The Biotin–Avidin Interaction in Biotinylated Gold Nanoparticles and the Modulation of Their Aggregation
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Physical Measurements
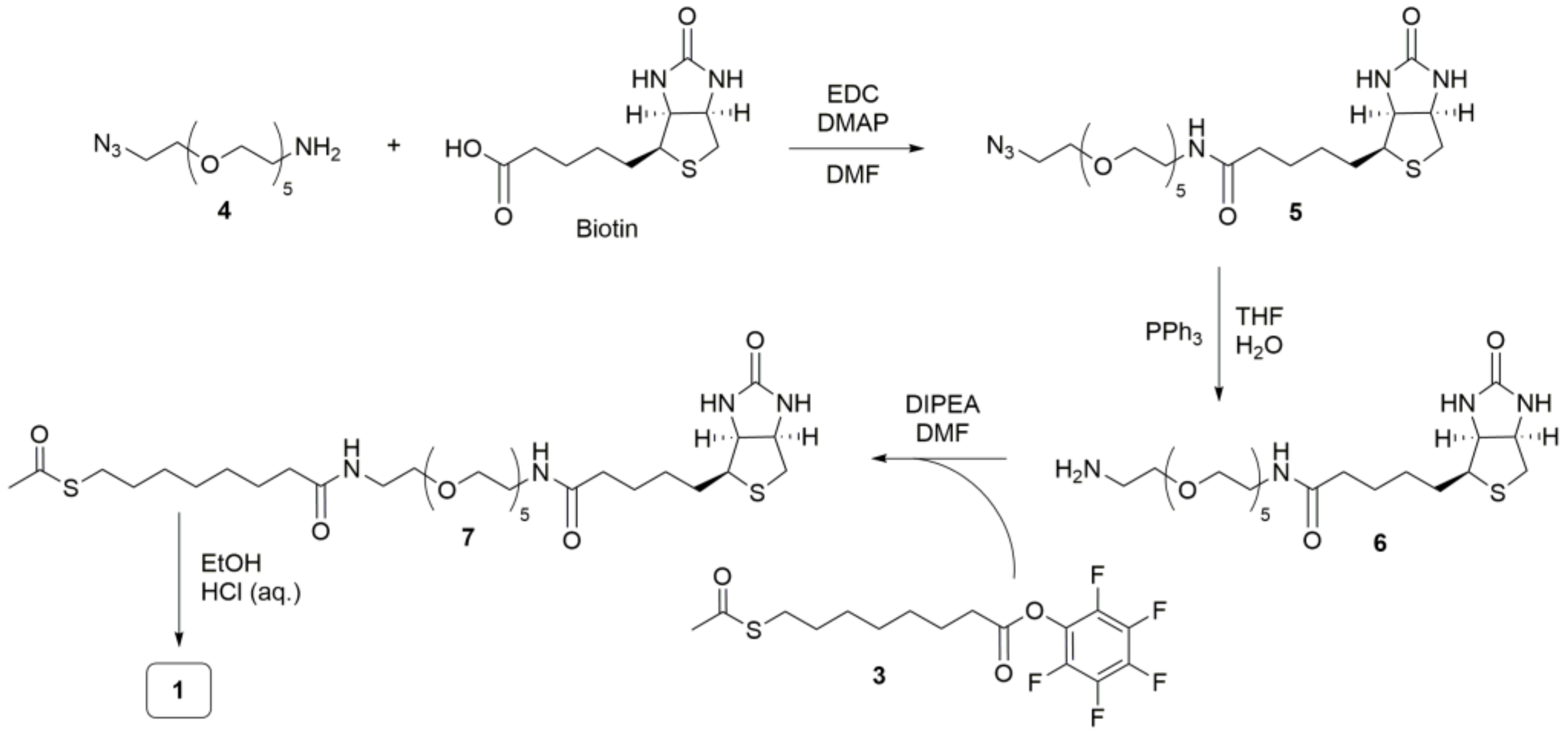
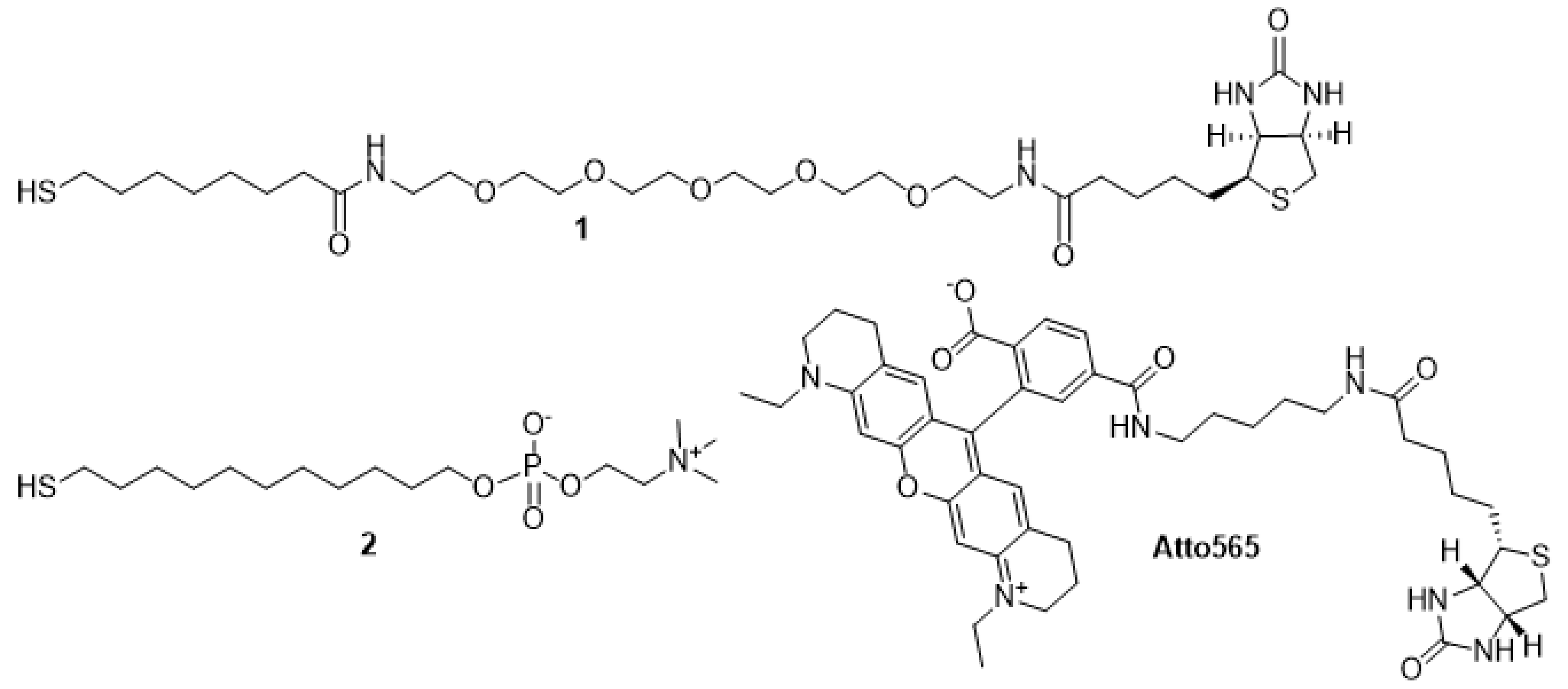
2.3. Synthesis and Characterization of Chemical Compounds
2.4. AuNPs Synthesis and Characterization
2.5. Thiol Exchange Protocol
2.6. Assessment of Biotinylated AuNPs—Avidin Interaction
3. Results and Discussion
3.1. Synthesis and Characterization of Biotinylated AuNPs
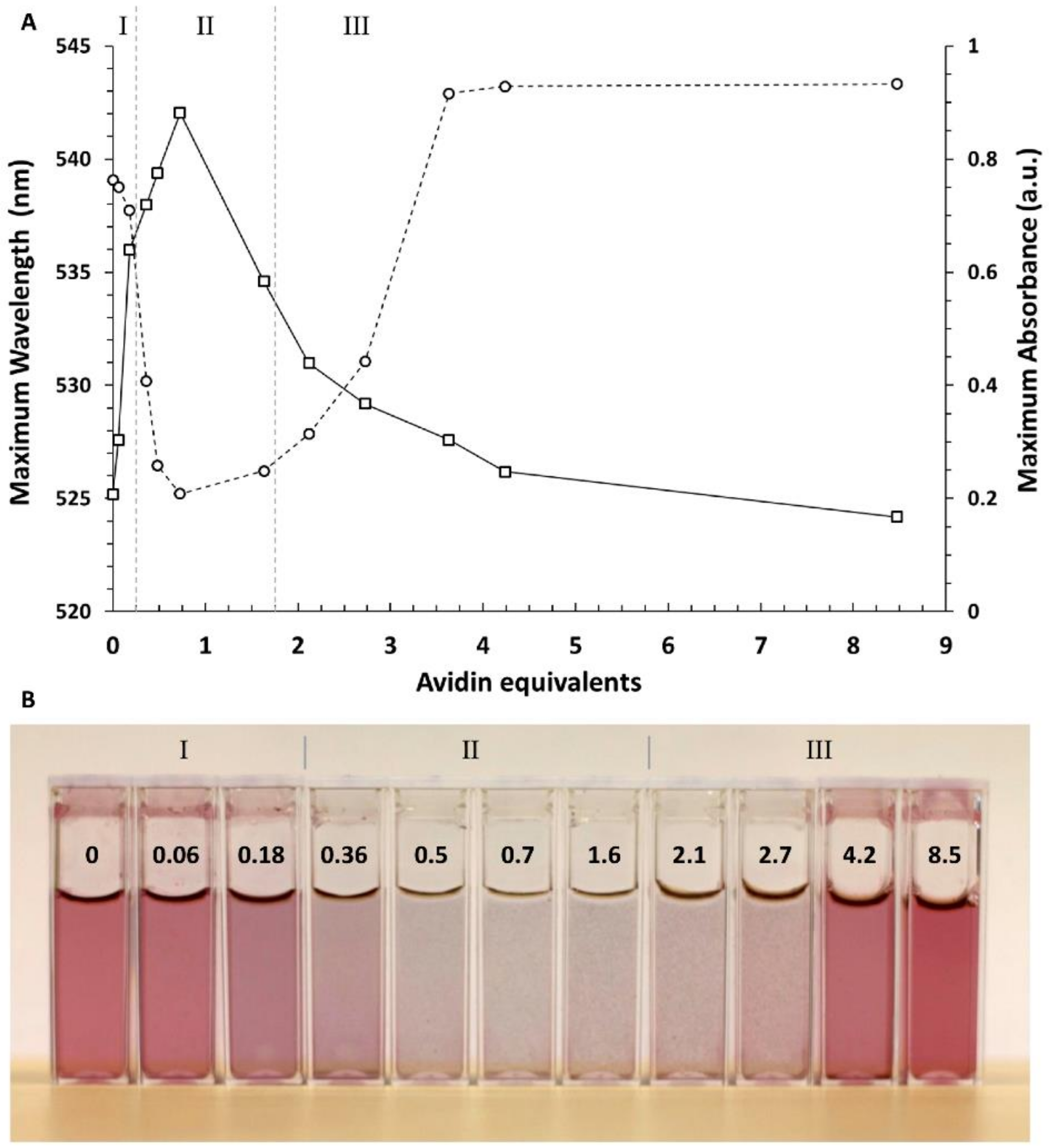
3.2. Biotinylated AuNPs Binding to Avidin
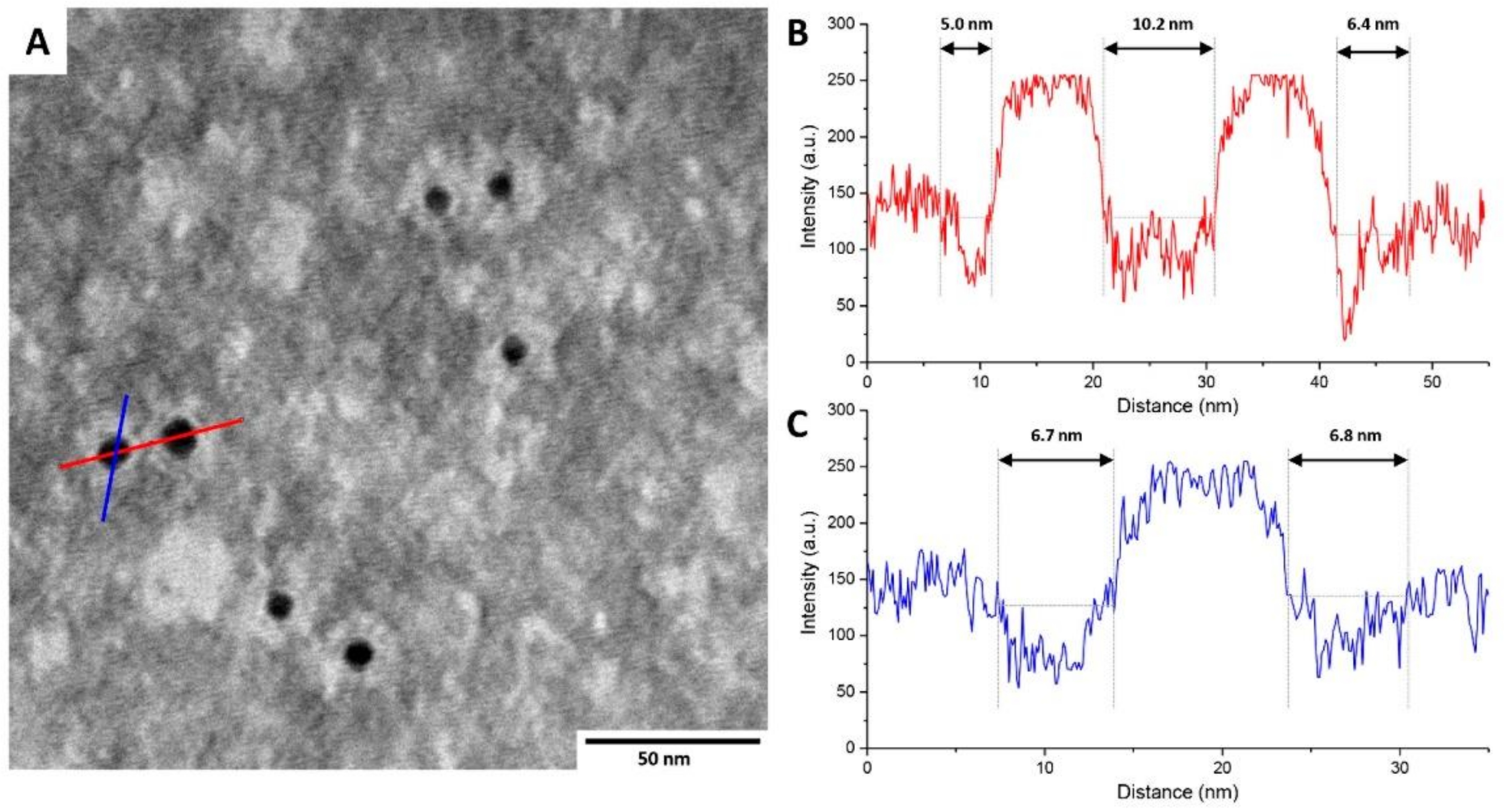
TEM Micrography
3.3. Avidin Modulation of Biotinylated AuNPs Crosslinking
3.4. Kinetic Control of the Cluster Formation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Livnah, O.; Bayer, E.A.; Wilchek, M.; Sussman, J.L. Three-Dimensional Structures of Avidin and the Avidin-Biotin Complex. Proc. Natl. Acad. Sci. USA 1993, 90, 5076–5080. [Google Scholar] [CrossRef] [Green Version]
- Korpela, J. Avidin, a high affinity biotin-binding protein, as a tool and subject of biological research. Med. Biol. 1984, 62, 5–26. [Google Scholar] [PubMed]
- Jain, A.; Cheng, K. The Principles and Applications of Avidin-Based Nanoparticles in Drug Delivery and Diagnosis. J. Control. Rel. 2017, 245, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, W.; Han, J.; Uhm, S.; Jang, Y.; Kang, C.; Kim, J.-H.; Kim, J. Recent Development of Biotin Conjugation in Biological Imaging, Sensing, and Target Delivery. Chem. Commun. 2015, 51, 10403–10418. [Google Scholar] [CrossRef]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin–Biotin Technology: Improvements and Innovations in Chemical and Biological Applications. Appl. Microbiol. Biot. 2013, 97, 9343–9353. [Google Scholar] [CrossRef]
- Lesch, H.P.; Kaikkonen, M.U.; Pikkarainen, J.T.; Ylä-Herttuala, S. Avidin-Biotin Technology in Targeted Therapy. Expert Opin. Drug Del. 2010, 7, 551–564. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lim, S.; Gunasekaran, S. Streptavidin-Coated Au Nanoparticles Coupled with Biotinylated Antibody-Based Bifunctional Linkers as Plasmon-Enhanced Immunobiosensors. ACS Appl. Nano Mater. 2020, 3, 1900–1909. [Google Scholar] [CrossRef]
- Pramanik, A.K.; Siddikuzzaman; Palanimuthu, D.; Somasundaram, K.; Samuelson, A.G. Biotin Decorated Gold Nanoparticles for Targeted Delivery of a Smart-Linked Anticancer Active Copper Complex: In Vitro and In Vivo Studies. Biocon. Chem. 2016, 27, 2874–2885. [Google Scholar] [CrossRef]
- Li, M.; Lam, J.W.; Mahtab, F.; Chen, S.; Zhang, W.; Hong, Y.; Xiong, J.; Zheng, Q.; Tang, B. Biotin-Decorated Fluorescent Silica Nanoparticles with Aggregation-Induced Emission Characteristics: Fabrication, Cytotoxicity and Biological Applications. J. Mat. Chem. B 2012, 1, 676–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gole, A.; Murphy, C.J. Biotin-Streptavidin-Induced Aggregation of Gold Nanorods: Tuning Rod-Rod Orientation. Langmuir 2005, 21, 10756–10762. [Google Scholar] [CrossRef]
- Caswell, K.; Wilson, J.N.; Bunz, U.H.; Murphy, C.J. Preferential End-to-End Assembly of Gold Nanorods by Biotin−Streptavidin Connectors. J. Am. Chem. Soc. 2003, 125, 13914–13915. [Google Scholar] [CrossRef]
- Lala, N.; Chittiboyina, A.G.; Chavan, S.P.; Sastry, M. Biotinylation of Colloidal Gold Particles Using Interdigitated Bilayers: A UV–Visible Spectroscopy and TEM Study of the Biotin–Avidin Molecular Recognition Process. Coll. Surf. A 2002, 205, 15–20. [Google Scholar] [CrossRef]
- Connolly, S.; Cobbe, S.; Fitzmaurice, D. Effects of Ligand−Receptor Geometry and Stoichiometry on Protein-Induced Aggregation of Biotin-Modified Colloidal Gold. J. Phys. Chem. B 2001, 105, 2222–2226. [Google Scholar] [CrossRef]
- Aslan, K.; Luhrs, C.C.; Pérez-Luna, V.H. Controlled and Reversible Aggregation of Biotinylated Gold Nanoparticles with Streptavidin. J. Phys. Chem. B 2004, 108, 15631–15639. [Google Scholar] [CrossRef]
- Raal, A.; Meos, A.; Hinrikus, T.; Heinämäki, J.; Romāne, E.; Gudienė, V.; Tas, V.J.; Koshovyi, O.; Kovaleva, A.; Fursenco, C.; et al. Dragendorff’s Reagent: Historical Perspectives and Current Status of a Versatile Reagent Introduced over 150 Years Ago at the University of Dorpat, Tartu, Estonia. Die Pharm. 2020, 75, 299–306. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S.; Ferretti, A.; Puglisi, A.; Nanoscale, P.-A. PEBBLES and PEBBLEJUGGLER: Software for Accurate, Unbiased, and Fast Measurement and Analysis of Nanoparticle Morphology from Transmission electron microscopy (TEM) micrographs. Nanoscale 2012, 4, 5356–5372. [Google Scholar] [CrossRef] [PubMed]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMs that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Guarino, G.; Rastrelli, F.; Scrimin, P.; Mancin, F. Lanthanide-based NMR: A tool to investigate component distribution in mixed-monolayer-protected nanoparticles. J. Am. Chem. Soc. 2012, 134, 7200–7203. [Google Scholar] [CrossRef]
- Goswami, L.N.; Houston, Z.H.; Sarma, S.J.; Jalisatgi, S.S.; Hawthorne, M.F. Efficient synthesis of diverse heterobifunctionalized clickable oligo (ethylene glycol) linkers: Potential applications in bioconjugation and targeted drug delivery. Org. Biomol. Chem. 2013, 11, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-Coated “Stealth” Nanoparticles for Biomedical Applications: Recent Advances in Countering Biomolecular Corona Formation and Uptake by the Mononuclear Phagocyte System. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.-S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and Biofunctionalizable Gold Nanoparticles. ACS Appl. Mater. Inter. 2016, 8, 14096–14101. [Google Scholar] [CrossRef] [Green Version]
- Agasti, S.S.; You, C.-C.; Arumugam, P.; Rotello, V.M. Structural Control of the Monolayer Stability of Water -Soluble Gold Nanoparticles. J. Mater. Chem. 2007, 18, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Pecina, A.; Rosa-Gastaldo, D.; Riccardi, L.; Franco-Ulloa, S.; Milan, E.; Scrimin, P.; Mancin, F.; De Vivo, M. On the Metal-Aided Catalytic Mechanism for Phosphodiester Bond Cleavage Performed by Nanozymes ACS Catalysis. 2021; in press. [Google Scholar]
- Xia, H.; Bai, S.; Hartmann, J.; Wang, D. Synthesis of monodisperse quasi-spherical gold nanoparticles in water via silver (I)-assisted citrate reduction. Langmuir 2010, 26, 3585–3589. [Google Scholar] [CrossRef] [PubMed]
- Martínez, Á.; Lyu, Y.; Mancin, F.; Scrimin, P. Glucosamine Phosphate Induces AuNPs Aggregation and Fusion into Easily Functionalizable Nanowires. Nanomaterials 2019, 9, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef] [PubMed]
- Gilles, M.; Brun, E.; Sicard-roselli, C. Gold nanoparticles functionalization notably decreases radiosensitization through hydroxyl radical production under ionizing radiation. Coll. Surf. B Biointerfaces 2014, 123, 770–777. [Google Scholar] [CrossRef]
- Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying Thiol Ligand Density of Self-Assembled Monolayers on Gold Nanoparticles by Inductively Coupled Plasma–Mass Spectrometry. ACS Nano 2013, 7, 1129–1136. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Wingate, J.E.; Zhong, C.-J.; Harris, J.E.; Vachet, R.W.; Clark, M.R.; Londono, D.J.; Green, S.J.; Stokes, J.J.; Wignall, G.D.; et al. Alkanethiolate Gold Cluster Molecules with Core Diameters from 1.5 to 5.2 Nm: Core and Monolayer Properties as a Function of Core Size. Langmuir 1998, 14, 17–30. [Google Scholar] [CrossRef]
- Repo, S.; Paldanius, T.A.; Hytönen, V.P.; Nyholm, T.K.M.; Halling, K.K.; Huuskonen, J.; Pentikäinen, O.T.; Rissanen, K.; Slotte, J.P.; Airenne, T.T.; et al. Binding Properties of HABA-Type Azo Derivatives to Avidin and Avidin-Related Protein 4. Chem. Biol. 2006, 13, 1029–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raikar, U.S.; Tangod, V.B.; Mastiholi, B.M.; Fulari, V.J. Fluorescence Quenching Using Plasmonic Gold Nanoparticles. Opt. Commun. 2011, 284, 4761–4765. [Google Scholar] [CrossRef]
- Pugliese, L.; Coda, A.; Malcovati, M.; Bolognesi, M. Three-dimensional structure of the tetragonal crystal form of egg-white avidin in its functional complex with biotin at 2.7 Å resolution. J. Mol. Biol. 1993, 231, 698–710. [Google Scholar]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction Coefficient of Gold Nanoparticles with Different Sizes and Different Capping Ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef]
- Omichi, M.; Asano, A.; Tsukuda, S.; Takano, K.; Sugimoto, M.; Saeki, A.; Sakamaki, D.; Onoda, A.; Hayashi, T.; Seki, S. Fabrication of Enzyme-Degradable and Size-Controlled Protein Nanowires Using Single Particle Nano-Fabrication Technique. Nat. Commun. 2014, 5, 3718. [Google Scholar] [CrossRef] [Green Version]
- Piserchia, A.; Zerbetto, M.; Salvia, M.-V.; Salassa, G.; Gabrielli, L.; Mancin, F.; Rastrelli, F.; Frezzato, D. Conformational Mobility in Monolayer-Protected Nanoparticles: From Torsional Free Energy Profiles to NMR Relaxation. J. Phys. Chem. C 2015, 119, 20100–20110. [Google Scholar] [CrossRef]
- Cui, M.; Liu, R.; Deng, Z.; Ge, G.; Liu, Y.; Xie, L. Quantitative Study of Protein Coronas on Gold Nanoparticles with Different Surface Modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Yokoyama, K.; Brown, K.; Shevlin, P.; Jenkins, J.; D’Ambrosio, E.; Ralbovsky, N.; Battaglia, J.; Deshmukh, I.; Ichiki, A. Examination of Adsorption Orientation of Amyloidogenic Peptides Over Nano-Gold Colloidal Particle Surfaces. Int. J. Mol. Sci. 2019, 20, 5354. [Google Scholar] [CrossRef] [Green Version]
- Zon, V.B.; Sachsenhauser, M.; Rant, U. Preparation of Gold Nanoparticle Dimers via Streptavidin-Induced Interlinking. J. Nanopart. Res. 2013, 15, 1974. [Google Scholar] [CrossRef]
- Li, M.; Wong, K.K.; Mann, S. Organization of Inorganic Nanoparticles Using Biotin−Streptavidin Connectors. Chem. Mat. 1999, 11, 23–26. [Google Scholar] [CrossRef]
- Connolly, S.; Fitzmaurice, D. Programmed Assembly of Gold Nanocrystals in Aqueous Solution. Adv. Mat. 1999, 11, 1202–1205. [Google Scholar] [CrossRef]
- Das, K.; Gabrielli, L.; Prins, L.J. Chemically-fueled Self-assembly in Biology and Chemistry. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]


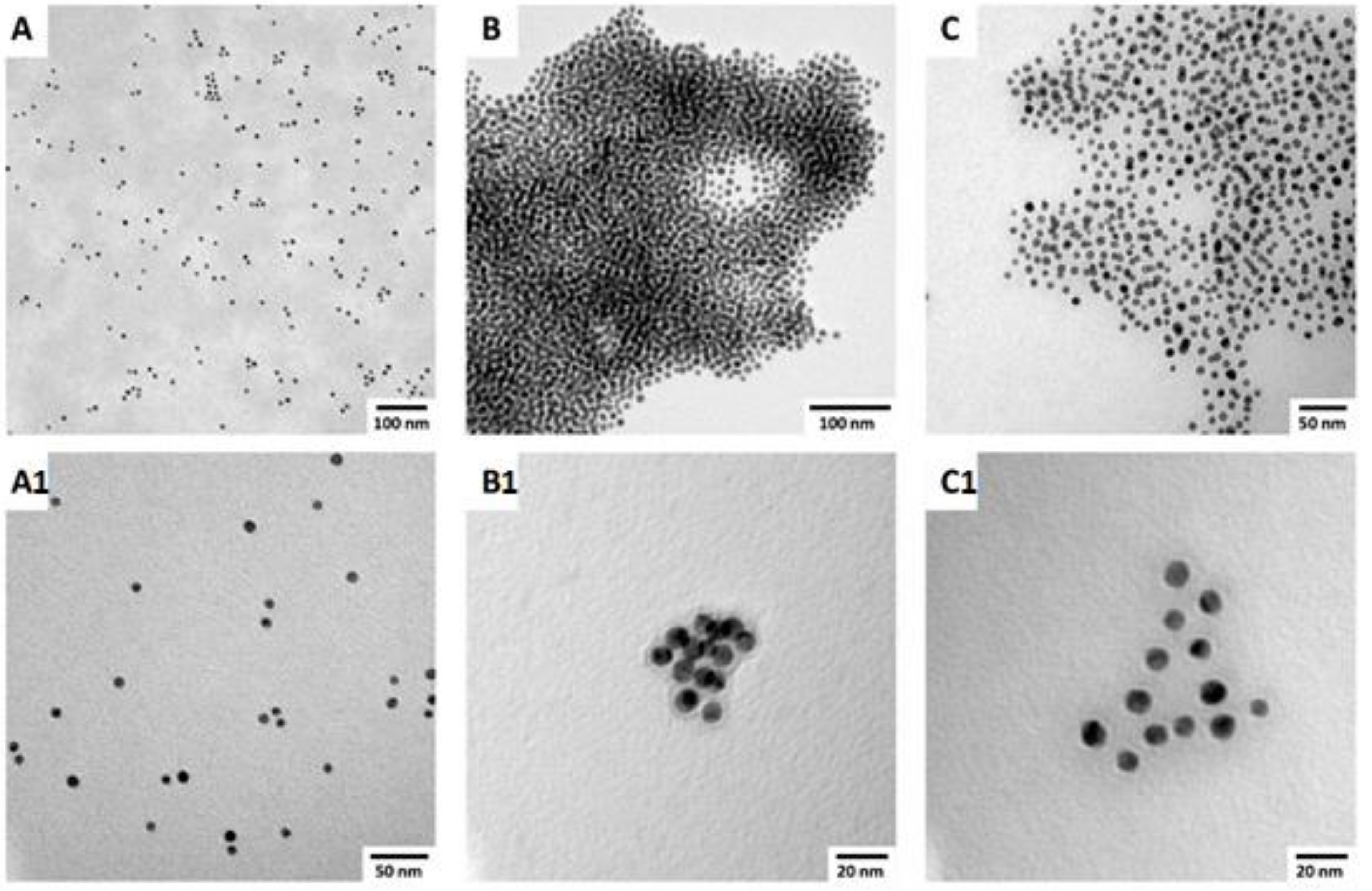
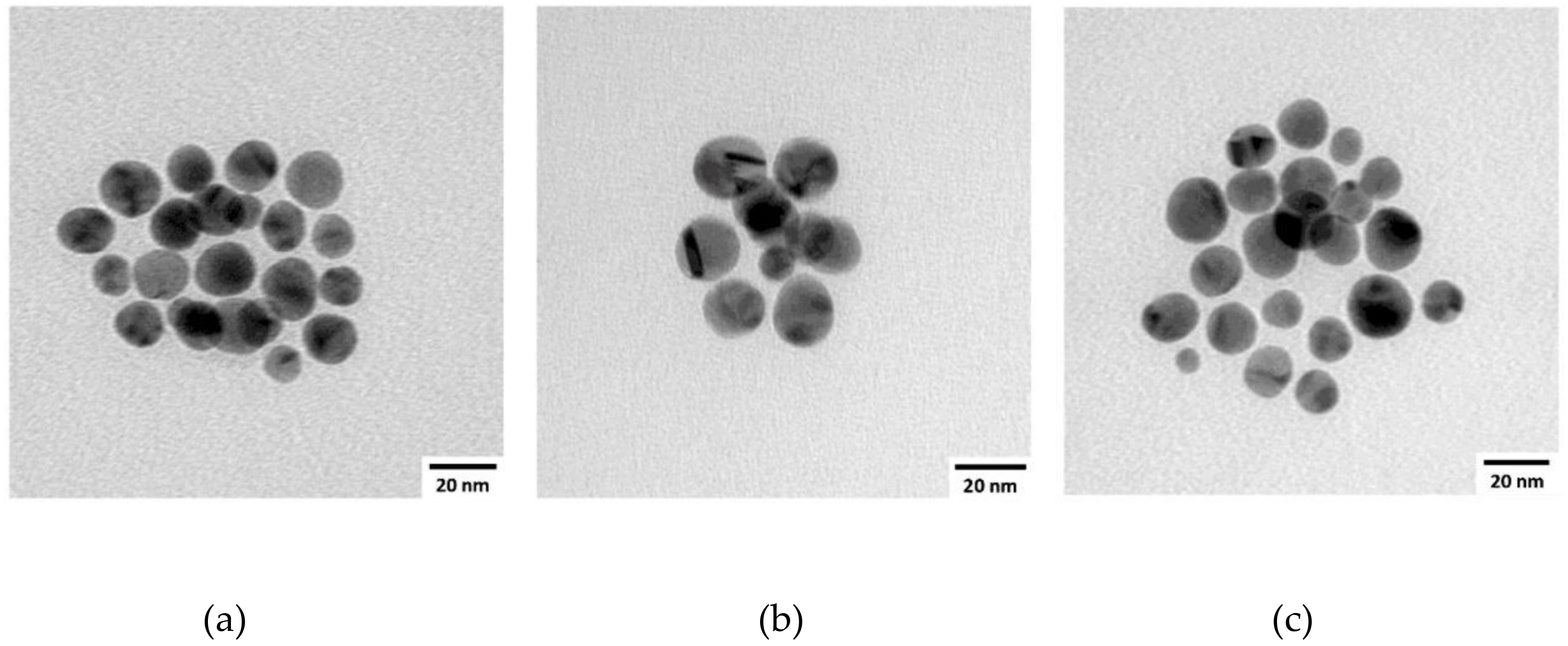
| Sample | AuNP Diameter a (nm) | % Biotin Thiol in Monolayer b,c | Estimated Average AuNP Formula |
|---|---|---|---|
| AuNP1 | 11.7 ± 1.7 | 11.5% | Au49k(SR)2150 |
| AuNP2 | 8.9 ± 0.8 | 13.5% | Au21k(SR)1244 |
| AuNP3 | 18.6 ± 3.1 | 13.8% | Au200k(SR)5434 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Y.; Martínez, Á.; D’Incà, F.; Mancin, F.; Scrimin, P. The Biotin–Avidin Interaction in Biotinylated Gold Nanoparticles and the Modulation of Their Aggregation. Nanomaterials 2021, 11, 1559. https://doi.org/10.3390/nano11061559
Lyu Y, Martínez Á, D’Incà F, Mancin F, Scrimin P. The Biotin–Avidin Interaction in Biotinylated Gold Nanoparticles and the Modulation of Their Aggregation. Nanomaterials. 2021; 11(6):1559. https://doi.org/10.3390/nano11061559
Chicago/Turabian StyleLyu, Yanchao, Álvaro Martínez, Federica D’Incà, Fabrizio Mancin, and Paolo Scrimin. 2021. "The Biotin–Avidin Interaction in Biotinylated Gold Nanoparticles and the Modulation of Their Aggregation" Nanomaterials 11, no. 6: 1559. https://doi.org/10.3390/nano11061559
APA StyleLyu, Y., Martínez, Á., D’Incà, F., Mancin, F., & Scrimin, P. (2021). The Biotin–Avidin Interaction in Biotinylated Gold Nanoparticles and the Modulation of Their Aggregation. Nanomaterials, 11(6), 1559. https://doi.org/10.3390/nano11061559







