Robust LSPR Sensing Using Thermally Embedded Au Nanoparticles in Glass Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Au NPs Deposition and Thermal Annnealing above Tg
2.2. Au NPs’ Dissolution
2.3. Adhesion and Stability Tests
2.4. Determination of the Refractive Index Sensitivity (RIS)
2.5. Structure and Surface Morphology
3. Results and Discussion
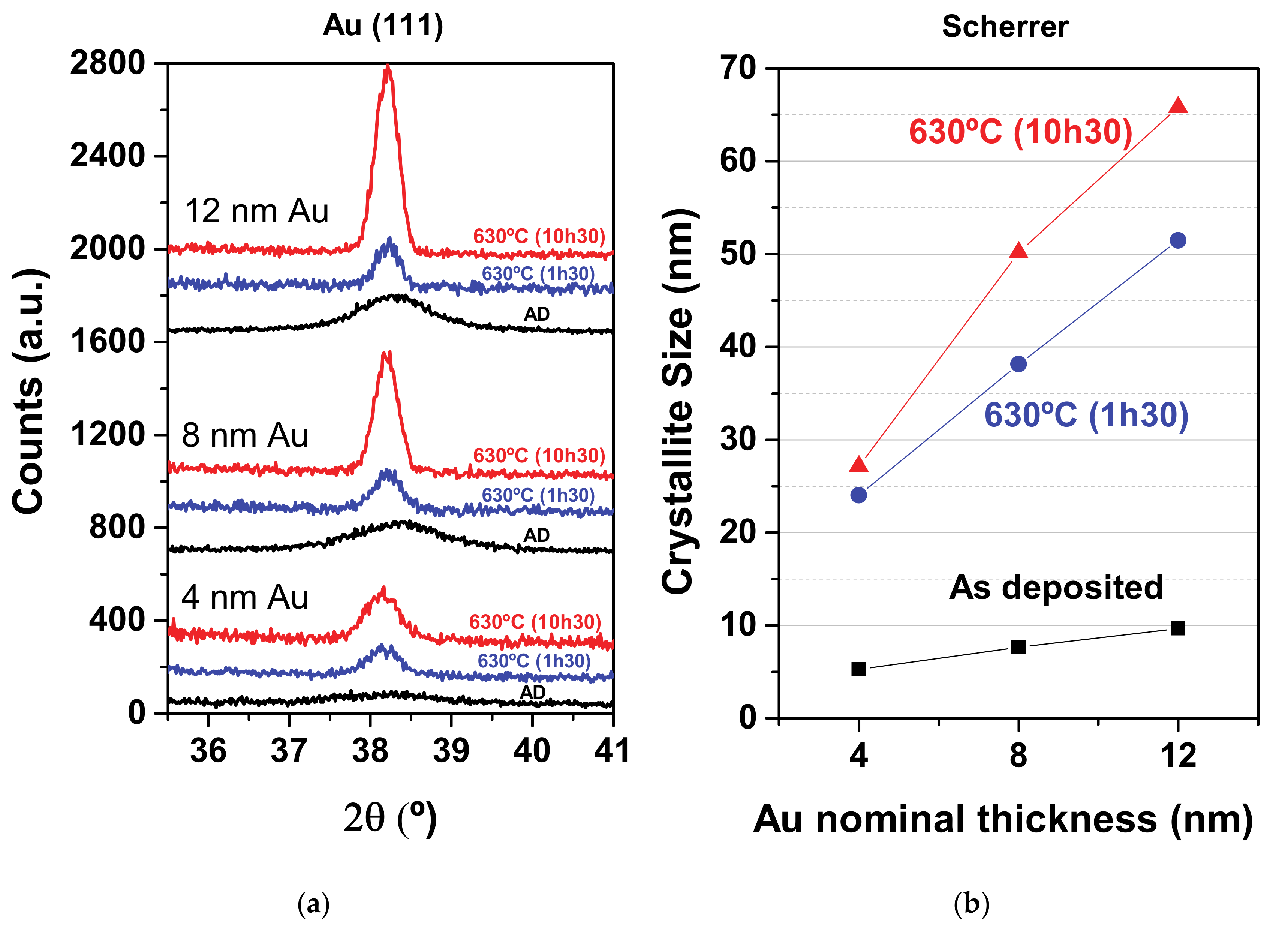
3.1. Structure and Crystallite Size
3.2. Surface Morphology
3.3. Reflectivity
3.4. Refractive Index Sensitivity (RIS)
3.5. Structural Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hvolbaek, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Norskov, J.K. Catalytic activity of Au nanoparticles. Nanotoday 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Figueiredo, N.M.; Cavaleiro, A. Dielectric Properties of Shape-Distributed Ellipsoidal Particle Systems. Plasmonics 2020, 15, 379–397. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, N.M.; Serra, R.; Manninen, N.K.; Cavaleiro, A. Production of Au clusters by plasma gas condensation and their incorporation in oxide matrixes by sputtering. Appl. Surf. Sci. 2018, 15, 144–152. [Google Scholar] [CrossRef]
- Font, F.; Myers, T.G. Spherically symmetric nanoparticle melting with a variable phase change temperature. J. Nanopart. Res. 2013, 15, 2086. [Google Scholar] [CrossRef]
- Lu, H.M.; Li, P.Y.; Cao, Z.H.; Meng, X.K. Size-, Shape-, and Dimensionality-Dependent Melting Temperatures of Nanocrystals. J. Phys. Chem. C 2009, 113, 7598–7602. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef] [Green Version]
- Cox, A.J.; Louderback, J.G.; Bloomfield, L.A. Experimental observation of magnetism in rhodium clusters. Phys. Rev. Lett. 1993, 71, 923. [Google Scholar] [CrossRef]
- Louis, C.; Pluchery, O. Gold Nanoparticles for Physics, Chemistry and Biology; Imperial College Press: London, UK, 2012. [Google Scholar]
- Figueiredo, N.M.; Louro, C.; Escobar-Galindo, R.; Climent-Font, A.; Cavaleiro, A. Structural and mechanical properties of Au alloyed Al-O sputter deposited coatings. Surf. Coat. Technol. 2012, 206, 2740–2745. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kimura, K. Instability of gold nano-islands in several organic media. Nanostruct. Mater. 1997, 9, 555–558. [Google Scholar] [CrossRef]
- Hashimoto, N.; Hashimoto, T.; Teranishib, T.; Nasu, H.; Kamiya, K. Cycle performance of sol–gel optical sensor based on localized surface plasmon resonance of silver particles. Sens. Actuators B 2006, 113, 382–388. [Google Scholar] [CrossRef]
- Gubicza, J.; Lábár, J.L.; Quynh, L.M.; Nam, N.H.; Luong, N.H. Evolution of size and shape of gold nanoparticles during long-time aging. Mater. Chem. Phys. 2013, 138, 449–453. [Google Scholar] [CrossRef]
- Henriquez, R.; Bravo, S.; Roco, R.; Del Campo, V.; Kroeger, D.; Haberle, P. Electrical Percolation and Aging of Gold Films. Metall. Mater. Trans. A 2019, 50A, 493–503. [Google Scholar] [CrossRef]
- Albrecht, G.; Kaiser, S.; Giessen, H.; Hentschel, M. Refractory Plasmonics without Refractory Materials. Nano Lett. 2017, 17, 6402–6408. [Google Scholar] [CrossRef]
- Chaikin, Y.; Kedem, O.; Raz, J.; Vaskevich, A.; Rubinstein, I. Stabilization of Metal Nanoparticle Films on Glass Surfaces Using Ultrathin Silica Coating. Anal. Chem. 2013, 85, 10022–10027. [Google Scholar] [CrossRef]
- Figueiredo, N.M.; Kubart, T.; Sanchez-García, J.A.; Escobar-Galindo, R.; Climent-Font, A.; Cavaleiro, A. Optical properties and refractive index sensitivity of reactive sputtered oxide coatings with embedded Au clusters. J. Appl. Phys. 2014, 115, 063512. [Google Scholar] [CrossRef] [Green Version]
- Aouani, H.; Wenger, J.; Gérard, D.; Rigneault, H.; Devaux, E.; Ebbesen, T.W.; Mahdavi, F.; Xu, T.; Blair, S. Crucial Role of the Adhesion Layer on the Plasmonic Fluorescence Enhancement. ACS Nano 2009, 7, 2043–2048. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Lieberman, S.H. Comparison of Three Methods to Improve Adherence of Thin Gold Films to Glass Substrates and Their Effect on the SERS Response. Appl. Spectrosc. 1999, 53, 862–873. [Google Scholar] [CrossRef]
- Todeschini, M.; Fanta, A.B.S.; Jensen, F.; Wagner, J.B.; Han, A. Influence of Ti and Cr Adhesion Layers on Ultrathin Au Films. ACS Appl. Mater. Interfaces 2017, 9, 37374–37385. [Google Scholar] [CrossRef] [Green Version]
- Bendikov, T.A.; Rabinkov, A.; Karakouz, T.; Vaskevich, A.; Rubinstein, I. Biological Sensing and Interface Design in Gold Island Film Based Localized Plasmon Transducers. Anal. Chem. 2008, 80, 7487–7498. [Google Scholar] [CrossRef]
- Karakouz, T.; Holder, D.; Goomanovsky, M.; Vaskevich, A.; Rubinstein, I. Morphology and Refractive Index Sensitivity of Gold Island Films. Chem. Mater. 2009, 21, 5875–5885. [Google Scholar] [CrossRef]
- Karakouz, T.; Maoz, B.M.; Lando, G.; Vaskevich, A.; Rubinstein, I. Stabilization of Gold Nanoparticle Films on Glass by Thermal Embedding. ACS Appl. Mater. Interfaces 2011, 3, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Karakouz, T.; Tesler, A.B.; Bendikov, T.A.; Lando, G.; Vaskevich, A.; Rubinstein, I. Highly Stable Localized Plasmon Transducers Obtained by Thermal Embedding of Gold Island Films on Glass. Adv. Mater. 2008, 20, 3893–3899. [Google Scholar] [CrossRef]
- Karakouz, T.; Tesler, A.B.; Sannomiya, T.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Mechanism of morphology transformation during annealing of nanostructured gold films on glass. Phys. Chem. Chem. Phys. 2013, 15, 4656–4665. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.J.; Vincett, P.S. Subsurface particle monolayer and film formation in softenable substrates: Techniques and thermodynamic criteria. Thin Solid Films 1984, 111, 65–81. [Google Scholar] [CrossRef]
- Bonde, H.C.; Fojan, P.; Popok, V.N. Controllable embedding of size-selected copper nanoparticles into polymer films. Plasma Process. Polym. 2020, 17, e1900237. [Google Scholar] [CrossRef]
- Ida, T.; Ando, M.; Toraya, H. Extended pseudo-Voigt function for approximating the Voigt profile. J. Appl. Cryst. 2000, 33, 1311–1316. [Google Scholar] [CrossRef]
- Birkholz, M. Thin Film Analysis by X-ray Scattering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Available online: http://gwyddion.net/ (accessed on 1 January 2021).
- Available online: https://www.wolfram.com/mathematica/ (accessed on 1 March 2020).
- Kosinova, A.; Kovalenko, O.; Klinger, L.; Rabkin, E. Mechanisms of solid-state dewetting of thin Au films in different annealing atmospheres. Acta Mater. 2015, 83, 91–101. [Google Scholar] [CrossRef]
- Müller, C.M.; Spolenak, R. Dewetting of Au and AuPt alloy films: A dewetting zone model. J. Appl. Phys. 2013, 113, 094301. [Google Scholar] [CrossRef]
- Tesler, A.B.; Maoz, B.M.; Feldman, Y.; Vaskevich, A.; Rubinstein, I. Solid-State Thermal Dewetting of Just-Percolated Gold Films Evaporated on Glass: Development of the Morphology and Optical Properties. J. Phys. Chem. C 2013, 117, 11337–11346. [Google Scholar] [CrossRef]
- Moharrami, N.; Bull, S.J. A Comparison of Nanoindentation Pile-up in Bulk Materials and Thin Films. Thin Solid Films 2014, 572, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Canet-Ferrer, J.; Coronado, E.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E. Correction of the tip convolution effects in the imaging of nanostructures studied through scanning force microscopy. Nanotechnology 2014, 25, 395703. [Google Scholar] [CrossRef]
- Jang, H.K.; Chung, Y.D.; Whangbo, S.W.; Lyo, I.W.; Whang, C.N. Effects of chemical etching with hydrochloric acid on a glass surface. J. Vac. Sci. Technol. A 2000, 18, 2563–2567. [Google Scholar] [CrossRef]
- Jang, H.K.; Chung, Y.D.; Whangbo, S.W.; Kim, T.G.; Whang, C.N. Effects of chemical etching with nitric acid on glass surfaces. J. Vac. Sci. Technol. A 2001, 19, 267–274. [Google Scholar] [CrossRef]
- Axelevitch, A.; Apter, B.; Golan, G. Simulation and experimental investigation of optical transparency in gold island films. Opt. Express 2013, 21, 4126–4138. [Google Scholar] [CrossRef]
- Dmitruk, N.L.; Korovin, A.V. Physical Nature of Anomalous Optical Transmission of Thin Absorptive Corrugated Films. JETP Lett. 2009, 89, 68–72. [Google Scholar] [CrossRef]
- Schwartzkopf, M.; Santoro, G.; Brett, C.J.; Rothkirch, A.; Polonskyi, O.; Hinz, A.; Metwalli, E.; Yao, Y.; Strunskus, T.; Faupel, F.; et al. Real-Time Monitoring of Morphology and Optical Properties during Sputter Deposition for Tailoring Metal−Polymer Interfaces. ACS Appl. Mater. Interfaces 2015, 7, 13547–13556. [Google Scholar] [CrossRef] [Green Version]
- Marks, R.S.; Cullen, D.C.; Karube, I.; Lowe, C.R.; Weetall, H.H. Handbook of Biosensors and Biochips; Volume 1, Wiley: Chichester, UK, 2008. [Google Scholar]
- Garoli, D.; Calandrini, E.; Giovannini, G.; Hubarevich, A.; Caligiuri, V.; De Angelis, F. Nanoporous gold metamaterials for high sensitivity plasmonic sensing. Nanoscale Horiz. 2019, 4, 1153–1157. [Google Scholar] [CrossRef]
- Meli, M.-V.; Lennox, R.B. Surface Plasmon Resonance of Gold Nanoparticle Arrays Partially Embedded in Quartz Substrates. J. Phys. Chem. C 2007, 111, 3658–3664. [Google Scholar] [CrossRef]





| Sample | Au 4 nm | Au 8 nm | Au 12 nm | |||
|---|---|---|---|---|---|---|
| Annealing Time | 1 h 30 | 10 h 30 | 1 h 30 | 10 h 30 | 1 h 30 | 10 h 30 |
| NP height (nm) | 11.5 ± 3.4 | 12.6 ± 5.9 | 12.1 ± 16.2 | 11.8 ± 13.4 | 12.1 ± 9.8 | 11.6 ± 12.7 |
| Indentation depth (nm) | 13.7 ± 7.0 | 18.8 ± 12.2 | 37.5 ± 19.8 | 55.9 ± 29.5 | 58.8 ± 37.2 | 80.9 ± 56.4 |
| Total NP height (nm) | 25.2 ± 7.8 | 31.4 ± 13.5 | 49.6 ± 25.6 | 67.7 ± 32.4 | 70.9 ± 38.5 | 92.4 ± 57.8 |
| % NP embedding | 54 | 60 | 76 | 83 | 83 | 88 |
| NP minor axis (nm) | 38.0 ± 15.7 | 51.5 ± 21.4 | 67.6 ± 72.3 | 58.8 ± 66.0 | 36.8 ± 58.2 | 61.7 ± 66.2 |
| NP Major axis (nm) | 49.7 ± 28.8 | 70.9 ± 31.7 | 100.3 ± 109.4 | 79.7 ± 93.0 | 48.4 ± 83.4 | 75.5 ± 73.2 |
| AR1 (top view) 1 | 1.3 ± 0.3 | 1.4 ± 0.3 | 1.5 ± 0.4 | 1.4 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.2 |
| AR2 (cross section view) 2 | 0.7 ± 0.3 | 0.6 ± 0.4 | 0.7 ± 0.9 | 1.2 ± 1.4 | 1.9 ± 3.2 | 1.5 ± 1.9 |
| Indentation minor axis (nm) | 30.7 ± 10.5 | 34.6 ± 10.5 | 98.8 ± 81.1 | 94.5 ± 58.1 | 96.3 ± 62.6 | 115.4 ± 85.9 |
| Indentation Major axis (nm) | 42.8 ± 19.2 | 45.5 ± 13.8 | 149.1 ±134.5 | 154.9 ± 100.3 | 175.9 ± 123.0 | 181.2 ± 141.8 |
| AR1 (top view) 1 | 1.4 ± 0.3 | 1.3 ± 0.2 | 1.6 ± 0.4 | 1.6 ± 0.3 | 1.9 ± 1.2 | 1.6 ± 0.4 |
| AR2 (cross section view) 2 | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.5 ± 0.5 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0.8 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, N.M.; Serra, R.; Cavaleiro, A. Robust LSPR Sensing Using Thermally Embedded Au Nanoparticles in Glass Substrates. Nanomaterials 2021, 11, 1592. https://doi.org/10.3390/nano11061592
Figueiredo NM, Serra R, Cavaleiro A. Robust LSPR Sensing Using Thermally Embedded Au Nanoparticles in Glass Substrates. Nanomaterials. 2021; 11(6):1592. https://doi.org/10.3390/nano11061592
Chicago/Turabian StyleFigueiredo, Nuno M., Ricardo Serra, and Albano Cavaleiro. 2021. "Robust LSPR Sensing Using Thermally Embedded Au Nanoparticles in Glass Substrates" Nanomaterials 11, no. 6: 1592. https://doi.org/10.3390/nano11061592







