: A review of theory, design, and applications. ACS Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ha, S.H.; Park, S.J.; Kim, J. Effect of surface cleaning on laser texturing of multicrystalline silicon wafer. Sci. Adv. Mater. 2018, 10, 690–693. [Google Scholar] [CrossRef]
- Budakci, M.; Saygin, E.; Senol, S. Effect of resin cleaning process on adhesion strength of water-based varnishes. Bioresources 2019, 14, 1317–1332. [Google Scholar]
- Huang, Y.; Lv, Z.; Cao, Z.; Huang, C. A green and facile method to fabricate superhydrophobic coatings. Surf. Eng. 2019, 35, 435–439. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Q.; Li, Y.; Hou, B. Facile fluorine-free one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chem. Eng. J. 2018, 352, 625–633. [Google Scholar] [CrossRef]
- Li, S.; Page, K.; Sathasivam, S.; Heale, F.; He, G.; Lu, Y.; Lai, Y.; Chen, G.; Carmalt, C.; Parkin, I. Efficiently texturing hierarchical superhydrophobic fluoride-free translucent films by AACVD with excellent durability and self-cleaning ability. J. Mater. Chem. A. 2018, 6, 17633–17641. [Google Scholar] [CrossRef]
- Wang, W.; Vahabi, H.; Movafaghi, S.; Kota, A. Superomniphobic surfaces with improved mechanical durability: Synergy of hierarchical texture and mechanical interlocking. Adv. Mater. Interfaces 2019, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, Z. A mechanically robust transparent coating for anti-icing and self-cleaning applications. J. Mater. Chem. A 2018, 6, 16043–16052. [Google Scholar] [CrossRef]
- Li, J.; Yan, X.; Yi, L.; Cai, Y.; Tang, Z. Novel coating system on poly(ethylene terephthalate) fabrics with mechanically durable liquid-repellence: Application as flexible materials with striking loading capacity. Appl. Surf. Sci. 2018, 457, 332–341. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Liu, L.; Fu, J.; Jin, L.; Shang, L.; Xiao, L.; Ao, Y. Directly coating silanized nanocrystalline cellulose on carbon fiber for enhancing the interfacial adhesion of carbon fiber/epoxy resin composites. Polym. Compos. 2018, 40, 744–752. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Yang, M.; Pan, Y.; Yin, Q.; Tang, J.; Qi, H.; Suo, Z. Printing hydrogels and elastomers in arbitrary sequence with strong adhesion. Adv. Funct. Mater. 2019, 29, 1–8. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, T.; Liu, F.; Zhang, M.; Gan, W.; Zhai, X.; Di, X.; Wang, Y.; Liu, G.; Wang, C. Facile design and fabrication of superwetting surfaces with excellent wear-resistance. ACS Appl. Mater. Interfaces 2017, 9, 15776–15784. [Google Scholar] [CrossRef] [PubMed]
- Masood, M.T.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Palazon, F.; Athanssiou, A.; Bayer, I.S. Superhydrophobic high impact polystyrene (HIPS) nanocomposites with wear abrasion resistance. Chem. Eng. J. 2017, 322, 10–21. [Google Scholar] [CrossRef]
- Xie, J.; Hu, J.; Lin, X.; Fang, L.; Wu, F.; Liao, X.; Luo, H.; Shi, L. Robust and anti-corrosive PDMS/SiO2 superhydrophobic coatings fabricated on magnesium alloys with different-sized SiO2 nanoparticles. Appl. Surf. Sci. 2018, 457, 870–880. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, K.; Zhang, D.; Guo, Z. Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method. Nanomaterials 2021, 11, 1506. https://doi.org/10.3390/nano11061506
Liu X, Chen K, Zhang D, Guo Z. Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method. Nanomaterials. 2021; 11(6):1506. https://doi.org/10.3390/nano11061506
Chicago/Turabian StyleLiu, Xiang, Kai Chen, Dekun Zhang, and Zhiguang Guo. 2021. "Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method" Nanomaterials 11, no. 6: 1506. https://doi.org/10.3390/nano11061506
APA StyleLiu, X., Chen, K., Zhang, D., & Guo, Z. (2021). Stable and Durable Conductive Superhydrophobic Coatings Prepared by Double-Layer Spray Coating Method. Nanomaterials, 11(6), 1506. https://doi.org/10.3390/nano11061506





