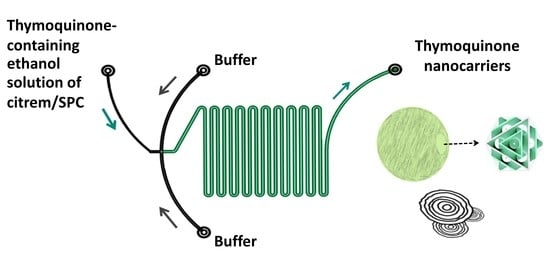
Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
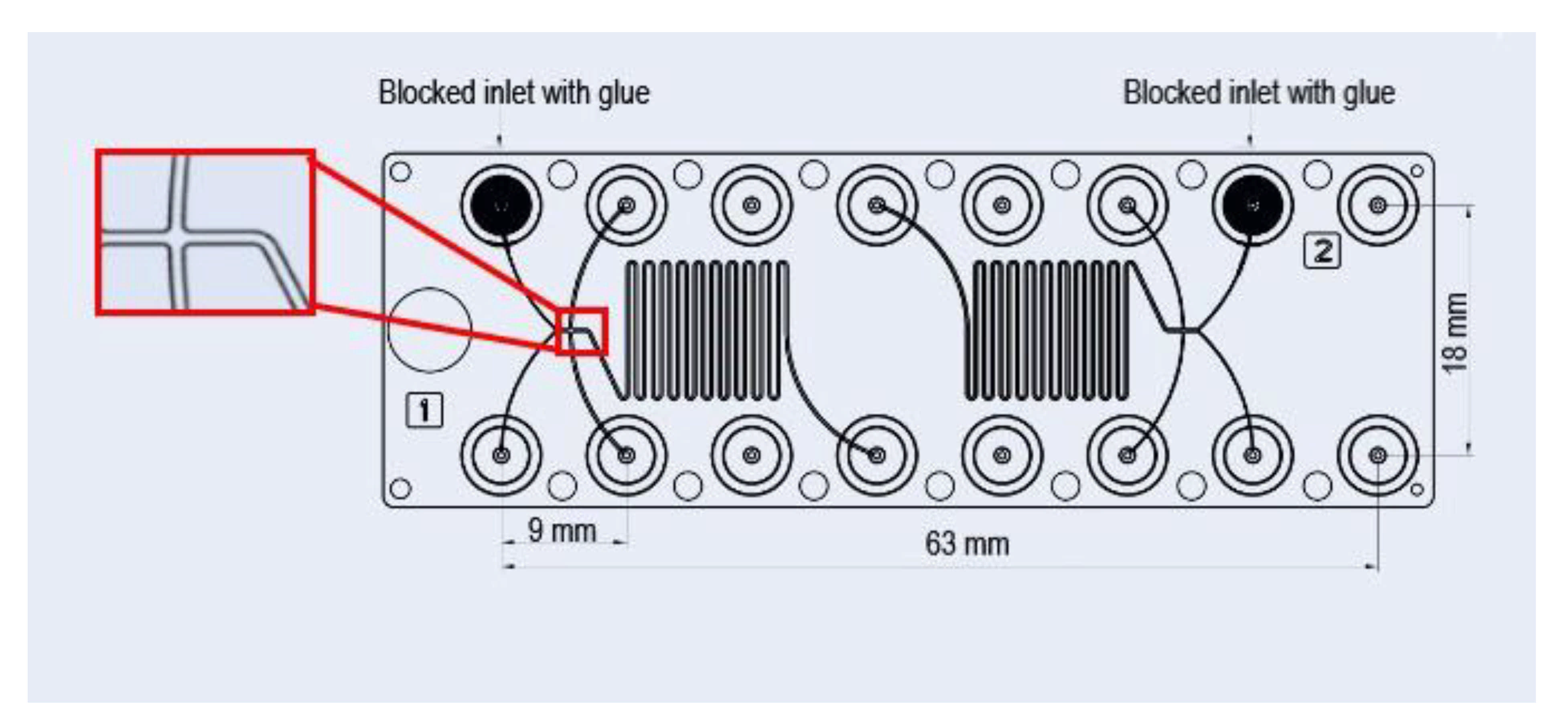
2.2. Continuous Production of Citrem/SPC Nano-Self-Assemblies
2.3. Low-Energy Batch Production of Citrem/SPC Nano-Self-Assemblies
2.4. NTA Measurements
2.5. Synchrotron Small Angle X-ray Scattering (SAXS)
3. Results and Discussion
3.1. Size Characteristics of TQ-Free and TQ-Loaded Citrem/SPC Nano Self-Assemblies
3.2. Effects of Loading Drug and EtOH Concentration on Citrem/SPC Nano Self-Assemblies Produced by the Batch Emulsification Method
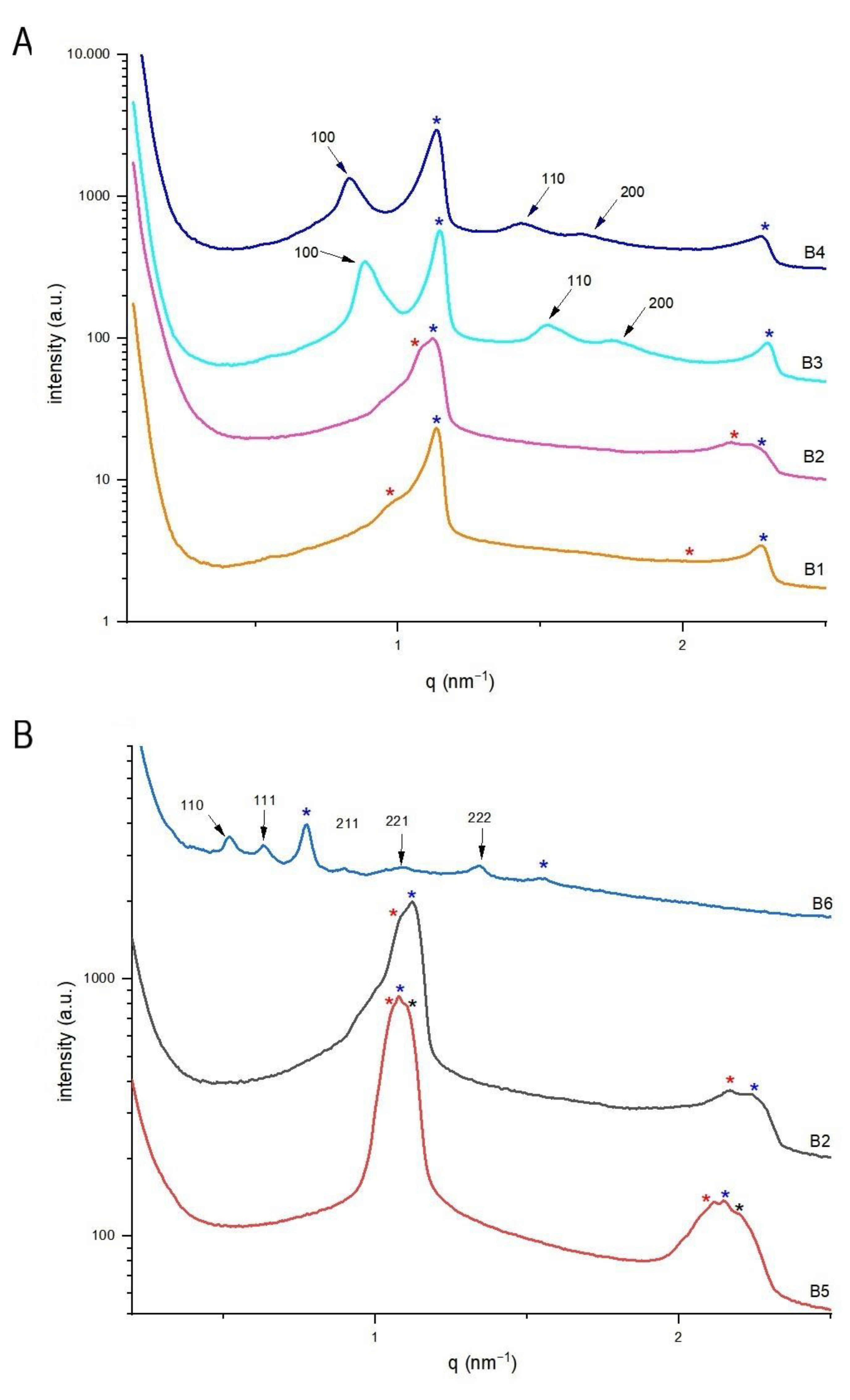
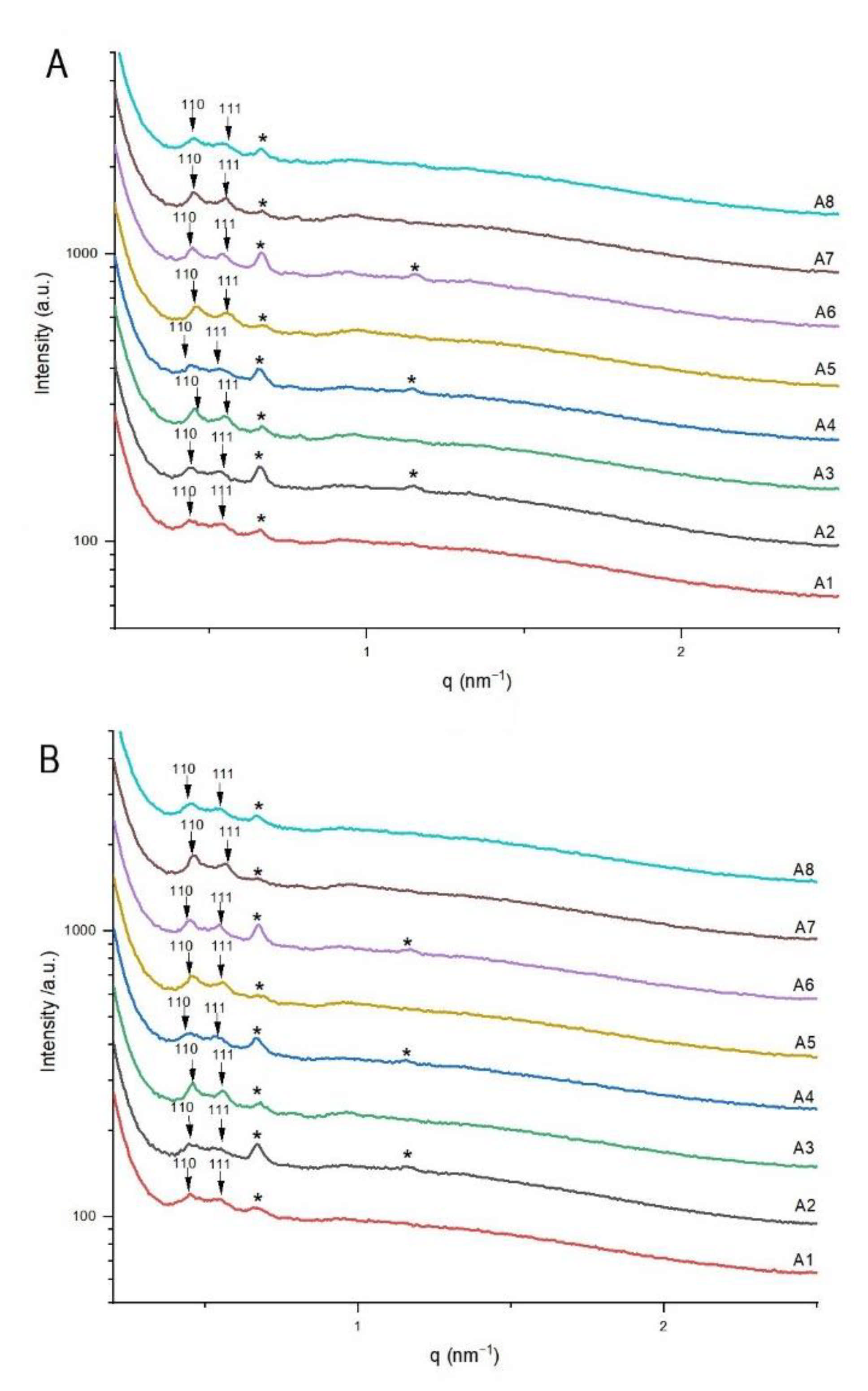
3.3. Structural Characteristics of Continuously Produced Citrem/SPC Nano-Self-Assemblies Using Microfluidics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bangham, A.D.; Horne, R.W. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J. Mol. Biol. 1964, 8, 660–668. [Google Scholar] [CrossRef]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-lamellar lyotropic liquid crystalline lipid nanoparticles for the next generation of nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, A.V.P.; Caron, A.L.; Viegas, J.; Praça, F.G.; Bentley, M.V.L.B. Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin. Drug Deliv. 2020, 17, 1781–1805. [Google Scholar] [CrossRef]
- Murgia, S.; Biffi, S.; Mezzenga, R. Recent advances of non-lamellar lyotropic liquid crystalline nanoparticles in nanomedicine. Curr. Opin. Colloid Interface Sci. 2020, 48, 28–39. [Google Scholar] [CrossRef]
- Yaghmur, A.; Glatter, O. Characterization and potential applications of nanostructured aqueous dispersions. Adv. Colloid Interface Sci. 2009, 147–148, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef]
- Glatter, O.; Salentinig, S. Inverting structures: From micelles via emulsions to internally self-assembled water and oil continuous nanocarriers. Curr. Opin. Colloid Interface Sci. 2020, 49, 82–93. [Google Scholar] [CrossRef]
- Meikle, T.G.; Dharmadana, D.; Hoffmann, S.V.; Jones, N.C.; Drummond, C.J.; Conn, C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid Interface Sci. 2021, 587, 90–100. [Google Scholar] [CrossRef]
- Zabara, M.; Senturk, B.; Gontsarik, M.; Ren, Q.; Rottmar, M.; Maniura-Weber, K.; Mezzenga, R.; Bolisetty, S.; Salentinig, S. Multifunctional nano-biointerfaces: Cytocompatible antimicrobial nanocarriers from stabilizer-free cubosomes. Adv. Funct. Mater. 2019, 29, 1904007. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Bizien, T.; Gorshkova, Y.E.; Deng, Y. Plasmalogen-based liquid crystalline multiphase structures involving docosapentaenoyl derivatives inspired by biological cubic membranes. Front. Cell Dev. Biol. 2021, 9, 617984. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Østergaard, J.; Stürup, S.; Gammelgaard, B.; Urtti, A.; Moghimi, S.M.; Yaghmur, A. Cisplatin encapsulation generates morphologically different multicompartments in the internal nanostructures of nonlamellar liquid-crystalline self-assemblies. Langmuir 2018, 34, 6570–6581. [Google Scholar] [CrossRef]
- Bor, G.; Azmi, I.D.M.; Yaghmur, A. Nanomedicines for cancer therapy: Current status, challenges and future prospects. Ther. Deliv. 2019, 10, 113–132. [Google Scholar] [CrossRef]
- Angelov, B.; Garamus, V.M.; Drechsler, M.; Angelova, A. Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J. Mol. Liq. 2017, 235, 83–89. [Google Scholar] [CrossRef]
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-lamellar liquid crystalline nanocarriers for thymoquinone encapsulation. Molecules 2020, 25, 16. [Google Scholar] [CrossRef]
- Nilsson, C.; Barrios-Lopez, B.; Kallinen, A.; Laurinmäki, P.; Butcher, S.J.; Raki, M.; Weisell, J.; Bergström, K.; Larsen, S.W.; Østergaard, J.; et al. SPECT/CT imaging of radiolabeled cubosomes and hexosomes for potential theranostic applications. Biomaterials 2013, 34, 8491–8503. [Google Scholar] [CrossRef]
- Alcaraz, N.; J Boyd, B. Cubosomes as carriers for MRI contrast agents. Curr. Med. Chem. 2017, 24, 470–482. [Google Scholar] [CrossRef]
- Helvig, S.Y.; Andersen, H.; Antopolsky, M.; Airaksinen, A.J.; Urtti, A.; Yaghmur, A.; Moghimi, S.M. Hexosome engineering for targeting of regional lymph nodes. Materialia 2020, 11, 100705. [Google Scholar] [CrossRef]
- Akbar, S.; Anwar, A.; Ayish, A.; Elliott, J.M.; Squires, A.M. Phytantriol based smart nano-carriers for drug delivery applications. Eur. J. Pharm. Sci. 2017, 101, 31–42. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Pispas, S.; Chrysina, E.D.; Forys, A.; Trzebicka, B.; Demetzos, C. Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies. Int. J. Pharm. 2018, 550, 57–70. [Google Scholar] [CrossRef]
- Gontsarik, M.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. From structure to function: pH-switchable antimicrobial nano-self-assemblie. ACS Appl. Mater. Interfaces 2019, 11, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Gontsarik, M.; Yaghmur, A.; Salentinig, S. pH-responsive nano-self-assemblies of the anticancer drug 2-hydroxyoleic acid. Langmuir 2019, 35, 7954–7961. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as PEGylated reservoirs of omega-3 polyunsaturated fatty acids: Structural ınsights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef]
- Gontsarik, M.; Buhmann, M.T.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. Antimicrobial peptide-driven colloidal transformations in liquid-crystalline nanocarriers. J. Phys. Chem. Lett. 2016, 7, 3482–3486. [Google Scholar] [CrossRef]
- Gontsarik, M.; Yaghmur, A.; Salentinig, S. Dispersed liquid crystals as pH-adjustable antimicrobial peptide nanocarriers. J. Colloid Interface Sci. 2021, 583, 672–682. [Google Scholar] [CrossRef]
- Lancelot, A.; Sierra, T. Nanostructured liquid-crystalline particles for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, R.; Larsen, S.W.; Yaghmur, A. Citrem-phosphatidylcholine nano-self-assemblies: Solubilization of bupivacaine and its role in triggering a colloidal transition from vesicles to cubosomes and hexosomes. Phys. Chem. Chem. Phys. 2019, 21, 15142–15150. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Angelova, A. Cubic liquid crystalline nanostructures involving catalase and curcumin: bioSAXS study and catalase peroxidatic function after cubosomal nanoparticle treatment of differentiated SH-SY5Y cells. Molecules 2019, 24, 3058. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.M.; Wibroe, P.P.; Wu, L.-P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Control. Release 2016, 239, 1–9. [Google Scholar] [CrossRef]
- Wibroe, P.P.; Mat Azmi, I.D.; Nilsson, C.; Yaghmur, A.; Moghimi, S.M. Citrem modulates internal nanostructure of glyceryl monooleate dispersions and bypasses complement activation: Towards development of safe tunable intravenous lipid nanocarriers. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1909–1914. [Google Scholar] [CrossRef]
- Khaliqi, K.; Ghazal, A.; Azmi, I.D.M.; Amenitsch, H.; Mortensen, K.; Salentinig, S.; Yaghmur, A. Direct monitoring of lipid transfer on exposure of citrem nanoparticles to an ethanol solution containing soybean phospholipids by combining synchrotron SAXS with microfluidics. Analyst 2017, 142, 3118–3126. [Google Scholar] [CrossRef]
- Prajapati, R.; Salentinig, S.; Yaghmur, A. Temperature triggering of kinetically trapped self-assemblies in citrem-phospholipid nanoparticles. Chem. Phys. Lipids 2018, 216, 30–38. [Google Scholar] [CrossRef]
- Yaghmur, A.; Al-Hosayni, S.; Amenitsch, H.; Salentinig, S. Structural investigation of bulk and dispersed inverse lyotropic hexagonal liquid crystalline phases of eicosapentaenoic acid monoglyceride. Langmuir 2017, 33, 14045–14057. [Google Scholar] [CrossRef]
- Jahn, A.; Vreeland, W.N.; Gaitan, M.; Locascio, L.E. Controlled vesicle self-assembly in microfluidic channels with hydrodynamic focusing. J. Am. Chem. Soc. 2004, 126, 2674–2675. [Google Scholar] [CrossRef]
- Sedighi, M.; Sieber, S.; Rahimi, F.; Shahbazi, M.A.; Rezayan, A.H.; Huwyler, J.; Witzigmann, D. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv. Transl. Res. 2019, 9, 404–413. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef]
- Khan, I.U.; Serra, C.A.; Anton, N.; Vandamme, F.T. Production of nanoparticle drug delivery systems with microfluidics tools. Expert Opin. Drug Deliv. 2015, 12, 547–562. [Google Scholar] [CrossRef]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; Devoe, D.L.; Gaitan, M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar] [CrossRef]
- Ghazal, A.; Gontsarik, M.; Kutter, J.P.; Lafleur, J.P.; Ahmadvand, D.; Labrador, A.; Salentinig, S.; Yaghmur, A. Microfluidic platform for the continuous production and characterization of multilamellar vesicles: A synchrotron small-angle x-ray scattering (SAXS) study. J. Phys. Chem. Lett. 2017, 8, 73–79. [Google Scholar] [CrossRef]
- Koh, C.G.; Zhang, X.; Liu, S.; Golan, S.; Yu, B.; Yang, X.; Guan, J.; Jin, Y.; Talmon, Y.; Muthusamy, N.; et al. Delivery of antisense oligodeoxyribonucleotide lipopolyplex nanoparticles assembled by microfluidic hydrodynamic focusing. J. Control. Release 2010, 141, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.G.; Kang, X.; Xie, Y.; Fei, Z.; Guan, J.; Yu, B.; Zhang, X.; Lee, L.J. Delivery of polyethylenimine/DNA complexes assembled in a microfluidics device. Mol. Pharm. 2009, 6, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.T.; Jahn, A.; Locascio, L.E.; Vreeland, W.N. Controlled self-assembly of monodisperse niosomes by microfluidic hydrodynamic focusing. Langmuir 2010, 26, 8559–8566. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sung, J.; Chang, Y.; Alfeche, A.; Leal, C. Microfluidics synthesis of gene silencing cubosomes. ACS Nano 2018, 12, 9196–9205. [Google Scholar] [CrossRef]
- Yaghmur, A.; Ghazal, A.; Ghazal, R.; Dimaki, M.; Svendsen, W.E. A hydrodynamic flow focusing microfluidic device for the continuous production of hexosomes based on docosahexaenoic acid monoglyceride. Phys. Chem. Chem. Phys. 2019, 21, 13005–13013. [Google Scholar] [CrossRef]
- Sharifi, F.; Yesil-Celiktas, O.; Kazan, A.; Maharjan, S.; Saghazadeh, S.; Firoozbakhsh, K.; Firoozabadi, B.; Zhang, Y.S. A hepatocellular carcinoma–bone metastasis-on-a-chip model for studying thymoquinone-loaded anticancer nanoparticles. Bio-Design Manuf. 2020, 3, 189–202. [Google Scholar] [CrossRef]
- Ilhan-Ayisigi, E.; Yaldiz, B.; Bor, G.; Yaghmur, A.; Yesil-Celiktas, O. Advances in microfluidic synthesis and coupling with synchrotron SAXS for continuous production and real-time structural characterization of nano-self-assemblies. Colloids Surfaces B 2021, 201, 111633. [Google Scholar] [CrossRef]
- Pilkington, C.P.; Seddon, J.M.; Elani, Y. Microfluidic technologies for the synthesis and manipulation of biomimetic membranous nano-assemblies. Phys. Chem. Chem. Phys. 2021, 23, 3693–3706. [Google Scholar] [CrossRef]
- Silva, B.F.B. SAXS on a chip: From dynamics of phase transitions to alignment phenomena at interfaces studied with microfluidic devices. Phys. Chem. Chem. Phys. 2017, 19, 23690–23703. [Google Scholar] [CrossRef]
- Khader, M.; Eckl, P.M. Thymoquinone: An emerging natural drug with a wide range of medical applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957. [Google Scholar] [CrossRef]
- Helvig, S.Y.; Woythe, L.; Pham, S.; Bor, G.; Andersen, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of glycerol monooleate/oleic acid non-lamellar liquid crystalline nanodispersions stabilized with nonionic methoxypoly(ethylene glycol) (mPEG)-lipids showing variable complement activation properties. J. Colloid Interface Sci. 2021, 582, 906–917. [Google Scholar] [CrossRef]
- Odeh, F.; Ismail, S.I.; Abu-Dahab, R.; Mahmoud, I.S.; Al Bawab, A. Thymoquinone in liposomes: A study of loading efficiency and biological activity towards breast cancer. Drug Deliv. 2012, 19, 371–377. [Google Scholar] [CrossRef]
- Rajput, S.; Puvvada, N.; Kumar, B.N.P.; Sarkar, S.; Konar, S.; Bharti, R.; Dey, G.; Mazumdar, A.; Pathak, A.; Fisher, P.B.; et al. Overcoming Akt ınduced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multilamellar gold niosomes. Mol. Pharm. 2015, 12, 4214–4225. [Google Scholar] [CrossRef]
- Kazan, A.; Yesil-Celiktas, O.; Zhang, Y.S. Fabrication of thymoquinone-loaded albumin nanoparticles by microfluidic particle synthesis and their effect on planarian regeneration. Macromol. Biosci. 2019, 19, 1900182. [Google Scholar] [CrossRef]
- Amara, S.; Patin, A.; Giuffrida, F.; Wooster, T.J.; Thakkar, S.K.; Bénarouche, A.; Poncin, I.; Robert, S.; Point, V.; Molinari, S.; et al. In vitro digestion of citric acid esters of mono- and diglycerides (CITREM) and CITREM-containing infant formula/emulsions. Food Funct. 2014, 5, 1409–1421. [Google Scholar] [CrossRef]
- Burian, M.; Meisenbichler, C.; Naumenko, D.; Amenitsch, H. SAXSDOG: Open software for real-time azimuthal integration of 2D scattering images. arXiv 2020, arXiv:2007.02022. [Google Scholar]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome dispersions as delivery systems for percutaneous administration of indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef]
- Yu, B.; Zhu, J.; Xue, W.; Wu, Y.; Huang, X.; Lee, L.J.; Lee, R.J. Microfluidic assembly of lipid-based oligonucleotide nanoparticles. Anticancer Res. 2011, 31, 771–776. [Google Scholar]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Fathordoobady, F.; Sannikova, N.; Guo, Y.; Singh, A.; Kitts, D.D.; Pratap-Singh, A. Comparing microfluidics and ultrasonication as formulation methods for developing hempseed oil nanoemulsions for oral delivery applications. Sci. Rep. 2021, 11, 72. [Google Scholar] [CrossRef]
- Ghazal, A.; Gontsarik, M.; Kutter, J.P.; Lafleur, J.P.; Labrador, A.; Mortensen, K.; Yaghmur, A. Direct monitoring of calcium-triggered phase transitions in cubosomes using small-angle X-ray scattering combined with microfluidics research papers. J. Appl. Crystallogr. 2016, 49, 2005–2014. [Google Scholar] [CrossRef]
- Nilsson, C.; Edwards, K.; Eriksson, J.; Larsen, S.W.; Østergaard, J.; Larsen, C.; Urtti, A.; Yaghmur, A. Characterization of oil-free and oil-loaded liquid-crystalline particles stabilized by negatively charged stabilizer citrem. Langmuir 2012, 28, 11755–11766. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, S.F.; Nilsson, C.; Laurinmäki, P.; Butcher, S.; Urtti, A.; Yaghmur, A. Nanostructured aqueous dispersions of citrem interacting with lipids and PEGylated lipids. RSC Adv. 2013, 3, 24576–24585. [Google Scholar] [CrossRef]
- Qiu, H.; Caffrey, M. The phase diagram of the monoolein/water system: Metastability and equilibrium aspects. Biomaterials 2000, 21, 223–234. [Google Scholar] [CrossRef]
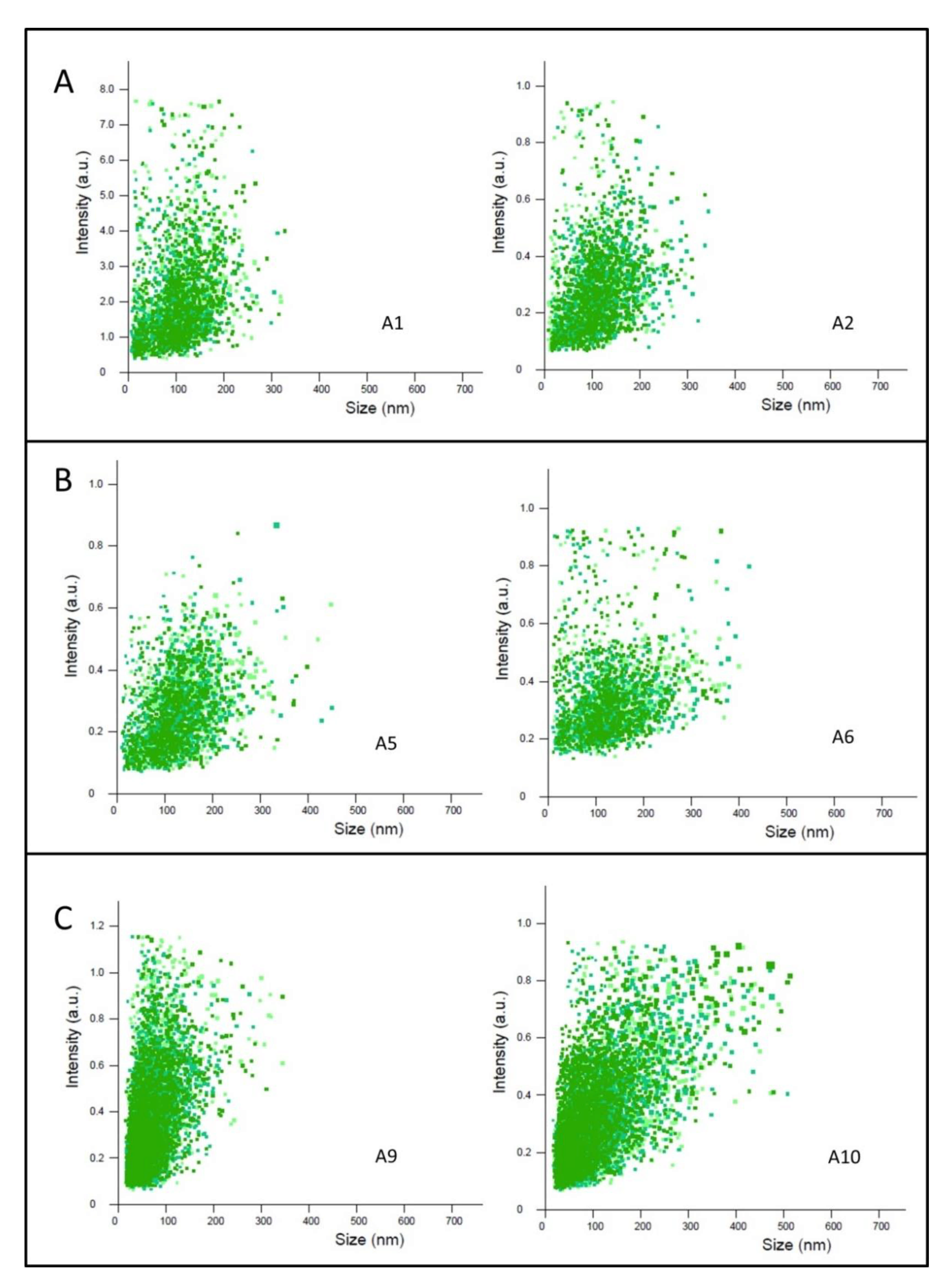
| Sample Name a | TQ (mg/mL) | TFR (µL/min) | Space Group | Lattice Parameter (nm ± Error%) 25 °C 37 °C | Size (nm) c Mean ± SD Mode ± SE | ||
|---|---|---|---|---|---|---|---|
| A1 | 0 | 100 | Lα Pn3m | 9.63 20.18 ± 0.03 | 9.48 19.70 ± 0.05 | 115.5 ± 42 | 134.8 ± 3.6 |
| A2 | 0 | 50 | Lα Pn3m | 9.51 20.18 ± 0.03 | 9.39 19.70 ± 0.05 | 119.7 ± 42 | 114.4 ± 3.8 |
| A3 | 1 | 100 | Lα Pn3m | 9.42 19.62 ± 0.02 | 9.43 19.36 ± 0.02 | 124.3 ± 38 | 126 ± 5.5 |
| A4 | 1 | 50 | Lα Pn3m | 9.56 20.18 ± 0.03 | 9.43 19.70 ± 0.05 | 139.8 ± 48 | 136.7 ± 3.2 |
| A5 | 2.5 | 100 | Lα Pn3m | 9.52 19.26 ± 0.04 | 9.36 19.27 ± 0.03 | 141.2 ± 49 | 145.5 ± 6.0 |
| A6 | 2.5 | 50 | Lα Pn3m | 9.46 19.87 ± 0.02 | 9.33 19.74 ± 0.02 | 150.1 ± 61 | 140.3 ± 2.7 |
| A7 | 5 | 100 | Lα Pn3m | 9.46 19.6 ± 0.02 | 9.44 19.18 ± 0.01 | 147.7 ± 45 | 150.5 ± 7. |
| A8 | 5 | 50 | Lα Pn3m | 9.48 19.71 ± 0.04 | 9.41 19.62 ± 0.06 | 164 ± 54 | 144.6 ± 8.1 |
| A9 | 0 | Batch | N.I. | N.I. b | 116.8 ± 45 | 102.3 ± 14.1 | |
| A10 | 2.5 | Batch | N.I. | N.I. b | 141.2 ± 44 | 135.1 ± 3.7 | |
| Sample Name a | EtOH % | Lipid % | TQ (mg/mL) | Space Group | Lattice Parameter (nm ± Error%) |
|---|---|---|---|---|---|
| B1 | 1.09 | 8.0 | 0 | Lα(1) Lα(2) | 5.55 ± 0.06 7.22 ± 2.63 |
| B2 | 1.09 | 8.0 | 2.5 | Lα(1) Lα(2) | 5.61 ± 0.05 5.72 ± 0.24 |
| B3 | 1.09 | 8.0 | 7.5 | Lα H2 | 5.50 ± 0.06 8.13 ± 0.24 |
| B4 | 1.09 | 8.0 | 10.0 | Lα H2 | 5.56 ± 0.02 8.68 ± 0.27 |
| B5 | 0 | 9.09 | 2.5 | Lα(1) Lα(2) Lα(3) | 5.93 ± 0.006 5.83 ± 0.06 5.75 ± 0.13 |
| B6 | 5.45 | 3.64 | 2.5 | Lα Pn3m | 8.14 ± 0.02 17.07 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilhan-Ayisigi, E.; Ghazal, A.; Sartori, B.; Dimaki, M.; Svendsen, W.E.; Yesil-Celiktas, O.; Yaghmur, A. Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery. Nanomaterials 2021, 11, 1510. https://doi.org/10.3390/nano11061510
Ilhan-Ayisigi E, Ghazal A, Sartori B, Dimaki M, Svendsen WE, Yesil-Celiktas O, Yaghmur A. Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery. Nanomaterials. 2021; 11(6):1510. https://doi.org/10.3390/nano11061510
Chicago/Turabian StyleIlhan-Ayisigi, Esra, Aghiad Ghazal, Barbara Sartori, Maria Dimaki, Winnie Edith Svendsen, Ozlem Yesil-Celiktas, and Anan Yaghmur. 2021. "Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery" Nanomaterials 11, no. 6: 1510. https://doi.org/10.3390/nano11061510
APA StyleIlhan-Ayisigi, E., Ghazal, A., Sartori, B., Dimaki, M., Svendsen, W. E., Yesil-Celiktas, O., & Yaghmur, A. (2021). Continuous Microfluidic Production of Citrem-Phosphatidylcholine Nano-Self-Assemblies for Thymoquinone Delivery. Nanomaterials, 11(6), 1510. https://doi.org/10.3390/nano11061510