Resolving Site-Specific Energy Levels of Small-Molecule Donor-Acceptor Heterostructures Close to Metal Contacts
Abstract
:1. Introduction
2. Methods and Experimental Results
2.1. Experimental Methods
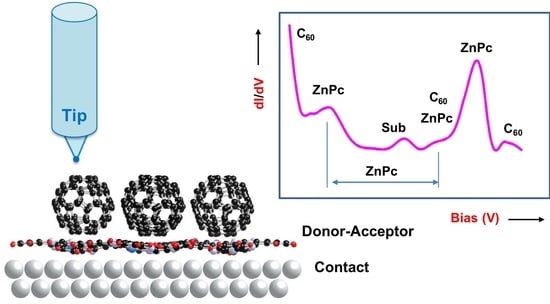
2.2. Experimental Results
3. Model Calculation and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Kippelen, B.; Bredas, J.L. Organic photovoltaics. Energy Environ. Sci. 2009, 2, 251–261. [Google Scholar] [CrossRef]
- Ostroverkhova, O. (Ed.) Handbook of Organic Materials for Optical and (Opto)Electronic Devices; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Qiao, Q. (Ed.) Organic Solar Cells: Materials, Devices, Interfaces, and Modeling; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Wang, R.; Yuan, J.; Wang, R.; Han, G.; Huang, T.; Huang, W.; Xue, J.; Wang, H.; Zhang, C.; Zhu, C.; et al. Rational Tuning of Molecular Interaction and Energy Level Alignment Enables High-Performance Organic Photovoltaics. Adv. Mater. 2019, 31, 1904215. [Google Scholar] [CrossRef]
- Sun, J.W.; Lee, J.-H.; Moon, C.-K.; Kim, K.-H.; Shin, H.; Kim, J.J. A Fluorescent Organic Light-Emitting Diode with 30% External Quantum Efficiency. Adv. Mater. 2014, 26, 5684–5688. [Google Scholar] [CrossRef]
- Tan, J.K.; Png, R.Q.; Zhao, C.; Ho, P.K.H. Ohmic transition at contacts key to maximizing fill factor and performance of organic solar cells. Nat. Comm. 2018, 9, 3269. [Google Scholar] [CrossRef]
- D’Avino, G.; Muccioli, L.; Castet, F.; Poelking, C.; Andrienko, D.; Soos, Z.G.; Cornil, J.; Beljonne, D. Electrostatic phenomena in organic semiconductors: Fundamentals and implications for photovoltaics. J. Phys. Condens. Matter 2016, 28, 433002. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Zacher, B.; Armstrong, N.R. Selective Interlayers and Contacts in Organic Photovoltaic Cells. J. Phys. Chem. Lett. 2011, 2, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.-H.; Tsang, S.W.; Manders, J.R.; Chen, S.; So, F. Properties of interlayer for organic photovoltaics. Mater. Today 2013, 16, 424–432. [Google Scholar] [CrossRef]
- Fleetham, T.B.; Mudrick, J.; Cao, W.; Klimes, K.; Xue, J.; Li, J. Efficient Zinc Phthalocyanine/C60 Heterojunction Photovoltaic Devices Employing Tetracene Anode Interfacial Layers. ACS Appl. Mater. Interfaces 2014, 6, 7254–7259. [Google Scholar] [CrossRef] [PubMed]
- Schubert, S.; Hermenau, M.; Meiss, J.; Müller-Meskamp, L.; Leo, K. Oxide Sandwiched Metal Thin-Film Electrodes for Long-Term Stable Organic Solar Cells. Adv. Funct. Mater. 2012, 22, 4993–4999. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Connor, S.T.; Cui, Y.; Peumans, P. Solution-Processed Metal Nanowire Mesh Transparent Electrodes. Nano Lett. 2008, 8, 689–692. [Google Scholar] [CrossRef]
- Chiba, T.; Kumagai, D.; Udagawa, K.; Watanabe, Y.; Kido, J. Dual mode OPV-OLED device with photovoltaic and light-emitting functionalities. Sci. Rep. 2018, 8, 11472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Ye, L.; Hou, J. Breaking the 10% Efficiency Barrier in Organic Photovoltaics: Morphology and Device Optimization of Well-Known PBDTTT Polymers. Adv. Energy Mater. 2016, 6, 1502529. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganäs, O.; Gao, F.; Hou, J. Fullerene-Free Polymer Solar Cells with over 11% Efficiency and Excellent Thermal Stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.G.; Feng, J.; Ji, J.-H.; Yi, F.-S.; Li, Y.-F.; Liu, Y.-F.; Zhang, X.-L.; Sun, H.-B. Nanostructures induced light harvesting enhancement in organic photovoltaics. Nanophotonics 2018, 7, 371–391. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Jeong, J.G.; Kim, H.-J.; Park, S.-H.; Cho, M.-H.; Cho, S.W.; Yi, Y.; Heo, M.Y.; Sohn, H. The electronic structure of C60/ZnPc interface for organic photovoltaic device with blended layer architecture. Appl. Phys. Lett. 2010, 96, 013302. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Yong, K.S.; Kam, Z.M.; Zhu, F.; Li, Y. Effect of blend layer morphology on performance of ZnPc:C60-based photovoltaic cells. Appl. Phys. Lett. 2010, 97, 133304. [Google Scholar] [CrossRef]
- Tietze, M.L.; Tress, W.; Pfutzner, S.; Schunemann, C.; Burtone, L.; Riede, M.; Leo, K. Correlation of open-circuit voltage and energy levels in zinc-phthalocyanine: C60 bulk heterojunction solar cells with varied mixing ratio. Phys. Rev. B Condens. Matter Mater. Phys. 2013, 88, 085119. [Google Scholar] [CrossRef]
- Stöhr, M.; Wagner, T.; Gabriel, M.; Weyers, B.; Möller, R. Binary Molecular Layers of C60 and Copper Phthalocyanine on Au(111): Self-Organized Nanostructuring. Adv. Funct. Mater. 2001, 11, 175. [Google Scholar] [CrossRef]
- Samuely, T.; Liu, S.-X.; Haas, M.; Decurtins, S.; Jung, T.A.; Stöhr, M. Self-Assembly of Individually Addressable Complexes of C60 and Phthalocyanines on a Metal Surface: Structural and Electronic Investigations. J. Phys. Chem. C 2009, 113, 19373–19375. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Liu, Q.; Dougherty, D.B.; Cullen, W.G.; Reutt-Robey, J.E.; Weeks, J.; Robey, S.W. C60 chain phases on ZnPc/Ag(111) surfaces: Supramolecular organization driven by competing interactions. J. Chem. Phys. 2015, 142, 101910. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Zhou, K.; Ma, W. Control of the molecular orientation in small molecule-based organic photovoltaics. Sustain. Energy Fuels 2020, 4, 4934–4955. [Google Scholar] [CrossRef]
- Onoe, J.; Watanabe, S.; Kato, S.; Nakaya, M.; Bucher, J.P. Spectroscopic and theoretical studies on the structural, electronic, and optical properties of zinc octaethylporphyrin/C60 co-deposited films. J. Chem. Phys. 2017, 147, 214701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Miyadera, T.; Yamanari, T.; Yoshida, Y. Templating Effects in Molecular Growth of Blended Films for Efficient Small-Molecule Photovoltaics. ACS Appl. Mater. Interfaces 2014, 6, 6369–6377. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Vázquez de Parga, A.L.; Gallego, J.M. Electronic, structural and chemical effects of charge-transfer at organic/inorganic interfaces. Surf. Sci. Rep. 2017, 72, 105–145. [Google Scholar] [CrossRef]
- Hesper, R.; Tjeng, L.H.; Sawatzky, G.A. Strongly reduced band gap in a correlated insulator in close proximity to a metal. Europhys. Lett. 1997, 40, 177. [Google Scholar] [CrossRef]
- Schwedhelm, R.; Kipp, L.; Dallmeyer, A.; Skibowski, M. Experimental band gap and core-hole electron interaction in epitaxial C60 films. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 58, 13176. [Google Scholar] [CrossRef]
- Lu, X.; Grobis, M.; Khoo, K.H.; Louie, S.G.; Crommie, M.F. Spatially Mapping the Spectral Density of a Single C60 Molecule. Phys. Rev. Lett. 2003, 90, 096802. [Google Scholar] [CrossRef]
- Lu, X.; Grobis, M.; Khoo, K.H.; Louie, S.G.; Crommie, M.F. Charge transfer and screening in individual C60 molecules on metal substrates: A scanning tunneling spectroscopy and theoretical study. Phys. Rev. B Condens. Matter Mater. Phys. 2004, 70, 115418. [Google Scholar] [CrossRef]
- Fernández Torrente, I.; Franke, K.J.; Pascual, J.I. Spectroscopy of C60 single molecules: The role of screening on energy level alignment. J. Phys. Condens. Matter 2008, 20, 184001. [Google Scholar] [CrossRef] [Green Version]
- Schulze, G.; Franke, K.J.; Pascual, J.I. Resonant heating and substrate-mediated cooling of a single C60 molecule in a tunnel junction. New J. Phys. 2008, 10, 065005. [Google Scholar] [CrossRef]
- Tuerhong, R.; Ngassam, F.; Watanabe, S.; Onoe, J.; Alouani, M.; Bucher, J.P. Two-Dimensional Organometallic Kondo Lattice with Long-Range Antiferromagnetic Order. J. Phys. Chem. C 2018, 122, 20046–20054. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Brumme, T.; Kröger, J.; Toher, C.; Cuniberti, G.; Berndt, R. Coverage-Driven Electronic Decoupling of Fe-Phthalocyanine from a Ag(111) Substrate. J. Phys. Chem. C 2011, 115, 12173–12179. [Google Scholar] [CrossRef]
- Dolbin, A.V.; Esel’son, V.B.; Gavrilko, V.G.; Manzhelii, V.G.; Vinnikov, N.A.; Basnukaeva, R.M. The effect of glass transition in fullerite C60 on Ar impurity diffusion. Low Temp. Phys. 2013, 39, 370. [Google Scholar] [CrossRef] [Green Version]
- De Menech, M.; Saalmann, U.; Garcia, M.E. Energy-resolved STM mapping of C60 on metal surfaces: A theoretical study. Phys. Rev. B 2006, 73, 155407. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. Cryst. Mater. 2005, 220, 567. [Google Scholar] [CrossRef] [Green Version]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B Condens. Matter Mater. Phys. 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.; Pack, J. Special points for Brillouin-zone integrations. Phys. Rev. B Condens. Matter Mater. Phys. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]




| ZnPc/Ag(111) | C60/Ag(111) | C60/ZnPc/Ag(111) | ZnPc * | C60 * | 7C60 + 4ZnPc/Ag(111) ** | |
|---|---|---|---|---|---|---|
| LUMO+2 | - | +1.6 | +1.6 | +2.35 | ||
| LUMO+1 | +1.1 | - | +1.1 | +1.39 | ||
| LUMO | +0.48 | +0.46 | +0.4 | +0.82 | +0.4 | +0.32 |
| SS | −0.3 | - | −0.3 | |||
| HOMO | −1.6 | −1.9 | −1.7 | −1.16 | −1.9 | −1.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benhnia, A.; Watanabe, S.; Tuerhong, R.; Nakaya, M.; Onoe, J.; Bucher, J.-P. Resolving Site-Specific Energy Levels of Small-Molecule Donor-Acceptor Heterostructures Close to Metal Contacts. Nanomaterials 2021, 11, 1618. https://doi.org/10.3390/nano11061618
Benhnia A, Watanabe S, Tuerhong R, Nakaya M, Onoe J, Bucher J-P. Resolving Site-Specific Energy Levels of Small-Molecule Donor-Acceptor Heterostructures Close to Metal Contacts. Nanomaterials. 2021; 11(6):1618. https://doi.org/10.3390/nano11061618
Chicago/Turabian StyleBenhnia, Amani, Shinta Watanabe, Rouzhaji Tuerhong, Masato Nakaya, Jun Onoe, and Jean-Pierre Bucher. 2021. "Resolving Site-Specific Energy Levels of Small-Molecule Donor-Acceptor Heterostructures Close to Metal Contacts" Nanomaterials 11, no. 6: 1618. https://doi.org/10.3390/nano11061618