Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review
Abstract
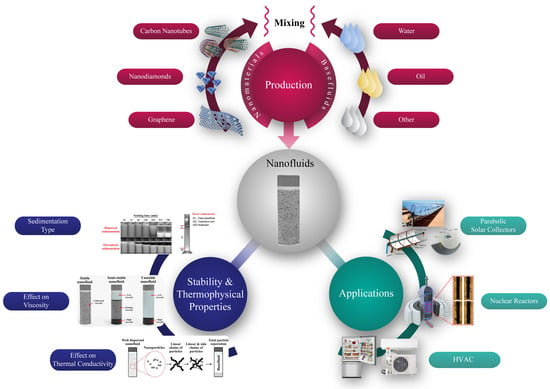
:1. Introduction
2. Synthesis of Nanoscaled Carbon-Based Materials
2.1. Nanodiamonds
2.2. Graphene
2.3. Carbon Nanotubes
3. Preparation of Nanofluids
3.1. One-Step Method
3.2. Two-Step Method
3.3. Carbon-Based Nanofluids Fabrication
4. Nanofluids Stability
4.1. Stability Mechanism and Evaluation
4.2. Stability Enhancements
5. Stability Effect on Thermophysical Properties
5.1. Effective Thermal Conductivity
5.2. Effective Viscosity
6. Thermal Applications
6.1. Parabolic Trough Solar Collectors
6.2. Nuclear Reactors
6.2.1. Nanofluids Influence on Flow Boiling
6.2.2. Dispersions Effect on Pool Boiling
6.3. Air Conditioning and Refrigeration Systems
6.3.1. Influence of Carbon-Based Nanoparticles on the Thermophysical Properties of Working Fluid in AC&R Systems
6.3.2. Influence of Carbon-Based Nanofluids on the COP and Overall Cooling Performance of AC&R Systems
7. Environmental Consideration and Potential Health Issues
8. Discussion and Future Directions
8.1. Challenges in Carbon-Based Nanofluids
8.2. Limitations in Parabolic Trough Solar Collector Systems
8.3. Limitations in Nuclear Reactor Systems
8.4. Limitations in Air Conditioning and Refrigeration Systems
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Area (nm2) | |
| AC | Air conditioning |
| AC&R | Air conditioning and refrigeration |
| AG | Arabic gum |
| ALWR | Advanced light water reactor |
| ANL | Argonne National Laboratory |
| BAC | Benzalkonium chloride |
| BWR | Boiling water reactor |
| Nanoparticle random motion velocity (nm/s) | |
| Self-crowding factor | |
| CFD | Computational fluid dynamics |
| CHF | Critical heat flux (W/m2) |
| CNT | Carbon nanotube |
| COP | Coefficient of performance |
| Specific heat capacity (J/kg∙K) | |
| CSPP | Concentrated solar power plant |
| CTAB | Cetyltrimethyl ammonium bromide |
| CVD | Chemical vapour deposition |
| DASC | Direct absorber solar collector |
| Diameter of the base fluid molecule (nm) | |
| DND | Detonation nanodiamond |
| Direct normal irradiance | |
| Nanoparticles mean diameter | |
| DSC | Differential scanning calorimetry |
| DSDMAC | Distearyl dimethyl ammonium chloride |
| DWCNT | Double-walled carbon nanotube |
| DX | Direct expansion |
| EG | Ethylene glycol |
| Maximum attainable concentration | |
| Packing fraction of the particles | |
| Particles volumetric fraction | |
| FVM | Finite volume method |
| GCR | Gas-cooled reactor |
| GO | Graphene oxide |
| HFC | Hydrofluorocarbon |
| HPHT | High-pressure and high-temperature |
| HTC | Heat transfer coefficient (W/m2∙K) |
| IPH | Industrial process heat |
| Equivalent thermal conductivity of the ellipsoids particle (W/m∙K) | |
| Boltzmann constant (1.381 10−23 J/K) | |
| Huggins coefficient | |
| Matrix conductivity (W/m∙K) | |
| Equivalent particle thermal conductivity (W/m∙K) | |
| Mean-free path of the base fluid molecule (nm) | |
| LOCA | Loss-of-coolant accident |
| Mass (Kg) | |
| MCRT | Monte Carlo ray tracing |
| MSR | Molten solid cooled reactor |
| MWCNT | Multiwalled carbon nanotube |
| ND | Nanodiamond |
| Nusselt number | |
| PHWR | Pressurized heavy water reactor |
| POE | Polyolester oil |
| Prandtl number | |
| PTSC | Parabolic trough solar collector |
| PVA | Polyvinyl alcohol |
| PVP | Polyvinylpyrrolidone |
| PWR | Pressurized water reactor |
| Volume ratio | |
| Impact of interfacial resistance (Km2/W) | |
| Particle apparent radius (nm) | |
| RGO | Reduced graphene oxide |
| Reynolds number | |
| Kaptiza radius (8 10−8 m2 K/W) | |
| Radius of the fluid medium particles (nm) | |
| SANSS | Submerged arc nanoparticle synthesis system |
| SDBS | Sodium dodecyl benzenesulfonate |
| SDS | Sodium dodecyl sulfate |
| SEM | Scanning electron microscopy |
| SWCNH | Single-walled carbon nanohorn |
| SWCNT | Single-walled carbon nanotube |
| Temperature (K or °C) | |
| Reference temperature (273 K) | |
| Mean temperature (K or °C) | |
| Tmin | Minimum film boiling temperature (K or °C) |
| Thickness of the nanolayer surrounding the particle (nm) | |
| to | Starting time (s) |
| tf | Finishing time (s) |
| TEM | Transmission electron microscopy |
| TWCNT | Triple-walled carbon nanotube |
| Volume (m3) | |
| VERSO | Vacuum evaporation onto a running oil substrate |
| vol. % | Volume percentage |
| WCR | Water-cooled reactor |
| wt % | Weight percentage |
| Greek letters | |
| Ratio of the nanolayer thickness to the particle radius | |
| Difference | |
| Average flatness ratio of the graphene nanoplatelet | |
| Intrinsic viscosity | |
| Dynamic viscosity (kg/m∙s) | |
| Empirical shape factor | |
| Kinematic viscosity (m2/s) | |
| ψ | Particle sphericity |
| Density (kg/m3) | |
| Thermal conductivity (W/m∙K) | |
| Subscripts | |
| Ambient | |
| Base fluid | |
| Carbon nanotube | |
| Effective | |
| Minimum | |
| Nanofluid | |
| Nanoparticles | |
| Saturated | |
| Super-heated | |
| Water |
References
- Ahuja, A.S. Augmentation of heat transport in laminar flow of polystyrene suspensions. I. Experiments and results. J. Appl. Phys. 1975, 46, 3408–3416. [Google Scholar] [CrossRef]
- Ahuja, A.S. Augmentation of heat transport in laminar flow of polystyrene suspensions. II. Analysis of the data. J. Appl. Phys. 1975, 46, 3417–3425. [Google Scholar] [CrossRef]
- Liu, K.V.; Choi, U.S.; Kasza, K.E. Measurements of Pressure Drop and Heat Transfer in Turbulent Pipe Flows of Particulate Slurries; Argonne National Lab: Lemont, IL, USA, 1988. [Google Scholar]
- Choi, S.U.; Cho, Y.I.; Kasza, K.E. Degradation effects of dilute polymer solutions on turbulent friction and heat transfer behavior. J. Non-Newton Fluid Mech. 1992, 41, 289–307. [Google Scholar] [CrossRef]
- Choi, U.; France, D.M.; Knodel, B.D. Impact of Advanced Fluids on Costs of District Cooling Systems; Argonne National Lab: Lemont, IL, USA, 1992. [Google Scholar]
- Choi, U.; Tran, T. Experimental Studies of the Effects of Non-Newtonian Surfactant Solutions on the Performance of a Shell-and-Tube Heat Exchanger. In Recent Developments in Non-Newtonian Flows and Industrial Applications; The American Society of Mechanical Engineers New York: New York, NY, USA; FED: Atlanta, GA, USA, 1991; pp. 47–52. [Google Scholar]
- Maxwell, J.C. A Treatise on Electricity and Magnetism, 2nd ed.; Clarendon Press: Oxford, UK, 1881. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A. Aluminium Nanofluids Stability: A Comparison between the Conventional Two-Step Fabrication Approach and the Controlled Sonication Bath Temperature Method. J. Nanomater. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Masuda, H.; Ebata, A.; Teramae, K. Alteration of Thermal Conductivity and Viscosity of Liquid by Dispersing Ultra-Fine Particles. Dispersion of Al2o3, Sio2 and Tio2 Ultra-Fine Particles; Netsu Bussei: Tokyo, Japan, 1993; Volume 7, pp. 227–233. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles. In Proceedings of the 1995 International Mechanical Engineering Congress and Exhibition, San Francisco, CA, USA, 12–17 November 1995; PBD: Washington, DC, USA; Argonne National Lab: Lemont, IL, USA, 1995; 8p. [Google Scholar]
- Naser, A.; Teixeira, J.A.; Ali, N. New pH Correlations for Stainless Steel 316L, Alumina, and Copper(I) Oxide Nanofluids Fabricated at Controlled Sonication Temperatures. J. Nano Res. 2019, 58, 125–138. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Choi, S.U.S. Application of Metallic Nanoparticle Suspensions in Advanced Cooling Systems; American Society of Mechanical Engineers, Materials Division: Atlanta, GA, USA, 1996; Volume 72, pp. 227–234. [Google Scholar]
- Ali, N.; Teixeira, J.A.; Addali, A. A Review on Nanofluids: Fabrication, Stability, and Thermophysical Properties. J. Nanomater. 2018, 2018, 1–33. [Google Scholar] [CrossRef]
- Pop, E.; Varshney, V.; Roy, A.K. Thermal properties of graphene: Fundamentals and applications. MRS Bull. 2012, 37, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef] [Green Version]
- Sezer, N.; Atieh, M.; Koc, M. A comprehensive review on synthesis, stability, thermophysical properties, and characterization of nanofluids. Powder Technol. 2019, 344, 404–431. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D.; Johnson, W.; Nawaz, K.; Cunningham, G. Thermo-physical properties of diamond nanofluids: A review. Int. J. Heat Mass Transf. 2019, 129, 1123–1135. [Google Scholar] [CrossRef]
- Schwamb, T.; Burg, B.R.; Schirmer, N.C.; Poulikakos, D. An electrical method for the measurement of the thermal and electrical conductivity of reduced graphene oxide nanostructures. Nanotechnology 2009, 20, 405704. [Google Scholar] [CrossRef]
- Mahanta, N.K.; Abramson, A.R. Thermal conductivity of graphene and graphene oxide nanoplatelets. In Proceedings of the 13th InterSociety Conference on Thermal and Thermomechanical Phenomena in Electronic Systems, San Diego, CA, USA, 30 May–1 June 2012; IEEE: New York, NY, USA, 2012; pp. 1–6. [Google Scholar]
- Zhang, H.; Fonseca, A.F.; Cho, K. Tailoring Thermal Transport Property of Graphene through Oxygen Functionalization. J. Phys. Chem. C 2014, 118, 1436–1442. [Google Scholar] [CrossRef]
- Eastman, J.A.; Choi, U.S.; Li, S.; Thompson, L.J.; Lee, S. Enhanced thermal conductivity through the development of nanofluids. In Proceedings of the 1996 MRS Fall Symposium; George, E.P., Gotthardt, R., Otsuka, K., Trolier-McKinstry, S., Wun-Fogle, M., Eds.; Materials Research Society: Pittsburgh, PA, USA; Boston, MA, USA, 1997; pp. 3–11. [Google Scholar]
- Li, Z.X.; Khaled, U.; Al-Rashed, A.A.A.A.; Goodarzi, M.; Sarafraz, M.M.; Meer, R. Heat transfer evaluation of a micro heat exchanger cooling with spherical carbon-acetone nanofluid. Int. J. Heat Mass Transf. 2020, 149, 119124. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Shuib, A.; Marneni, N. A review on the viscous and thermal transport properties of nanofluids. In International Conference on Process Engineering and Advanced Materials, ICPEAM 2012; Trans Tech Publications Ltd.: Kuala Lumpur, Malaysia, 2014; pp. 18–27. [Google Scholar]
- Shanthi, R.; Anandan, S.; Ramalingam, V. Heat transfer enhancement using nanofluids: An overview. Therm. Sci. 2012, 16, 423–444. [Google Scholar] [CrossRef]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Vékás, L.; Bica, D.; Avdeev, M.V. Magnetic nanoparticles and concentrated magnetic nanofluids: Synthesis, properties and some applications. China Particuol. 2007, 5, 43–49. [Google Scholar] [CrossRef]
- Reddy, K.S.; Kamnapure, N.R.; Srivastava, S. Nanofluid and nanocomposite applications in solar energy conversion systems for performance enhancement: A review. Int. J. Low Carbon Technol. 2016, 12, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Sheikholeslami, M.; Ganji, D.D. Application of Nanofluids. In Applications of Semi Analytical Methods for Nanofluid Flow and Heat Transfer; Sheikholeslami, M., Ganji, D.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–44. [Google Scholar]
- Mansoury, D.; Doshmanziari, F.I.; Kiani, A.; Chamkha, A.J.; Sharifpur, M. Heat Transfer and Flow Characteristics of Al2O3/Water Nanofluid in Various Heat Exchangers: Experiments on Counter Flow. Heat Transf. Eng. 2018, 41, 1–36. [Google Scholar] [CrossRef]
- Chamkha, A.J.; Molana, M.; Rahnama, A.; Ghadami, F. On the nanofluids applications in microchannels: A comprehensive review. Powder Technol. 2018, 332, 287–322. [Google Scholar] [CrossRef]
- Alsayegh, A.; Ali, N. Gas Turbine Intercoolers: Introducing Nanofluids—A Mini-Review. Processes 2020, 8, 1572. [Google Scholar] [CrossRef]
- Mannu, R.; Karthikeyan, V.; Velu, N.; Arumugam, C.; Roy, V.A.L.; Gopalan, A.-I.; Saianand, G.; Sonar, P.; Lee, K.-P.; Kim, W.-J.; et al. Polyethylene Glycol Coated Magnetic Nanoparticles: Hybrid Nanofluid Formulation, Properties and Drug Delivery Prospects. Nanomaterials 2021, 11, 440. [Google Scholar] [CrossRef]
- Martínez-Merino, P.; Sánchez-Coronilla, A.; Alcántara, R.; Martín, E.I.; Carrillo-Berdugo, I.; Gómez-Villarejo, R.; Navas, J. The Role of the Interactions at the Tungsten Disulphide Surface in the Stability and Enhanced Thermal Properties of Nanofluids with Application in Solar Thermal Energy. Nanomaterials 2020, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Rostami, S.; Aghakhani, S.; Pordanjani, A.H.; Afrand, M.; Cheraghian, G.; Oztop, H.F.; Shadloo, M.S. A Review on the Control Parameters of Natural Convection in Different Shaped Cavities with and Without Nanofluid. Processes 2020, 8, 1011. [Google Scholar] [CrossRef]
- Scopus-Database, Nanofluid Analyze Search Results for Documents Published from 1995 to 2020 with the Word ‘Nanofluid’; Elsevier: Amsterdam, The Netherlands. 2021. Available online: www.scopus.com (accessed on 1 April 2021).
- Mukherjee, S.; Mishra, P.C.; Chaudhuri, P. Stability of Heat Transfer Nanofluids—A Review. ChemBioEng Rev. 2018, 5, 312–333. [Google Scholar] [CrossRef]
- Almurtaji, S.; Ali, N.; Teixeira, J.A.; Addali, A. On the Role of Nanofluids in Thermal-hydraulic Performance of Heat Exchangers-A Review. Nanomaterials 2020, 10, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.Y.; Bhadeshia, H.; Suh, D.-W. Stability of stainless-steel nanoparticle and water mixtures. Powder Technol. 2015, 272, 34–44. [Google Scholar] [CrossRef]
- Ebrahimnia-Bajestan, E.; Niazmand, H.; Duangthongsuk, W.; Wongwises, S. Numerical investigation of effective parameters in convective heat transfer of nanofluids flowing under a laminar flow regime. Int. J. Heat Mass Transf. 2011, 54, 4376–4388. [Google Scholar] [CrossRef]
- Martínez-Cuenca, R.; Mondragón, R.; Hernández, L.; Segarra, C.; Jarque, J.C.; Hibiki, T.; Juliá, J.E. Forced-convective heat-transfer coefficient and pressure drop of water-based nanofluids in a horizontal pipe. Appl. Therm. Eng. 2016, 98, 841–849. [Google Scholar] [CrossRef]
- Benedict, L.X.; Louie, S.G.; Cohen, M.L. Heat capacity of carbon nanotubes. Solid State Commun. 1996, 100, 177–180. [Google Scholar] [CrossRef]
- Yazid, M.N.A.W.M.; Sidik, N.A.C.; Mamat, R.; Najafi, G. A review of the impact of preparation on stability of carbon nanotube nanofluids. Int. Commun. Heat Mass Transf. 2016, 78, 253–263. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Z.; Eres, G. Order in vertically aligned carbon nanotube arrays. Appl. Phys. Lett. 2006, 88, 213111. [Google Scholar] [CrossRef] [Green Version]
- Hong, P.N.; Minh, D.N.; Van Hung, N.; Minh, P.N.; Khoi, P.H. Carbon Nanotube and Graphene Aerogels—The World’s 3D Lightest Materials for Environment Applications: A Review. Int. J. Mater. Sci. Appl. 2017, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Askari, S.; Lotfi, R.; Seifkordi, A.; Rashidi, A.; Koolivand, H. A novel approach for energy and water conservation in wet cooling towers by using MWNTs and nanoporous graphene nanofluids. Energy Convers. Manag. 2016, 109, 10–18. [Google Scholar] [CrossRef]
- Neuberger, N.; Adidharma, H.; Fan, M. Graphene: A review of applications in the petroleum industry. J. Pet. Sci. Eng. 2018, 167, 152–159. [Google Scholar] [CrossRef]
- Shanbedi, M.; Heris, S.Z.; Amiri, A.; Hosseinipour, E.; Eshghi, H.; Kazi, S. Synthesis of aspartic acid-treated multi-walled carbon nanotubes based water coolant and experimental investigation of thermal and hydrodynamic properties in circular tube. Energy Convers. Manag. 2015, 105, 1366–1376. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Latibari, S.T.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on graphene nanofluids: Recent research, development and applications. Energy Convers. Manag. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Tam, N.T.; Phuong, N.V.; Khoi, P.H.; Minh, P.N.; Afrand, M.; Van Trinh, P.; Thang, B.H.; Żyła, G.; Estellé, P. Carbon Nanomaterial-Based Nanofluids for Direct Thermal Solar Absorption. Nanomaterials 2020, 10, 1199. [Google Scholar] [CrossRef]
- Ambreen, T.; Saleem, A.; Park, C. Homogeneous and Multiphase Analysis of Nanofluids Containing Nonspherical MWCNT and GNP Nanoparticles Considering the Influence of Interfacial Layering. Nanomaterials 2021, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Giwa, S.O.; Sharifpur, M.; Ahmadi, M.H.; Murshed, S.M.S.; Meyer, J.P. Experimental Investigation on Stability, Viscosity, and Electrical Conductivity of Water-Based Hybrid Nanofluid of MWCNT-Fe2O3. Nanomaterials 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Pontes, P.; Cautela, R.; Bahadur, V.; Miranda, J.; Ribeiro, A.P.C.; Souza, R.R.; Oliveira, J.D.; Copetti, J.B.; Lima, R.; et al. Article pool boiling of nanofluids on biphilic surfaces: An experimental and numerical study. Nanomaterials 2021, 11, 125. [Google Scholar] [CrossRef]
- Karagiannakis, N.P.; Skouras, E.D.; Burganos, V.N. Modelling Thermal Conduction in Nanoparticle Aggregates in the Presence of Surfactants. Nanomaterials 2020, 10, 2288. [Google Scholar] [CrossRef]
- Khan, H.; Soudagar, M.E.M.; Kumar, R.H.; Safaei, M.R.; Farooq, M.; Khidmatgar, A.; Banapurmath, N.R.; Farade, R.A.; Abbas, M.M.; Afzal, A.; et al. Effect of Nano-Graphene Oxide and n-Butanol Fuel Additives Blended with Diesel—Nigella sativa Biodiesel Fuel Emulsion on Diesel Engine Characteristics. Symmetry 2020, 12, 961. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Safaei, M.R.; Manokar, A.M.; El-Seesy, A.I.; Mujtaba, M.A.; Samuel, O.D.; Badruddin, I.A.; Ahmed, W.; Shahapurkar, K.; et al. Investigation on the effect of cottonseed oil blended with different percentages of octanol and suspended MWCNT nanoparticles on diesel engine characteristics. J. Therm. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, Q.Z. Carbon and other light element contents in the Earth’s core based on first-principles molecular dynamics. Proc. Natl. Acad. Sci. USA 2012, 109, 19579–19583. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J.; Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Kuo, P.K.; Thomas, R.L.; Anthony, T.R.; Banholzer, W.F. Thermal conductivity of isotopically modified single crystal diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef]
- Hodkiewicz, J.; Scientific, T. Characterizing Carbon Materials with Raman Spectroscopy; Thermo Scientific Application Note; Thermo Fisher Scientific: Madison, WI, USA, 2010. [Google Scholar]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Ishii, C.; Ruike, M.; Kuwabara, H. Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 1992, 30, 1075–1088. [Google Scholar] [CrossRef]
- Pang, J.; Bachmatiuk, A.; Ibrahim, I.; Fu, L.; Placha, D.; Martynková, G.S.; Trzebicka, B.; Gemming, T.; Eckert, J.; Rümmeli, M.H. CVD growth of 1D and 2D sp2 carbon nanomaterials. J. Mater. Sci. 2015, 51, 640–667. [Google Scholar] [CrossRef]
- van Thiel, M.; Ree, F.H. Properties of carbon clusters in TNT detonation products: Graphite-diamond transition. J. Appl. Phys. 1987, 62, 1761–1767. [Google Scholar] [CrossRef]
- Morita, Y.; Takimoto, T.; Yamanaka, H.; Kumekawa, K.; Morino, S.; Aonuma, S.; Kimura, T.; Komatsu, N. A Facile and Scalable Process for Size-Controllable Separation of Nanodiamond Particles as Small as 4 nm. Small 2008, 4, 2154–2157. [Google Scholar] [CrossRef]
- Ali, M.S.; Metwally, A.; Fahmy, R.H.; Osman, R. Nanodiamonds: Minuscule gems that ferry antineoplastic drugs to resistant tumors. Int. J. Pharm. 2019, 558, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Bovenkerk, H.P.; Bundy, F.P.; Hall, H.T.; Strong, H.M.; Wentorf, R.H. Preparation of Diamond. Nat. Cell Biol. 1959, 184, 1094–1098. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Kumar, A.; Lin, P.A.; Xue, A.; Hao, B.; Yap, Y.K.; Sankaran, R.M. Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour. Nat. Commun. 2013, 4, 2618. [Google Scholar] [CrossRef] [Green Version]
- Frenklach, M.; Howard, W.; Huang, D.; Yuan, J.; Spear, K.E.; Koba, R. Induced nucleation of diamond powder. Appl. Phys. Lett. 1991, 59, 546–548. [Google Scholar] [CrossRef]
- Yang, G.-W.; Wang, J.-B.; Liu, Q.-X. Preparation of nano-crystalline diamonds using pulsed laser induced reactive quenching. J. Phys. Condens. Matter 1998, 10, 7923–7927. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Mechanically Induced Graphite-Nanodiamonds-Phase Transformations During High-Energy Ball Milling. J. Mater. Eng. Perform. 2017, 26, 2974–2982. [Google Scholar] [CrossRef]
- Lin, C.R.; Wei, D.H.; Dao, M.K.; Chung, R.J.; Chang, M.H. Nanocrystalline diamond particles prepared by high-energy ball milling method. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Schwyz, Switzerland, 2013; Volume 284, pp. 168–172. [Google Scholar] [CrossRef]
- Galimov, A.É.M.; Kudin, A.M.; Skorobogatskii, V.N.; Plotnichenko, V.G.; Bondarev, O.L.; Zarubin, B.G.; Strazdovskii, V.V.; Aronin, A.S.; Fisenko, A.V.; Bykov, I.; et al. Experimental corroboration of the synthesis of diamond in the cavitation process. Dokl. Phys. 2004, 49, 150–153. [Google Scholar] [CrossRef]
- Welz, S.; Gogotsi, Y.; McNallan, M.J. Nucleation, growth, and graphitization of diamond nanocrystals during chlorination of carbides. J. Appl. Phys. 2003, 93, 4207–4214. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Carbon onions as nanoscopic pressure cells for diamond formation. Nature 1996, 382, 433–435. [Google Scholar] [CrossRef]
- Daulton, T.; Kirk, M.; Lewis, R.; Rehn, L. Production of nanodiamonds by high-energy ion irradiation of graphite at room temperature. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2001, 175–177, 12–20. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Method for Synthesizing Nanodiamonds. U.S. Patent 9,540,245 B1, 10 January 2017. [Google Scholar]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Mashali, F.; Languri, E.M.; Davidson, J.; Kerns, D. Diamond Nanofluids: Microstructural Analysis and Heat Transfer Study. Heat Transf. Eng. 2020, 42, 479–491. [Google Scholar] [CrossRef]
- Crane, M.J.; Petrone, A.; Beck, R.A.; Lim, M.B.; Zhou, X.; Li, X.; Stroud, R.M.; Pauzauskie, P.J. High-pressure, high-temperature molecular doping of nanodiamond. Sci. Adv. 2019, 5, eaau6073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afandi, A.; Howkins, A.; Boyd, I.W.; Jackman, R.B. Nanodiamonds for device applications: An investigation of the properties of boron-doped detonation nanodiamonds. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Tinwala, H.; Wairkar, S. Production, surface modification and biomedical applications of nanodiamonds: A sparkling tool for theranostics. Mater. Sci. Eng. C 2019, 97, 913–931. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Zhou, Y.; Bi, H.; Yang, M.; Wang, J.; Liu, Q.; Huang, F. Multidimensional graphene structures and beyond: Unique properties, syntheses and applications. Prog. Mater. Sci. 2020, 113, 100665. [Google Scholar] [CrossRef]
- Moreau, E.; Ferrer, F.J.; Vignaud, D.; Godey, S.; Wallart, X. Graphene growth by molecular beam epitaxy using a solid carbon source. Phys. Status Solidi (a) 2010, 207, 300–303. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-Induced Graphene by Multiple Lasing: Toward Electronics on Cloth, Paper, and Food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, e1803621. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery. Langmuir 2010, 26, 3208–3213. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Mehdi, S.M.; Abbas, N.; Hussain, M.; Naqvi, R.A. Synthesis of graphene from solid carbon sources: A focused review. Mater. Chem. Phys. 2020, 248, 122924. [Google Scholar] [CrossRef]
- Tetsuka, H.; Nagoya, A.; Fukusumi, T.; Matsui, T. Molecularly Designed, Nitrogen-Functionalized Graphene Quantum Dots for Optoelectronic Devices. Adv. Mater. 2016, 28, 4632–4638. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Tochihara, H.; Pomprasit, A.; Kadowaki, T.; Mizuno, S.; Minagawa, H.; Hayakawa, K.; Toyoshima, I. Decomposition of the surface carbide on Ni(001) induced by copper adsorption and surface segregation of carbon. Surf. Sci. Lett. 1991, 257, L623–L627. [Google Scholar]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of Graphene Films on Copper Foils by Chemical Vapor Deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Subrahmanyam, K.S.; Matte, H.S.; Abdulhakeem, B.; Govindaraj, A.; Das, B.; Kumar, P.; Ghosh, A.; Late, D.J. A study of the synthetic methods and properties of graphenes. Sci. Technol. Adv. Mater. 2010, 11, 054502. [Google Scholar] [CrossRef]
- Reibold, M.; Paufler, P.; Levin, A.A.; Kochmann, W.; Patzke, N.; Meyer, D.C. Materials: Carbon nanotubes in an ancient Damascus sabre. Nature 2006, 444, 286. [Google Scholar] [CrossRef]
- Radushkevich, L.; Lukyanovich, V.Á. O strukture ugleroda, obrazujucegosja pri termiceskom razlozenii okisi ugleroda na zeleznom kontakte. Zurn Fisic Chim 1952, 26, 88–95. [Google Scholar]
- Boehm, H. Carbon from carbon monoxide disproportionation on nickel and iron catalysts: Morphological studies and possible growth mechanisms. Carbon 1973, 11, 583–590. [Google Scholar] [CrossRef]
- Oberlin, A.; Endo, M.; Koyama, T. Filamentous growth of carbon through benzene decomposition. J. Cryst. Growth 1976, 32, 335–349. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R. Characterization of carbon nanotubes by Raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Rafique, I.; Kausar, A.; Anwar, Z.; Muhammad, B. Exploration of Epoxy Resins, Hardening Systems, and Epoxy/Carbon Nanotube Composite Designed for High Performance Materials: A Review. Polym. Technol. Eng. 2015, 55, 312–333. [Google Scholar] [CrossRef]
- Da Cunha, T.H.; De Oliveira, S.; Martins, I.L.; Geraldo, V.; Miquita, D.; Ramos, S.L.; Lacerda, R.G.; Ladeira, L.O.; Ferlauto, A.S. High-yield synthesis of bundles of double- and triple-walled carbon nanotubes on aluminum flakes. Carbon 2018, 133, 53–61. [Google Scholar] [CrossRef]
- Xia, D.; Luo, Y.; Li, Q.; Xue, Q.; Zhang, X.; Liang, C.; Dong, M. Extracting the inner wall from nested double-walled carbon nanotube by platinum nanowire: Molecular dynamics simulations. RSC Adv. 2017, 7, 39480–39489. [Google Scholar] [CrossRef] [Green Version]
- Dresselhaus, G.; Riichiro, S. Physical Properties of Carbon Nanotubes; World Scientific: Singapore, 1998. [Google Scholar]
- Ibrahim, K.S. Carbon nanotubes-properties and applications: A review. Carbon Lett. 2013, 14, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Mubarak, N.; Abdullah, E.C.; Jayakumar, N.; Sahu, J. An overview on methods for the production of carbon nanotubes. J. Ind. Eng. Chem. 2014, 20, 1186–1197. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Kim, H.-J.; Jung, D.H. Carbon nanotubes synthesis using diffusion and premixed flame methods: A review. Carbon Lett. 2015, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.; Saeed, T.; Chu, Y.-M.; Ali, H.M.; Cheraghian, G.; Kalbasi, R. Comprehensive study concerned graphene nano-sheets dispersed in ethylene glycol: Experimental study and theoretical prediction of thermal conductivity. Powder Technol. 2021, 386, 51–59. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Goodarzi, M.; Amiri, A.; Sarsam, W.; Alehashem, M.; Dahari, M.; Kazi, S. Study of synthesis, stability and thermo-physical properties of graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 15–21. [Google Scholar] [CrossRef]
- You, X.; Li, S. Fully Developed Opposing Mixed Convection Flow in the Inclined Channel Filled with a Hybrid Nanofluid. Nanomaterials 2021, 11, 1107. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Panigrahi, P.K. Stability of nanofluid: A review. Appl. Therm. Eng. 2020, 174, 115259. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H. A Review on Nanofluids: Preparation, Stability Mechanisms, and Applications. J. Nanomater. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Okonkwo, E.C.; Wole-Osho, I.; Almanassra, I.W.; Abdullatif, Y.M.; Al-Ansari, T. An updated review of nanofluids in various heat transfer devices. J. Therm. Anal. Calorim. 2020, 1–56. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Tung, S.; Schneider, E.; Xi, S. A review on development of nanofluid preparation and characterization. Powder Technol. 2009, 196, 89–101. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Habib, K.; Saidur, R.; Saha, B.B.; Irshad, K. Comprehensive study on nanofluid and ionanofluid for heat transfer enhancement: A review on current and future perspective. J. Mol. Liq. 2020, 305, 112787. [Google Scholar] [CrossRef]
- Toghraie, D.; Chaharsoghi, V.A.; Afrand, M. Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluid. J. Therm. Anal. Calorim. 2016, 125, 527–535. [Google Scholar] [CrossRef]
- Abbasi, S.M.; Rashidi, A.; Nemati, A.; Arzani, K. The effect of functionalisation method on the stability and the thermal conductivity of nanofluid hybrids of carbon nanotubes/gamma alumina. Ceram. Int. 2013, 39, 3885–3891. [Google Scholar] [CrossRef]
- Yang, L.; Ji, W.; Mao, M.; Huang, J.-N. An updated review on the properties, fabrication and application of hybrid-nanofluids along with their environmental effects. J. Clean. Prod. 2020, 257, 120408. [Google Scholar] [CrossRef]
- Shenoy, U.S.; Shetty, A.N. A simple single-step approach towards synthesis of nanofluids containing cuboctahedral cuprous oxide particles using glucose reduction. Front. Mater. Sci. 2018, 12, 74–82. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.; Lee, C.; Jung, Y.; Cheong, S.; Ku, B.; Jang, S. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Sabiha, M.; Mostafizur, R.; Saidur, R.; Mekhilef, S. Experimental investigation on thermo physical properties of single walled carbon nanotube nanofluids. Int. J. Heat Mass Transf. 2016, 93, 862–871. [Google Scholar] [CrossRef]
- Alam Khairul, M.; Saidur, R.; Hossain, A.; Alim, M.A.; Mahbubul, I.M. Heat Transfer Performance of Different Nanofluids Flows in a Helically Coiled Heat Exchanger. Adv. Mater. Res. 2014, 832, 160–165. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, S.U.S.; Jang, S.P.; Lee, S.Y. Production of aqueous spherical gold nanoparticles using conventional ultrasonic bath. Nanoscale Res. Lett. 2012, 7, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duangthongsuk, W.; Wongwises, S. Measurement of temperature-dependent thermal conductivity and viscosity of TiO2-water nanofluids. Exp. Therm. Fluid Sci. 2009, 33, 706–714. [Google Scholar] [CrossRef]
- Sandhu, H.; Gangacharyulu, D. An experimental study on stability and some thermophysical properties of multiwalled carbon nanotubes with water–ethylene glycol mixtures. Part. Sci. Technol. 2016, 35, 547–554. [Google Scholar] [CrossRef]
- Drzazga, M.; Dzido, G.; Lemanowicz, M.; Gierczycki, A. Influence of nonionic surfactant on nanofluid properties. In Proceedings of the 14th European Conference on Mixing, Warszawa, Poland, 10–13 September 2012; pp. 89–94. [Google Scholar]
- Ilyas, S.U.; Pendyala, R.; Narahari, M.; Susin, L. Stability, rheology and thermal analysis of functionalized alumina- thermal oil-based nanofluids for advanced cooling systems. Energy Convers. Manag. 2017, 142, 215–229. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Experimental investigation into the pool boiling heat transfer of aqueous based γ-alumina nanofluids. J. Nanoparticle Res. 2005, 7, 265–274. [Google Scholar] [CrossRef]
- Fontes, D.H.; Ribatski, G.; Filho, E.P.B. Experimental evaluation of thermal conductivity, viscosity and breakdown voltage AC of nanofluids of carbon nanotubes and diamond in transformer oil. Diam. Relat. Mater. 2015, 58, 115–121. [Google Scholar] [CrossRef]
- Farbod, M.; Asl, R.K.; Abadi, A.R.N. Morphology dependence of thermal and rheological properties of oil-based nanofluids of CuO nanostructures. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 474, 71–75. [Google Scholar] [CrossRef]
- Ettefaghi, E.-O.-L.; Mohtasebi, S.S.; Alaei, M.; Ahmadi, H.; Rashidi, A. Experimental evaluation of engine oil properties containing copper oxide nanoparticles as a nanoadditive. Int. J. Ind. Chem. 2013, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.I.; Valan Arasu, A. A review of influence of nanoparticle synthesis and geometrical parameters on thermophysical properties and stability of nanofluids. Therm. Sci. Eng. Prog. 2019, 11, 334–364. [Google Scholar] [CrossRef]
- Noroozi, M.; Radiman, S.; Zakaria, A. Influence of Sonication on the Stability and Thermal Properties of Al2O3Nanofluids. J. Nanomater. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Asadi, A.; Pourfattah, F.; Szilágyi, I.M.; Afrand, M.; Żyła, G.; Ahn, H.S.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A.; Mahian, O. Effect of sonication characteristics on stability, thermophysical properties, and heat transfer of nanofluids: A comprehensive review. Ultrason. Sonochemistry 2019, 58, 104701. [Google Scholar] [CrossRef]
- Wciślik, S. Efficient Stabilization of Mono and Hybrid Nanofluids. Energies 2020, 13, 3793. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.; Mamat, R.; Usri, N.; Najafi, G. Investigation of Al2O3 Nanofluid Viscosity for Different Water/EG Mixture Based. Energy Procedia 2015, 79, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Asadi, A.; Alarifi, I.M.; Foong, L.K. An experimental study on characterization, stability and dynamic viscosity of CuO-TiO2/water hybrid nanofluid. J. Mol. Liq. 2020, 307, 112987. [Google Scholar] [CrossRef]
- Aghahadi, M.H.; Niknejadi, M.; Toghraie, D. An experimental study on the rheological behavior of hybrid Tungsten oxide (WO3)-MWCNTs/engine oil Newtonian nanofluids. J. Mol. Struct. 2019, 1197, 497–507. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Bahiraei, M.; Heshmatian, S. Graphene family nanofluids: A critical review and future research directions. Energy Convers. Manag. 2019, 196, 1222–1256. [Google Scholar] [CrossRef]
- Le Ba, T.; Mahian, O.; Wongwises, S.; Szilágyi, I.M. Review on the recent progress in the preparation and stability of graphene-based nanofluids. J. Therm. Anal. Calorim. 2020, 142, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Texter, J. Graphene dispersions. Curr. Opin. Colloid Interface Sci. 2014, 19, 163–174. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.U.; Kim, S.H.; Oh, J.M. Estimation of Thermal Conductivity of Nanofluid Using Experimental Effective Particle Volume. Exp. Heat Transf. 2006, 19, 181–191. [Google Scholar] [CrossRef]
- Xie, H.; Yu, W.; Li, Y.; Chen, L. Discussion on the thermal conductivity enhancement of nanofluids. Nanoscale Res. Lett. 2011, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Xie, H.; Li, Y.; Chen, L.; Wang, Q. Experimental investigation on the thermal transport properties of ethylene glycol based nanofluids containing low volume concentration diamond nanoparticles. Colloids Surfaces A: Physicochem. Eng. Asp. 2011, 380, 1–5. [Google Scholar] [CrossRef]
- Xie, H.; Yu, W.; Li, Y. Thermal performance enhancement in nanofluids containing diamond nanoparticles. J. Phys. D Appl. Phys. 2009, 42, 095413. [Google Scholar] [CrossRef]
- Branson, B.T.; Beauchamp, P.S.; Beam, J.C.; Lukehart, C.M.; Davidson, J.L. Nanodiamond Nanofluids for Enhanced Thermal Conductivity. ACS Nano 2013, 7, 3183–3189. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Narahari, M.; Pendyala, R. Rheological characteristics of ultrastable diamond-thermal oil nanofluids. J. Mol. Liq. 2020, 309, 113098. [Google Scholar] [CrossRef]
- Shukla, G.; Aiyer, H. Thermal conductivity enhancement of transformer oil using functionalized nanodiamonds. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2185–2190. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Experimental thermal conductivity and viscosity of nanodiamond-based propylene glycol and water mixtures. Diam. Relat. Mater. 2016, 69, 49–60. [Google Scholar] [CrossRef]
- Li, P.; Zheng, Y.; Wu, Y.; Qu, P.; Yang, R.; Zhang, A. Nanoscale ionic graphene material with liquid-like behavior in the absence of solvent. Appl. Surf. Sci. 2014, 314, 983–990. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, N.J. Influence of the oxidation treatment and the average particle diameter of graphene for thermal conductivity enhancement. J. Ind. Eng. Chem. 2014, 20, 1911–1915. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Moghaddam, M.B.; Goharshadi, E.K.; Entezari, M.H.; Nancarrow, P. Preparation, characterization, and rheological properties of graphene–glycerol nanofluids. Chem. Eng. J. 2013, 231, 365–372. [Google Scholar] [CrossRef]
- Babu, K.; Kumar, T.P. Effect of CNT concentration and agitation on surface heat flux during quenching in CNT nanofluids. Int. J. Heat Mass Transf. 2011, 54, 106–117. [Google Scholar] [CrossRef]
- Öndin, O.; Kıvak, T.; Sarıkaya, M.; Yıldırım, Ç.V. Investigation of the influence of MWCNTs mixed nanofluid on the machinability characteristics of PH 13-8 Mo stainless steel. Tribol. Int. 2020, 148, 106323. [Google Scholar] [CrossRef]
- Pourpasha, H.; Heris, S.Z.; Mahian, O.; Wongwises, S. The effect of multi-wall carbon nanotubes/turbine meter oil nanofluid concentration on the thermophysical properties of lubricants. Powder Technol. 2020, 367, 133–142. [Google Scholar] [CrossRef]
- Rahimi, A.; Kasaeipoor, A.; Malekshah, E.H.; Kolsi, L. Experimental and numerical study on heat transfer performance of three-dimensional natural convection in an enclosure filled with DWCNTs-water nanofluid. Powder Technol. 2017, 322, 340–352. [Google Scholar] [CrossRef]
- Shamaeil, M.; Firouzi, M.; Fakhar, A. The effects of temperature and volume fraction on the thermal conductivity of functionalized DWCNTs/ethylene glycol nanofluid. J. Therm. Anal. Calorim. 2016, 126, 1455–1462. [Google Scholar] [CrossRef]
- Said, Z. Thermophysical and optical properties of SWCNTs nanofluids. Int. Commun. Heat Mass Transf. 2016, 78, 207–213. [Google Scholar] [CrossRef]
- Harish, S.; Ishikawa, K.; Einarsson, E.; Aikawa, S.; Inoue, T.; Zhao, P.; Watanabe, M.; Chiashi, S.; Shiomi, J.; Maruyama, S. Temperature Dependent Thermal Conductivity Increase of Aqueous Nanofluid with Single Walled Carbon Nanotube Inclusion. Mater. Express 2012, 2, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for thermal transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. A review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Witharana, S.; Hodges, C.; Xu, D.; Lai, X.; Ding, Y. Aggregation and settling in aqueous polydisperse alumina nanoparticle suspensions. J. Nanoparticle Res. 2012, 14, 851. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Carrillo-Berdugo, I.; Zorrilla, D.; Sánchez-Márquez, J.; Aguilar, T.; Gallardo, J.J.; Gómez-Villarejo, R.; Alcántara, R.; Fernández-Lorenzo, C.; Navas, J. Interface-inspired formulation and molecular-level perspectives on heat conduction and energy storage of nanofluids. Sci. Rep. 2019, 9, 7595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunsong, M. Sedimentation Behavior of a Fine Kaolinite in the Presence of Fresh Fe Electrolyte. Clays Clay Miner. 1992, 40, 586–592. [Google Scholar] [CrossRef]
- Joni, I.M.; Purwanto, A.; Iskandar, F.; Okuyama, K. Dispersion Stability Enhancement of Titania Nanoparticles in Organic Solvent Using a Bead Mill Process. Ind. Eng. Chem. Res. 2009, 48, 6916–6922. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.-K.; Lee, J.-K.; Jeong, Y.-M.; Cheong, S.-I.; Ahn, Y.-C.; Kim, S.H. Production and dispersion stability of nanoparticles in nanofluids. Powder Technol. 2008, 186, 145–153. [Google Scholar] [CrossRef]
- Fedele, L.; Colla, L.; Bobbo, S.; Barison, S.; Agresti, F. Experimental stability analysis of different water-based nanofluids. Nanoscale Res. Lett. 2011, 6, 300. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Xie, H.; Lee, H.; Youn, W.; Choi, M. Nanofluids containing multiwalled carbon nanotubes and their enhanced thermal conductivities. J. Appl. Phys. 2003, 94, 4967. [Google Scholar] [CrossRef]
- Xian-Ju, W.; Xin-Fang, L. Influence of pH on Nanofluids’ Viscosity and Thermal Conductivity. Chin. Phys. Lett. 2009, 26, 056626. [Google Scholar] [CrossRef]
- Yu, H.; Hermann, S.; Schulz, S.E.; Gessner, T.; Dong, Z.; Li, W.J. Optimizing sonication parameters for dispersion of single-walled carbon nanotubes. Chem. Phys. 2012, 408, 11–16. [Google Scholar] [CrossRef]
- Xia, G.; Jiang, H.; Liu, R.; Zhai, Y. Effects of surfactant on the stability and thermal conductivity of Al2O3/de-ionized water nanofluids. Int. J. Therm. Sci. 2014, 84, 118–124. [Google Scholar] [CrossRef]
- Tang, E.; Cheng, G.; Ma, X.; Pang, X.; Zhao, Q. Surface modification of zinc oxide nanoparticle by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 2006, 252, 5227–5232. [Google Scholar] [CrossRef]
- Zhang, S.; Han, X. Effect of different surface modified nanoparticles on viscosity of nanofluids. Adv. Mech. Eng. 2018, 10. [Google Scholar] [CrossRef]
- Halelfadl, S.; Maré, T.; Estellé, P. Efficiency of carbon nanotubes water based nanofluids as coolants. Exp. Therm. Fluid Sci. 2014, 53, 104–110. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza-Gallego, M.J.; Casanova, C.; Páramo, R.; Barbés, B.; Legido, J.L.; Piñeiro, M.M. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. J. Appl. Phys. 2009, 106, 064301. [Google Scholar] [CrossRef]
- Saini, H.; Sandhu, A.; Sharma, S.; Dasaroju, G. Nanofluids: A Review Preparation, Stability, Properties and Applications. Int. J. Eng. Res. Technol. 2016, 5, 11–16. [Google Scholar]
- Zhou, S.; Ni, R. Measurement of the specific heat capacity of water-based Al2O3 nanofluid. Appl. Phys. Lett. 2008, 92, 093123. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Gracia-Fernández, C.; Legido, J.; Lugo, L. Specific heat of metal oxide nanofluids at high concentrations for heat transfer. Int. J. Heat Mass Transf. 2015, 88, 872–879. [Google Scholar] [CrossRef]
- Chandrasekar, M.; Suresh, S.; Bose, A.C. Experimental investigations and theoretical determination of thermal conductivity and viscosity of Al2O3/water nanofluid. Exp. Therm. Fluid Sci. 2010, 34, 210–216. [Google Scholar] [CrossRef]
- Utomo, A.T.; Poth, H.; Robbins, P.T.; Pacek, A.W. Experimental and theoretical studies of thermal conductivity, viscosity and heat transfer coefficient of titania and alumina nanofluids. Int. J. Heat Mass Transf. 2012, 55, 7772–7781. [Google Scholar] [CrossRef]
- Shin, D.; Banerjee, D. Enhancement of specific heat capacity of high-temperature silica-nanofluids synthesized in alkali chloride salt eutectics for solar thermal-energy storage applications. Int. J. Heat Mass Transf. 2011, 54, 1064–1070. [Google Scholar] [CrossRef]
- Starace, A.K.; Gomez, J.C.; Pradhan, S.; Glatzmaier, G.C.; Wang, J. Nanofluid heat capacities. J. Appl. Phys. 2011, 110, 124323. [Google Scholar] [CrossRef]
- Putnam, S.A.; Cahill, D.G.; Braun, P.V.; Ge, Z.; Shimmin, R.G. Thermal conductivity of nanoparticle suspensions. J. Appl. Phys. 2006, 99, 084308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Gu, H.; Fujii, M. Effective thermal conductivity and thermal diffusivity of nanofluids containing spherical and cylindrical nanoparticles. J. Appl. Phys. 2006, 100, 44325. [Google Scholar] [CrossRef]
- Eapen, J.; Williams, W.C.; Buongiorno, J.; Hu, L.-W.; Yip, S.; Rusconi, R.; Piazza, R. Mean-Field Versus Microconvection Effects in Nanofluid Thermal Conduction. Phys. Rev. Lett. 2007, 99, 095901. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, E.V.; Gavrilov, A.N.; McCloskey, J.M.; Tolmachev, Y.; Sprunt, S.; Lopatina, L.M.; Selinger, J. Thermal conductivity and particle agglomeration in alumina nanofluids: Experiment and theory. Phys. Rev. E 2007, 76, 061203. [Google Scholar] [CrossRef] [Green Version]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.J.; Townsend, J.; Christianson, R.J.; Tolmachev, Y.; Keblinski, P.; Hu, L.-W.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef] [Green Version]
- Barai, D.P.; Bhanvase, B.A.; Sonawane, S.H. A Review on Graphene Derivatives-Based Nanofluids: Investigation on Properties and Heat Transfer Characteristics. Ind. Eng. Chem. Res. 2020, 59, 10231–10277. [Google Scholar] [CrossRef]
- Ambreen, T.; Kim, M.-H. Influence of particle size on the effective thermal conductivity of nanofluids: A critical review. Appl. Energy 2020, 264, 114684. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Wang, X. Enhanced Thermal Conductivity of Liquid Paraffin Based Nanofluids Containing Copper Nanoparticles. J. Dispers. Sci. Technol. 2011, 32, 948–951. [Google Scholar] [CrossRef]
- Haghighi, E.B.; Nikkam, N.; Saleemi, M.; Behi, M.; Mirmohammadi, S.A.; Poth, H.; Khodabandeh, R.; Toprak, M.; Muhammed, M.; Palm, B. Shelf stability of nanofluids and its effect on thermal conductivity and viscosity. Meas. Sci. Technol. 2013, 24. [Google Scholar] [CrossRef]
- Li, X.; Zhu, D.; Wang, X.; Wang, N.; Gao, J.; Li, H. Thermal conductivity enhancement dependent pH and chemical surfactant for Cu-H2O nanofluids. Thermochim. Acta 2008, 469, 98–103. [Google Scholar] [CrossRef]
- Prasher, R.; Evans, W.; Meakin, P.; Fish, J.; Phelan, P.; Keblinskia, P. Effect of aggregation on thermal conduction in colloidal nanofluids. Appl. Phys. Lett. 2006, 89, 143119. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, R.; Gao, J.; Chen, G. Heat conduction mechanisms in nanofluids and suspensions. Nano Today 2012, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.; Wright, B.; Wensel, J.; Jin, S.; Ye, X.R.; Roy, W. Enhanced thermal conductivity by the magnetic field in heat transfer nanofluids containing carbon nanotube. Synth. Met. 2007, 157, 437–440. [Google Scholar] [CrossRef]
- Wright, B.; Thomas, D.; Hong, H.; Groven, L.; Puszynski, J.; Duke, E.; Ye, X.; Jin, S. Magnetic field enhanced thermal conductivity in heat transfer nanofluids containing Ni coated single wall carbon nanotubes. Appl. Phys. Lett. 2007, 91, 173116. [Google Scholar] [CrossRef]
- Wensel, J.; Wright, B.; Thomas, D.; Douglas, W.; Mannhalter, B.; Cross, W.; Hong, H.; Kellar, J.J.; Smith, P.; Roy, W. Enhanced thermal conductivity by aggregation in heat transfer nanofluids containing metal oxide nanoparticles and carbon nanotubes. Appl. Phys. Lett. 2008, 92, 023110. [Google Scholar] [CrossRef]
- Hong, H.; Luan, X.; Horton, M.; Li, C.; Peterson, G. Alignment of carbon nanotubes comprising magnetically sensitive metal oxides in heat transfer nanofluids. Thermochim. Acta 2011, 525, 87–92. [Google Scholar] [CrossRef]
- Younes, H.; Hong, H.; Peterson, G.P. A Novel Approach to Fabricate Carbon Nanomaterials–Nanoparticle Solids through Aqueous Solutions and Their Applications. Nanomanufacturing Metrol. 2021, 1–11. [Google Scholar] [CrossRef]
- Xue, L.; Keblinski, P.; Phillpot, S.; Choi, S.-S.; Eastman, J.; Xue, L.; Keblinski, P.; Phillpot, S.; Choi, S.-S.; Eastman, J. Effect of liquid layering at the liquid–solid interface on thermal transport. Int. J. Heat Mass Transf. 2004, 47, 4277–4284. [Google Scholar] [CrossRef]
- Keblinski, P.; Phillpot, S.; Choi, S.; Eastman, J. Mechanisms of heat flow in suspensions of nano-sized particles (nanofluids). Int. J. Heat Mass Transf. 2002, 45, 855–863. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transf. 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. Impact analysis of nanoparticle motion mechanisms on the thermal conductivity of nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1111–1118. [Google Scholar] [CrossRef]
- Domingues, G.; Voltz, S.; Joulain, K.; Greffet, J.-J. Heat Transfer between Two Nanoparticles Through Near Field Interaction. Phys. Rev. Lett. 2005, 94, 085901. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Narayanaswamy, A.; Chen, G. Surface Phonon Polaritons Mediated Energy Transfer between Nanoscale Gaps. Nano Lett. 2009, 9, 2909–2913. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.H.; Patel, H.E.; Kumar, V.R.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for Heat Conduction in Nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [Green Version]
- Keblinski, P.; Cahill, D.G. Comment on “Model for heat conduction in nanofluids”. Phys. Rev. Lett. 2005, 95, 209401. [Google Scholar] [CrossRef]
- Paul, G.; Chopkar, M.; Manna, I.; Das, P. Techniques for measuring the thermal conductivity of nanofluids: A review. Renew. Sustain. Energy Rev. 2010, 14, 1913–1924. [Google Scholar] [CrossRef]
- Tawfik, M.M. Experimental studies of nanofluid thermal conductivity enhancement and applications: A review. Renew. Sustain. Energy Rev. 2017, 75, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- [Pradhan, N.R.; Duan, H.; Liang, J.; Iannacchione, G.S. The specific heat and effective thermal conductivity of composites containing single-wall and multi-wall carbon nanotubes. Nanotechnology 2009, 20, 245705. [Google Scholar] [CrossRef]
- Che, J.; Çagin, T.; Goddard, W.A. Thermal conductivity of carbon nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Wang, X. Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys. Lett. A 2011, 375, 1323–1328. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Amiri, A.; Alehashem, M.S.; Dahari, M.; Kazi, S. Experimental investigation of thermo-physical properties, convective heat transfer and pressure drop of functionalized graphene nanoplatelets aqueous nanofluid in a square heated pipe. Energy Convers. Manag. 2016, 114, 38–49. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, J.; Fang, X.; Zhang, Z. Effect of morphology of carbon nanomaterials on thermo-physical characteristics, optical properties and photo-thermal conversion performance of nanofluids. Renew. Energy 2016, 99, 888–897. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Shariaty-Niasar, M.; Rashidi, A.M. Preparation of nanofluids from functionalized Graphene by new alkaline method and study on the thermal conductivity and stability. Int. Commun. Heat Mass Transf. 2013, 42, 89–94. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Rashidi, A.; Shariaty-Niassar, M. Convective heat transfer enhancement of graphene nanofluids in shell and tube heat exchanger. Exp. Therm. Fluid Sci. 2014, 53, 136–141. [Google Scholar] [CrossRef]
- Goodarzi, M.; Kherbeet, A.; Afrand, M.; Sadeghinezhad, E.; Mehrali, M.; Zahedi, P.; Wongwises, S.; Dahari, M. Investigation of heat transfer performance and friction factor of a counter-flow double-pipe heat exchanger using nitrogen-doped, graphene-based nanofluids. Int. Commun. Heat Mass Transf. 2016, 76, 16–23. [Google Scholar] [CrossRef]
- Liu, W.; Malekahmadi, O.; Bagherzadeh, S.A.; Ghashang, M.; Karimipour, A.; Hasani, S.; Tlili, I.; Goodarzi, M. A novel comprehensive experimental study concerned graphene oxide nanoparticles dispersed in water: Synthesise, characterisation, thermal conductivity measurement and present a new approach of RLSF neural network. Int. Commun. Heat Mass Transf. 2019, 109, 104333. [Google Scholar] [CrossRef]
- Maxwell, J.C. The Scientific Papers of James Clerk Maxwell; Cambridge University Press: London, UK, 1890. [Google Scholar]
- Jefferson, T.B.; Witzell, O.W.; Sibbitt, W.L. Thermal Conductivity of Graphite—Silicone Oil and Graphite-Water Suspensions. Ind. Eng. Chem. 1958, 50, 1589–1592. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal Conductivity of Heterogeneous Two-Component Systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Wasp, E.J.; Kenny, J.P.; Gandhi, R.L. Solid-liquid flow: Slurry pipeline transportation. Pumps, valves, mechanical equipment, economics. Ser. Bulk Mater. Handl. 1977, 1, 216–219. [Google Scholar]
- Yu, W.; Choi, S. The Role of Interfacial Layers in the Enhanced Thermal Conductivity of Nanofluids: A Renovated Maxwell Model. J. Nanoparticle Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggregation structure and thermal conductivity of nanofluids. AIChE J. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Nan, C.-W.; Shi, Z.; Lin, Y. A simple model for thermal conductivity of carbon nanotube-based composites. Chem. Phys. Lett. 2003, 375, 666–669. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Hamilton?Crosser model. J. Nanopart. Res. 2004, 6, 355–361. [Google Scholar] [CrossRef]
- Prasher, R.; Bhattacharya, P.; Phelan, P.E. Thermal Conductivity of Nanoscale Colloidal Solutions (Nanofluids). Phys. Rev. Lett. 2005, 94, 025901. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q. Model for thermal conductivity of carbon nanotube-based composites. Phys. B Condens. Matter 2005, 368, 302–307. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Leong, K.C.; Yang, C. A Model for Predicting the Effective Thermal Conductivity of Nanoparticle-Fluid Suspensions. Int. J. Nanosci. 2011, 5, 23–33. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K.; Kulkarni, D.P. Development of new correlations for convective heat transfer and friction factor in turbulent regime for nanofluids. Int. J. Heat Mass Transf. 2010, 53, 4607–4618. [Google Scholar] [CrossRef]
- Xing, M.; Yu, J.; Wang, R. Experimental investigation and modelling on the thermal conductivity of CNTs based nanofluids. Int. J. Therm. Sci. 2016, 104, 404–411. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, H.; Sasmito, A.P.; Mujumdar, A.S. Measurement and modeling of thermal conductivity of graphene nanoplatelet water and ethylene glycol base nanofluids. Int. J. Heat Mass Transf. 2018, 123, 97–109. [Google Scholar] [CrossRef]
- Li, M.-J.; He, Y.-L.; Tao, W.-Q. A novel semi-empirical model on predicting the thermal conductivity of diathermic oil-based nanofluid for solar thermal application. Int. J. Heat Mass Transf. 2019, 138, 1002–1013. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Dzido, G.; Zorȩbski, E.; Kolanowska, A.; Jȩdrysiak, R.; Dziadosz, J.; Libera, M.; Boncel, S.; Dzida, M. Remarkable Thermal Conductivity Enhancement in Carbon-Based Ionanofluids: Effect of Nanoparticle Morphology. ACS Appl. Mater. Interfaces 2020, 12, 38113–38123. [Google Scholar] [CrossRef]
- Pantzali, M.; Kanaris, A.; Antoniadis, K.; Mouza, A.; Paras, S. Effect of nanofluids on the performance of a miniature plate heat exchanger with modulated surface. Int. J. Heat Fluid Flow 2009, 30, 691–699. [Google Scholar] [CrossRef]
- Masoumi, N.; Sohrabi, N.; Behzadmehr, A. A new model for calculating the effective viscosity of nanofluids. J. Phys. D: Appl. Phys. 2009, 42, 055501. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.M.; Moghadassi, A.R.; Henneke, D.E. A new dimensionless group model for determining the viscosity of nanofluids. J. Therm. Anal. Calorim. 2010, 100, 873–877. [Google Scholar] [CrossRef]
- Chevalier, J.; Tillement, O.; Ayela, F. Structure and rheology of SiO2 nanoparticle suspensions under very high shear rates. Phys. Rev. E Stat. Nonlin Soft Matter Phys. 2009, 80, 051403. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Desgranges, F.; Galanis, N.; Roy, G.; Maré, T.; Boucher, S.; Mintsa, H.A. Viscosity data for Al2O3–water nanofluid—hysteresis: Is heat transfer enhancement using nanofluids reliable? Int. J. Therm. Sci. 2008, 47, 103–111. [Google Scholar] [CrossRef]
- Jarahnejad, M.; Haghighi, E.B.; Saleemi, M.; Nikkam, N.; Khodabandeh, R.; Palm, B.; Toprak, M.S.; Muhammed, M. Experimental investigation on viscosity of water-based Al2O3 and TiO2 nanofluids. Rheol. Acta 2015, 54, 411–422. [Google Scholar] [CrossRef]
- Dalkilic, A.; Küçükyıldırım, B.; Eker, A.A.; Çebi, A.; Tapan, S.; Jumpholkul, C.; Wongwises, S. Experimental investigation on the viscosity of Water-CNT and Antifreeze-CNT nanofluids. Int. Commun. Heat Mass Transf. 2017, 80, 47–59. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A. Dynamic viscosity of MWCNT/ZnO–engine oil hybrid nanofluid: An experimental investigation and new correlation in different temperatures and solid concentrations. Int. Commun. Heat Mass Transf. 2016, 76, 41–45. [Google Scholar] [CrossRef]
- Żyła, G.; Fal, J.; Estellé, P. The influence of ash content on thermophysical properties of ethylene glycol based graphite/diamonds mixture nanofluids. Diam. Relat. Mater. 2017, 74, 81–89. [Google Scholar] [CrossRef]
- Mena, J.B.; de Moraes, A.A.U.; Benito, Y.R.; Ribatski, G.; Parise, J.A.R. Extrapolation of Al2O3–water nanofluid viscosity for temperatures and volume concentrations beyond the range of validity of existing correlations. Appl. Therm. Eng. 2013, 51, 1092–1097. [Google Scholar] [CrossRef]
- Srivastava, S. Effect of aggregation on thermal conductivity and viscosity of nanofluids. Appl. Nanosci. 2012, 2, 325–331. [Google Scholar]
- Sadri, R.; Ahmadi, G.; Togun, H.; Dahari, M.; Kazi, S.N.; Sadeghinezhad, E.; Zubir, N. An experimental study on thermal conductivity and viscosity of nanofluids containing carbon nanotubes. Nanoscale Res. Lett. 2014, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, A.; Alarifi, I.M. Effects of ultrasonication time on stability, dynamic viscosity, and pumping power management of MWCNT-water nanofluid: An experimental study. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Doganay, S.; Alsangur, R.; Turgut, A. Effect of external magnetic field on thermal conductivity and viscosity of magnetic nanofluids: A review. Mater. Res. Express 2019, 6, 112003. [Google Scholar] [CrossRef]
- Yiamsawas, T.; Mahian, O.; Dalkilic, A.S.; Kaewnai, S.; Wongwises, S. Experimental studies on the viscosity of TiO2 and Al2O3 nanoparticles suspended in a mixture of ethylene glycol and water for high temperature applications. Appl. Energy 2013, 111, 40–45. [Google Scholar] [CrossRef]
- Walters, K.; Jones, W. Measurement of Viscosity. In Instrumentation Reference Book; Boyes, W., Ed.; Butterworth-Heinemann: Boston, MA, USA, 2010; pp. 69–75. [Google Scholar]
- Żyła, G.; Cholewa, M. On unexpected behavior of viscosity of diethylene glycol-based MgAl2O4 nanofluids. RSC Adv. 2014, 4, 26057–26062. [Google Scholar] [CrossRef]
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Lyu, Z.; Asadi, A.; Alarifi, I.M.; Ali, V.; Foong, L.K. Thermal and Fluid Dynamics Performance of MWCNT-Water Nanofluid Based on Thermophysical Properties: An Experimental and Theoretical Study. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Akhavan-Zanjani, H.; Saffar-Avval, M.; Mansourkiaei, M.; Ahadi, M.; Sharif, F. Turbulent Convective Heat Transfer and Pressure Drop of Graphene–Water Nanofluid Flowing Inside a Horizontal Circular Tube. J. Dispers. Sci. Technol. 2014, 35, 1230–1240. [Google Scholar] [CrossRef]
- Iranmanesh, S.; Mehrali, M.; Sadeghinezhad, E.; Ang, B.C.; Ong, H.C.; Esmaeilzadeh, A. Evaluation of viscosity and thermal conductivity of graphene nanoplatelets nanofluids through a combined experimental–statistical approach using respond surface methodology method. Int. Commun. Heat Mass Transf. 2016, 79, 74–80. [Google Scholar] [CrossRef]
- Ghozatloo, A.; Azimi, M.S.; Shariaty, N.M.; Morad, R.A. Investigation of nanoparticles morphology on viscosity of nanofluids and new correlation for prediction. J. Nanostructures 2015, 5, 161–168. [Google Scholar]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Einstein, A. Eine neue Bestimmung der Moleküldimensionen. Ann. Phys. Leipzig 1906, 324, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Hatschek, E. The general theory of viscosity of two-phase systems. Trans. Faraday Soc. 1913, 9, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Saitô, N. Concentration Dependence of the Viscosity of High Polymer Solutions. I. J. Phys. Soc. Jpn. 1950, 5, 4–8. [Google Scholar] [CrossRef]
- Mooney, M. The viscosity of a concentrated suspension of spherical particles. J. Colloid Sci. 1951, 6, 162–170. [Google Scholar] [CrossRef]
- Brinkman, H.C. The Viscosity of Concentrated Suspensions and Solutions. J. Chem. Phys. 1952, 20, 571. [Google Scholar] [CrossRef]
- Roscoe, R. The viscosity of suspensions of rigid spheres. Br. J. Appl. Phys. 1952, 3, 267–269. [Google Scholar] [CrossRef]
- Maron, S.H.; Pierce, P.E. Application of ree-eyring generalized flow theory to suspensions of spherical particles. J. Colloid Sci. 1956, 11, 80–95. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Frankel, N.; Acrivos, A. On the viscosity of a concentrated suspension of solid spheres. Chem. Eng. Sci. 1967, 22, 847–853. [Google Scholar] [CrossRef]
- Nielsen, L.E. Generalized Equation for the Elastic Moduli of Composite Materials. J. Appl. Phys. 1970, 41, 4626–4627. [Google Scholar] [CrossRef]
- Brenner, H.; Condiff, D.W. Transport mechanics in systems of orientable particles. IV. convective transport. J. Colloid Interface Sci. 1974, 47, 199–264. [Google Scholar] [CrossRef]
- Jeffrey, D.J.; Acrivos, A. The rheological properties of suspensions of rigid particles. AIChE J. 1976, 22, 417–432. [Google Scholar] [CrossRef]
- Graham, A.L. On the viscosity of suspensions of solid spheres. Flow Turbul. Combust. 1981, 37, 275–286. [Google Scholar] [CrossRef]
- Kitano, T.; Kataoka, T.; Shirota, T. An empirical equation of the relative viscosity of polymer melts filled with various inorganic fillers. Rheol. Acta 1981, 20, 207–209. [Google Scholar] [CrossRef]
- Bicerano, J.; Douglas, J.F.; Brune, D.A. Model for the Viscosity of Particle Dispersions. J. Macromol. Sci. Part C 1999, 39, 561–642. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Choi, S.U.S. Thermal Conductivity of Nanoparticle—Fluid Mixture. J. Thermophys. Heat Transf. 1999, 13, 474–480. [Google Scholar] [CrossRef]
- Bobbo, S.; Fedele, L.; Benetti, A.; Colla, L.; Fabrizio, M.; Pagura, C.; Barison, S. Viscosity of water based SWCNH and TiO2 nanofluids. Exp. Therm. Fluid Sci. 2012, 36, 65–71. [Google Scholar] [CrossRef]
- Esfe, M.H.; Saedodin, S.; Mahian, O.; Wongwises, S. Thermophysical properties, heat transfer and pressure drop of COOH-functionalized multi walled carbon nanotubes/water nanofluids. Int. Commun. Heat Mass Transf. 2014, 58, 176–183. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A.; Moravej, M.; Aberoumand, H.; Javaherdeh, K. Experimental study on the rheological behavior of silver-heat transfer oil nanofluid and suggesting two empirical based correlations for thermal conductivity and viscosity of oil based nanofluids. Appl. Therm. Eng. 2016, 101, 362–372. [Google Scholar] [CrossRef]
- Akbari, M.; Afrand, M.; Arshi, A.; Karimipour, A. An experimental study on rheological behavior of ethylene glycol based nanofluid: Proposing a new correlation as a function of silica concentration and temperature. J. Mol. Liq. 2017, 233, 352–357. [Google Scholar] [CrossRef]
- Esfe, M.H.; Raki, H.R.; Emami, M.R.S.; Afrand, M. Viscosity and rheological properties of antifreeze based nanofluid containing hybrid nano-powders of MWCNTs and TiO2 under different temperature conditions. Powder Technol. 2019, 342, 808–816. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Ciria, J.C.; Sanahuja-Parejo, O.; Víctor-Román, S.; González-Domínguez, J.M.; García-Bordejé, E.; Benito, A.M.; Maser, W.K. The viscosity of dilute carbon nanotube (1D) and graphene oxide (2D) nanofluids. Phys. Chem. Chem. Phys. 2020, 22, 11474–11484. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Tiwari, A.K.; Ghosh, S.K. Application of nanofluids in plate heat exchanger: A review. Energy Convers. Manag. 2015, 105, 1017–1036. [Google Scholar] [CrossRef]
- Duffie, J.A.; Beckman, W.A. Concentrating Collectors. In Solar Engineering of Thermal Processes, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 322–370. [Google Scholar]
- Fernández-García, A.; Zarza, E.; Valenzuela, L.; Pérez, M. Parabolic-trough solar collectors and their applications. Renew. Sustain. Energy Rev. 2010, 14, 1695–1721. [Google Scholar] [CrossRef]
- Lillo, I.; Pérez, E.; Moreno, S.; Silva, M. Process Heat Generation Potential from Solar Concentration Technologies in Latin America: The Case of Argentina. Energies 2017, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Kalogirou, S. Solar thermal collectors and applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Olia, H.; Torabi, M.; Bahiraei, M.; Ahmadi, M.H.; Goodarzi, M.; Safaei, M.R. Application of Nanofluids in Thermal Performance Enhancement of Parabolic Trough Solar Collector: State-of-the-Art. Appl. Sci. 2019, 9, 463. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Abid, M.; Ratlamwala, T.A.H. Energy, Exergy and Economic Feasibility Analyses of a 60 MW Conventional Steam Power Plant Integrated with Parabolic Trough Solar Collectors Using Nanofluids. Iran. J. Sci. Technol. Trans. Mech. Eng. 2018, 43, 193–209. [Google Scholar] [CrossRef]
- Abed, N.; Afgan, I.; Iacovides, H.; Cioncolini, A.; Khurshid, I.; Nasser, A. Thermal-Hydraulic Analysis of Parabolic Trough Collectors Using Straight Conical Strip Inserts with Nanofluids. Nanomaterials 2021, 11, 853. [Google Scholar] [CrossRef]
- Ebrazeh, S.; Sheikholeslami, M. Applications of nanomaterial for parabolic trough collector. Powder Technol. 2020, 375, 472–492. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daviran, S.; Azarian, R.D.; Rashidi, A. Performance evaluation and nanofluid using capability study of a solar parabolic trough collector. Energy Convers. Manag. 2015, 89, 368–375. [Google Scholar] [CrossRef]
- Kasaeian, A.; Daneshazarian, R.; Rezaei, R.; Pourfayaz, F.; Kasaeian, G. Experimental investigation on the thermal behavior of nanofluid direct absorption in a trough collector. J. Clean. Prod. 2017, 158, 276–284. [Google Scholar] [CrossRef]
- Mwesigye, A.; Yılmaz, İ.H.; Meyer, J.P. Numerical analysis of the thermal and thermodynamic performance of a parabolic trough solar collector using SWCNTs-Therminol®VP-1 nanofluid. Renew. Energy 2018, 119, 844–862. [Google Scholar] [CrossRef] [Green Version]
- Dugaria, S.; Bortolato, M.; Del Col, D. Modelling of a direct absorption solar receiver using carbon based nanofluids under concentrated solar radiation. Renew. Energy 2018, 128, 495–508. [Google Scholar] [CrossRef]
- Muhammad, M.; Che Sidik, N.A.; Umar, U.; Hamisu, M.; Sa’ad, A.; Malaysia, T.; Lumpur, K.; Sultan, J.; Petra, Y.; Semarak, J. Carbon Nanotube for Solar Energy Applications: A Review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 56, 233–247. [Google Scholar]
- Di Rosa, D.; Wanic, M.; Fal, J.; Żyła, G.; Mercatelli, L.; Sani, E. Optical and dielectric properties of ethylene glycol-based nanofluids containing nanodiamonds with various purities. Powder Technol. 2019, 356, 508–516. [Google Scholar] [CrossRef]
- Ho, M.; Obbard, E.; A Burr, P.; Yeoh, G. A review on the development of nuclear power reactors. Energy Procedia 2019, 160, 459–466. [Google Scholar] [CrossRef]
- Fernández-Arias, P.; Vergara, D.; Orosa, J.A. A Global Review of PWR Nuclear Power Plants. Appl. Sci. 2020, 10, 4434. [Google Scholar] [CrossRef]
- Rowinski, M.K.; White, T.; Zhao, J. Small and Medium sized Reactors (SMR): A review of technology. Renew. Sustain. Energy Rev. 2015, 44, 643–656. [Google Scholar] [CrossRef]
- Estrada-Domínguez, L.-A.; Espinosa-Paredes, G.; Nuñez-Carrera, A.; del Valle-Gallegos, E.; Vázquez-Rodriguez, R. Progress and utilization of small nuclear reactors. Energy Sources, Part A: Recover. Util. Environ. Eff. 2016, 38, 2362–2369. [Google Scholar] [CrossRef]
- Abu-Khader, M.M. Recent advances in nuclear power: A review. Prog. Nucl. Energy 2009, 51, 225–235. [Google Scholar] [CrossRef]
- Gu, Z. History review of nuclear reactor safety. Ann. Nucl. Energy 2018, 120, 682–690. [Google Scholar] [CrossRef]
- Buongiorno, J.; Hu, L.-W. Nanofluid heat transfer enhancement for nuclear reactor applications. In International Conference on Micro/Nanoscale Heat Transfer; ASME: Shanghai, China, 2009; pp. 517–522. [Google Scholar]
- Martin Callizo, C. Flow Boiling Heat Transfer in Single Vertical Channels of Small Diameter. Ph.D. Thesis, KTH, Brinellvägen, Stockholm, 2010. [Google Scholar]
- Beck, F.R.; Jin, Y.; Garrett, G.; Cheung, F.-B.; Bajorek, S.M.; Tien, K. Effect of Fouling on Quenching of Simulated Fuel Rods. Transactions 2019, 120, 1123–1125. [Google Scholar]
- Garrett, G.; Beck, F.R.; Jin, Y.; Cheung, F.B.; Bajorek, S.M.; Tien, K.; Hoxie, C. Effects of system parameters on the two-phase flow and heat transfer behavior in a rod bundle. In 4th Thermal and Fluids Engineering Conference; Begel House Inc.: Las Vegas, NV, USA, 2019; pp. 1383–1396. [Google Scholar]
- Mesquita, A.Z.; Rodrigues, R.R. Detection of the Departure from Nucleate Boiling in Nuclear Fuel Rod Simulators. Int. J. Nucl. Energy 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Kamel, M.S.; Lezsovits, F.; Hussein, A.K. Experimental studies of flow boiling heat transfer by using nanofluids. J. Therm. Anal. Calorim. 2019, 138, 4019–4043. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.S.; Kim, M.H. A Review on Critical Heat Flux Enhancement with Nanofluids and Surface Modification. J. Heat Transf. 2011, 134, 024001. [Google Scholar] [CrossRef]
- Dadhich, M.; Prajapati, O.S. A brief review on factors affecting flow and pool boiling. Renew. Sustain. Energy Rev. 2019, 112, 607–625. [Google Scholar] [CrossRef]
- Mukherjee, S.; Jana, S.; Mishra, P.C.; Chaudhuri, P.; Chakrabarty, S. Experimental investigation on thermo-physical properties and subcooled flow boiling performance of Al2O3/water nanofluids in a horizontal tube. Int. J. Therm. Sci. 2021, 159, 106581. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Abad, A.T.K. Statistical and experimental investigation on flow boiling heat transfer to carbon nanotube-therminol nanofluid. Phys. A Stat. Mech. Appl. 2019, 536, 122505. [Google Scholar] [CrossRef]
- Le Ba, T.; Alkurdi, A.Q.; Lukács, I.E.; Molnár, J.; Wongwises, S.; Gróf, G.; Szilágyi, I.M. A Novel Experimental Study on the Rheological Properties and Thermal Conductivity of Halloysite Nanofluids. Nanomaterials 2020, 10, 1834. [Google Scholar] [CrossRef] [PubMed]
- Murshed, S.M.S.; Nieto de Castro, C.A. Superior thermal features of carbon nanotubes-based nanofluids—A review. Renew. Sustain. Energy Rev. 2014, 37, 155–167. [Google Scholar] [CrossRef]
- Younes, H.; Al Ghaferi, A.; Saadat, I.; Hong, H. Nanofluids based on carbon nanostructures. In Advances in Carbon Nanostructures; IntechOpen: London, UK, 2016. [Google Scholar]
- Manthey, R.; Viereckl, F.; Shabestary, A.M.; Zhang, Y.; Ding, W.; Lucas, D.; Schuster, C.; Leyer, S.; Hurtado, A.; Hampel, U. Modelling of Passive Heat Removal Systems: A Review with Reference to the Framatome BWR Reactor KERENA: Part II. Energies 2020, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Hashemi, M.; Noie, S.H. Study of flow boiling heat transfer characteristics of critical heat flux using carbon nanotubes and water nanofluid. J. Therm. Anal. Calorim. 2017, 130, 2199–2209. [Google Scholar] [CrossRef]
- Hashemi, M.; Noie, S.H. Investigation of critical heat flux (CHF) enhancement in flow boiling using carbon nanotubes/water nanofluid. Transp. Phenom. Nano Micro. Scales 2020, 8, 81–88. [Google Scholar]
- Sarafraz, M.; Hormozi, F. Comparatively experimental study on the boiling thermal performance of metal oxide and multi-walled carbon nanotube nanofluids. Powder Technol. 2016, 287, 412–430. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, K.M.; Bang, I.C. Study on flow boiling critical heat flux enhancement of graphene oxide/water nanofluid. Int. J. Heat Mass Transf. 2013, 65, 348–356. [Google Scholar] [CrossRef]
- Park, S.D.; Bang, I.C. Flow boiling CHF enhancement in an external reactor vessel cooling (ERVC) channel using graphene oxide nanofluid. Nucl. Eng. Des. 2013, 265, 310–318. [Google Scholar] [CrossRef]
- Groeneveld, D.; Shan, J.; Vasić, A.; Leung, L.; Durmayaz, A.; Yang, J.; Cheng, S.; Tanase, A. The 2006 CHF look-up table. Nucl. Eng. Des. 2007, 237, 1909–1922. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, L.; Xu, H.; Wang, D.; Ye, B. Investigation of flow boiling performance and the resulting surface deposition of graphene oxide nanofluid in microchannels. Exp. Therm. Fluid Sci. 2017, 86, 1–10. [Google Scholar] [CrossRef]
- Mohammed, H.I.; Giddings, D.; Walker, G.S. Experimental investigation of nanoparticles concentration, boiler temperature and flow rate on flow boiling of zinc bromide and acetone solution in a rectangular duct. Int. J. Heat Mass Transf. 2019, 130, 710–721. [Google Scholar] [CrossRef]
- Kim, S.J.; McKrell, T.; Buongiorno, J.; Hu, L.-W. Experimental Study of Flow Critical Heat Flux in Alumina-Water, Zinc-Oxide-Water, and Diamond-Water Nanofluids. J. Heat Transf. 2009, 131, 043204. [Google Scholar] [CrossRef]
- Kim, S.J.; McKrell, T.; Buongiorno, J.; Hu, L.-W. Subcooled flow boiling heat transfer of dilute alumina, zinc oxide, and diamond nanofluids at atmospheric pressure. Nucl. Eng. Des. 2010, 240, 1186–1194. [Google Scholar] [CrossRef]
- DolatiAsl, K.; Bakhshan, Y.; Abedini, E.; Niazi, S. Correlations for estimating critical heat flux (CHF) of nanofluid flow boiling. Int. J. Heat Mass Transf. 2019, 139, 69–76. [Google Scholar] [CrossRef]
- Incropera, F.P.; Dewitt, D.P.; Bergman, T.; Lavine, A. Fundamentals of Mass and Heat Transfer; John Wiley and Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Peterson, L.J.; Bajorek, S.M. Experimental Investigation of Minimum Film Boiling Temperature for Vertical Cylinders at Elevated Pressure. In 10th International Conference on Nuclear Engineering; ASME: Arlington, VA, USA, 2002; pp. 883–892. [Google Scholar]
- Sinha, J. Effects of surface roughness, oxidation level, and liquid subcooling on the minimum film boiling temperature. Exp. Heat Transf. 2003, 16, 45–60. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chun, T.H.; In, W.K. Effect of change in surface condition induced by oxidation on transient pool boiling heat transfer of vertical stainless steel and copper rodlets. Int. J. Heat Mass Transf. 2014, 79, 397–407. [Google Scholar] [CrossRef]
- Henry, R. A correlation for the minimum film boiling temperature. AIChE Symp. Ser. 1974, 70, 81–90. [Google Scholar]
- Bahman, A.M.; Ebrahim, S.A. Prediction of the minimum film boiling temperature using artificial neural network. Int. J. Heat Mass Transf. 2020, 155, 119834. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Kim, T.K.; Lee, G.C.; Jo, H.; Kim, M.H.; Park, H.S. Impact of system parameters on quenching heat transfer in the candidate materials for accident tolerant fuel-cladding in LWRs. Ann. Nucl. Energy 2019, 129, 375–389. [Google Scholar] [CrossRef]
- Sakurai, A.; Shiotsu, M.; Hata, K. Transient boiling caused by vapor film collapse at minimum heat flux in film boiling. Nucl. Eng. Des. 1987, 99, 167–175. [Google Scholar] [CrossRef]
- Jun-Young, K.; Cheol, L.G.; Kaviany, M.; Sun, P.H.; Moriyama, K.; Hwan, K.M. Control of minimum film-boiling quench temperature of small spheres with micro-structured surface. Int. J. Multiph. Flow 2018, 103, 30–42. [Google Scholar] [CrossRef]
- Adler, M.R. The Influence of Water Purity and Subcooling on the Minimum Film Boiling Temperature; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 1979. [Google Scholar]
- Freud, R.; Harari, R.; Sher, E. Collapsing criteria for vapor film around solid spheres as a fundamental stage leading to vapor explosion. Nucl. Eng. Des. 2009, 239, 722–727. [Google Scholar] [CrossRef]
- Ebrahim, S.A.; Alat, E.; Sohag, F.A.; Fudurich, V.; Chang, S.; Cheung, F.-B.; Bajorek, S.M.; Tien, K.; Hoxie, C.L. Effects of Substrate Materials and Surface Conditions on the Minimum Film-Boiling Temperature. Nucl. Technol. 2018, 205, 226–238. [Google Scholar] [CrossRef]
- Ebrahim, S.A.; Chang, S.; Cheung, F.-B.; Bajorek, S.M. Parametric investigation of film boiling heat transfer on the quenching of vertical rods in water pool. Appl. Therm. Eng. 2018, 140, 139–146. [Google Scholar] [CrossRef]
- Carey, V.P. Liquid-Vapor Phase-Change Phenomena: An Introduction to the Thermophysics of Vaporization and Condensation Processes in Heat Transfer Equipment; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Shoji, M.; Witte, L.C.; Sankaran, S. The influence of surface conditions and subcooling on film-transition boiling. Exp. Therm. Fluid Sci. 1990, 3, 280–290. [Google Scholar] [CrossRef]
- Lee, C.Y.; Kim, S. Parametric investigation on transient boiling heat transfer of metal rod cooled rapidly in water pool. Nucl. Eng. Des. 2017, 313, 118–128. [Google Scholar] [CrossRef]
- Khan, A.; Ali, H.M. A comprehensive review on pool boiling heat transfer using nanofluids. Therm. Sci. 2019, 23, 3209–3237. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of pool boiling enhancement with additives and nanofluids. Int. J. Heat Mass Transf. 2018, 124, 423–453. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S. Parametric study of pool boiling heat transfer with nanofluids for the enhancement of critical heat flux: A review. Int. J. Therm. Sci. 2015, 87, 228–240. [Google Scholar] [CrossRef]
- Yazid, M.N.A.W.M.; Sidik, N.A.C.; Yahya, W.J. Heat and mass transfer characteristics of carbon nanotube nanofluids: A review. Renew. Sustain. Energy Rev. 2017, 80, 914–941. [Google Scholar] [CrossRef]
- Barber, J.; Brutin, D.; Tadrist, L. A review on boiling heat transfer enhancement with nanofluids. Nanoscale Res. Lett. 2011, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- Bang, I.C.; Chang, S.H. Boiling heat transfer performance and phenomena of Al2O3–water nano-fluids from a plain surface in a pool. Int. J. Heat Mass Transf. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Pham, Q.; Kim, T.; Lee, S.; Chang, S. Enhancement of critical heat flux using nano-fluids for Invessel Retention–External Vessel Cooling. Appl. Therm. Eng. 2012, 35, 157–165. [Google Scholar] [CrossRef]
- Sakashita, H. CHF and near-wall boiling behaviors in pool boiling of water on a heating surface coated with nanoparticles. Int. J. Heat Mass Transf. 2012, 55, 7312–7320. [Google Scholar] [CrossRef] [Green Version]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Characterization and Pool Boiling Heat Transfer Studies of Nanofluids. J. Heat Transf. 2009, 131, 081902. [Google Scholar] [CrossRef]
- Coursey, J.S.; Kim, J. Nanofluid boiling: The effect of surface wettability. Int. J. Heat Fluid Flow 2008, 29, 1577–1585. [Google Scholar] [CrossRef]
- Kim, S.; Bang, I.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Kwark, S.M.; Kumar, R.; Moreno, G.; Yoo, J.; You, S.M. Pool boiling characteristics of low concentration nanofluids. Int. J. Heat Mass Transf. 2010, 53, 972–981. [Google Scholar] [CrossRef]
- Yang, X.-F.; Liu, Z.-H. Pool boiling heat transfer of functionalized nanofluid under sub-atmospheric pressures. Int. J. Therm. Sci. 2011, 50, 2402–2412. [Google Scholar] [CrossRef]
- Okawa, T.; Takamura, M.; Kamiya, T. Boiling time effect on CHF enhancement in pool boiling of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 2719–2725. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Xiong, J.-G.; Bao, R. Boiling heat transfer characteristics of nanofluids in a flat heat pipe evaporator with micro-grooved heating surface. Int. J. Multiph. Flow 2007, 33, 1284–1295. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F.; Silakhori, M.; Peyghambarzadeh, S. On the fouling formation of functionalized and non-functionalized carbon nanotube nano-fluids under pool boiling condition. Appl. Therm. Eng. 2016, 95, 433–444. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, D.; Shim, S.E. Nucleate boiling heat transfer in aqueous solutions with carbon nanotubes up to critical heat fluxes. Int. J. Multiph. Flow 2009, 35, 525–532. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Yang, X.-F.; Xiong, J.-G. Boiling characteristics of carbon nanotube suspensions under sub-atmospheric pressures. Int. J. Therm. Sci. 2010, 49, 1156–1164. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, D. Enhancement of nucleate boiling heat transfer using carbon nanotubes. Int. J. Heat Mass Transf. 2007, 50, 4499–4502. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Bahrami, M.; Moraveji, M.K. Experimental investigation and CFD modeling of the dynamics of bubbles in nanofluid pool boiling. Int. Commun. Heat Mass Transf. 2014, 58, 12–24. [Google Scholar] [CrossRef]
- Sarafraz, M.; Hormozi, F. Experimental investigation on the pool boiling heat transfer to aqueous multi-walled carbon nanotube nanofluids on the micro-finned surfaces. Int. J. Therm. Sci. 2016, 100, 255–266. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Amiri, H.; Heris, S.Z.; Kazi, S.; Chew, B.; Eshghi, H. Pool boiling heat transfer of CNT/water nanofluids. Appl. Therm. Eng. 2014, 71, 450–459. [Google Scholar] [CrossRef]
- Park, S.-S.; Kim, N.-J. Critical heat flux enhancement in pool-boiling heat transfer using oxidized multi-wall carbon nanotubes. Int. J. Energy Res. 2015, 39, 1391–1401. [Google Scholar] [CrossRef]
- Kumar, R.; Milanova, D. Effect of surface tension on nanotube nanofluids. Appl. Phys. Lett. 2009, 94, 073107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Fan, L.; Yu, Z.; Cen, K. An experimental investigation of transient pool boiling of aqueous nanofluids with graphene oxide nanosheets as characterized by the quenching method. Int. J. Heat Mass Transf. 2014, 73, 410–414. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, J.M.; Kim, M.H. Experimental study of the effect of a reduced graphene oxide coating on critical heat flux enhancement. Int. J. Heat Mass Transf. 2013, 60, 763–771. [Google Scholar] [CrossRef]
- Park, S.D.; Won Lee, S.; Kang, S.; Bang, I.C.; Kim, J.H.; Shin, H.S.; Lee, D.W.; Won Lee, D. Effects of nanofluids containing graphene/graphene-oxide nanosheets on critical heat flux. Appl. Phys. Lett. 2010, 97, 023103. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Kim, T.; Kim, J.; Kim, M.H.; Ahn, H.S. Effect of a graphene oxide coating layer on critical heat flux enhancement under pool boiling. Int. J. Heat Mass Transf. 2014, 77, 919–927. [Google Scholar] [CrossRef]
- Park, S.D.; Bang, I.C. Experimental study of a universal CHF enhancement mechanism in nanofluids using hydrodynamic instability. Int. J. Heat Mass Transf. 2014, 70, 844–850. [Google Scholar] [CrossRef]
- Truong, B.; Hu, L.-W.; Buongiorno, J.; McKrell, T. Modification of sandblasted plate heaters using nanofluids to enhance pool boiling critical heat flux. Int. J. Heat Mass Transf. 2010, 53, 85–94. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R.; Jain, P.K. Pool Boiling Characteristics of Carbon Nanotube Based Nanofluids Over a Horizontal Tube. J. Therm. Sci. Eng. Appl. 2009, 1, 022001. [Google Scholar] [CrossRef]
- Jaikumar, A.; Kandlikar, S.G.; Gupta, A. Pool Boiling Enhancement through Graphene and Graphene Oxide Coatings. Heat Transf. Eng. 2016, 38, 1274–1284. [Google Scholar] [CrossRef]
- Gerardi, C.D. Investigation of the Pool Boiling Heat Transfer Enhancement of Nano-Engineered Fluids by Means of High-Speed Infrared Thermography. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2009. [Google Scholar]
- Fan, L.-W.; Li, J.-Q.; Wu, Y.-Z.; Zhang, L.; Yu, Z.-T. Pool boiling heat transfer during quenching in carbon nanotube (CNT)-based aqueous nanofluids: Effects of length and diameter of the CNTs. Appl. Therm. Eng. 2017, 122, 555–565. [Google Scholar] [CrossRef]
- Fan, L.-W.; Li, J.-Q.; Li, D.-Y.; Zhang, L.; Yu, Z.-T.; Cen, K.-F. The effect of concentration on transient pool boiling heat transfer of graphene-based aqueous nanofluids. Int. J. Therm. Sci. 2015, 91, 83–95. [Google Scholar] [CrossRef]
- Kim, H.; DeWitt, G.; McKrell, T.; Buongiorno, J.; Hu, L.-W. On the quenching of steel and zircaloy spheres in water-based nanofluids with alumina, silica and diamond nanoparticles. Int. J. Multiph. Flow 2009, 35, 427–438. [Google Scholar] [CrossRef]
- Wang, S.K. Handbook of Air Conditioning and Refrigeration, 2nd ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Pérez-Tello, C.; Campbell-Ramírez, H.; Suástegui-Macías, J.A.; Reinhardt, M.S. Methodology of Energy Management in Housing and Buildings of Regions with Hot and Dry Climates; IntechOpen: London, UK, 2018; pp. 13–29. [Google Scholar]
- Said, S.A.; El-Shaarawi, M.A.; Siddiqui, M.U. Alternative designs for a 24-h operating solar-powered absorption refrigeration technology. Int. J. Refrig. 2012, 35, 1967–1977. [Google Scholar] [CrossRef]
- Veera Raghavalu, K.; Govindha Rasu, N. Review on Applications of NanoFluids used in Vapour Compression Refrigeration System for Cop Enhancement. IOP Conf. Series: Mater. Sci. Eng. 2018, 330, 012112. [Google Scholar] [CrossRef]
- Nair, V.; Tailor, P.; Parekh, A. Nanorefrigerants: A comprehensive review on its past, present and future. Int. J. Refrig. 2016, 67, 290–307. [Google Scholar] [CrossRef]
- Bandgar, M.; Ragit, S.; Kolhe, K.; Biradar, N. Effect of Nano Lubricant on the Performance of Vapour Compression Refrigeration System: A Review. J. Emerg. Technol. Innov. Res. 2016, 3, 56–59. [Google Scholar]
- Azmi, W.; Sharif, M.; Yusof, T.; Mamat, R.; Redhwan, A. Potential of nanorefrigerant and nanolubricant on energy saving in refrigeration system—A review. Renew. Sustain. Energy Rev. 2017, 69, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Alawi, O.A.; Salih, J.M.; Mallah, A. Thermo-physical properties effectiveness on the coefficient of performance of Al2O3/R141b nano-refrigerant. Int. Commun. Heat Mass Transf. 2019, 103, 54–61. [Google Scholar] [CrossRef]
- Jwo, C.-S.; Jeng, L.-Y.; Teng, T.-P.; Chang, H. Effects of nanolubricant on performance of hydrocarbon refrigerant system. J. Vac. Sci. Technol. B: Microelectron. Nanometer Struct. 2009, 27, 1473. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Hwang, Y.; Lee, C.; Kim, S.H. Enhancement of Lubrication Properties of Nano-oil by Controlling the Amount of Fullerene Nanoparticle Additives. Tribol. Lett. 2007, 28, 203–208. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Rezaeianjouybari, B.; Darzi, M.; Shafee, A.; Li, Z.; Nguyen, T.K. Application of nano-refrigerant for boiling heat transfer enhancement employing an experimental study. Int. J. Heat Mass Transf. 2019, 141, 974–980. [Google Scholar] [CrossRef]
- Krishnan, R.S.; Arulprakasajothi, M.; Logesh, K.; Raja, N.D.; Rajendra, M. Analysis and Feasibilty of Nano-Lubricant in Vapour Compression Refrigeration System. Mater. Today Proc. 2018, 5, 20580–20587. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, Y.; Cheong, S.; Kwon, L.; Kim, S.; Lee, J. Performance evaluation of nano-lubricants of fullerene nanoparticles in refrigeration mineral oil. Curr. Appl. Phys. 2009, 9, e128–e131. [Google Scholar] [CrossRef]
- Lee, K.; Hwang, Y.; Cheong, S.; Choi, Y.; Kwon, L.; Lee, J.; Kim, S.H. Understanding the Role of Nanoparticles in Nano-oil Lubrication. Tribol. Lett. 2009, 35, 127–131. [Google Scholar] [CrossRef]
- Jia, T.; Wang, R.; Xu, R. Performance of MoFe2O4–NiFe2O4/Fullerene-added nano-oil applied in the domestic refrigerator compressors. Int. J. Refrig. 2014, 45, 120–127. [Google Scholar] [CrossRef]
- Saidur, R.; Kazi, S.; Hossain, M.; Rahman, M.; Mohammed, H. A review on the performance of nanoparticles suspended with refrigerants and lubricating oils in refrigeration systems. Renew. Sustain. Energy Rev. 2011, 15, 310–323. [Google Scholar] [CrossRef]
- Kasaeian, A.; Hosseini, S.M.; Sheikhpour, M.; Mahian, O.; Yan, W.-M.; Wongwises, S. Applications of eco-friendly refrigerants and nanorefrigerants: A review. Renew. Sustain. Energy Rev. 2018, 96, 91–99. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C.; Mohammed, H. A comprehensive review of fundamentals, preparation and applications of nanorefrigerants. Int. Commun. Heat Mass Transf. 2014, 54, 81–95. [Google Scholar] [CrossRef]
- Sun, B.; Yang, D. Flow boiling heat transfer characteristics of nano-refrigerants in a horizontal tube. Int. J. Refrig. 2014, 38, 206–214. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.; Xu, Z.; Huang, H.; Liu, Z.; Liu, C.; Long, X.; Ge, Z. Measurement and modeling of the thermal conductivity of nanorefrigerants with low volume concentrations. Thermochim. Acta 2020, 688, 178603. [Google Scholar] [CrossRef]
- Selimefendigil, F. Experimental investigation of nano compressor oil effect on the cooling performance of a vapor-compression refrigeration system. J. Therm. Eng. 2019, 5, 100–104. [Google Scholar] [CrossRef]
- Celen, A.; Çebi, A.; Aktas, M.; Mahian, O.; Dalkilic, A.S.; Wongwises, S. A review of nanorefrigerants: Flow characteristics and applications. Int. J. Refrig. 2014, 44, 125–140. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Ramana, E.V.; Singh, B.K.; Grácio, J.; Sousa, A. Enhanced Thermal Conductivity and Viscosity of Nanodiamond-Nickel Nanocomposite Nanofluids. Sci. Rep. 2014, 4, 4039. [Google Scholar] [CrossRef] [Green Version]
- Sundar, L.S.; Ramana, E.V.; Graca, M.P.; Singh, M.K.; Sousa, A. Nanodiamond-Fe 3 O 4 nanofluids: Preparation and measurement of viscosity, electrical and thermal conductivities. Int. Commun. Heat Mass Transf. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Improving the performance of refrigeration systems by using nanofluids: A comprehensive review. Renew. Sustain. Energy Rev. 2018, 82, 3656–3669. [Google Scholar] [CrossRef]
- Liu, M.; Lin, M.C.; Wang, C. Enhancements of thermal conductivities with Cu, CuO, and carbon nanotube nanofluids and application of MWNT/water nanofluid on a water chiller system. Nanoscale Res. Lett. 2011, 6, 297. [Google Scholar] [CrossRef] [Green Version]
- Maheshwary, P.B.; Handa, C.C.; Nemade, K.R. Preparation of nanorefrigerants using mono-, bi- and tri-layer graphene nanosheets in R134a refrigerant. AIP Conf. Proc. 2019, 2104, 020017. [Google Scholar] [CrossRef]
- Cantuario, T.E.; Fonseca, A.F. High Performance of Carbon Nanotube Refrigerators. Ann. der Phys. 2019, 531, 1800502. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, W.; Ji, W.; Mahian, O.; Bazri, S.; Sadri, R.; Badruddin, I.A.; Wongwises, S. A review of heating/cooling processes using nanomaterials suspended in refrigerants and lubricants. Int. J. Heat Mass Transf. 2020, 153, 119611. [Google Scholar] [CrossRef]
- Molana, M.; Wang, H. A critical review on numerical study of nanorefrigerant heat transfer enhancement. Powder Technol. 2020, 368, 18–31. [Google Scholar] [CrossRef]
- Hamisa, A.H.; Yusof, T.M.; Azmi, W.H.; Mamat, R.; Sharif, M. The stability of TiO2/POE nanolubricant for automotive air-conditioning system of hybrid electric vehicles. IOP Conf. Series: Mater. Sci. Eng. 2020, 863, 012050. [Google Scholar] [CrossRef]
- Nair, V.; Parekh, A.D.; Tailor, P.R. Performance analysis of Al2O3–R718 nanorefrigerant turbulent flow through a flooded chiller tube: A numerical investigation. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Safaei, M.R.; Ranjbarzadeh, R.; Hajizadeh, A.; Bahiraei, M.; Afrand, M.; Karimipour, A. Effects of cobalt ferrite coated with silica nanocomposite on the thermal conductivity of an antifreeze: New nanofluid for refrigeration condensers. Int. J. Refrig. 2019, 102, 86–95. [Google Scholar] [CrossRef]
- Aktas, M.; Dalkilic, A.S.; Celen, A.; Cebi, A.; Mahian, O.; Wongwises, S. A Theoretical Comparative Study on Nanorefrigerant Performance in a Single-Stage Vapor-Compression Refrigeration Cycle. Adv. Mech. Eng. 2014, 7, 138725. [Google Scholar] [CrossRef] [Green Version]
- Adelekan, D.S.; Ohunakin, O.; Babarinde, T.O.; Odunfa, M.K.; Leramo, R.O.; Oyedepo, S.O.; Badejo, D.C. Experimental performance of LPG refrigerant charges with varied concentration of TiO 2 nano-lubricants in a domestic refrigerator. Case Stud. Therm. Eng. 2017, 9, 55–61. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, D.K.; Chander, S. An experimental approach to study thermal and tribology behavior of LPG refrigerant and MO lubricant appended with ZnO nanoparticles in domestic refrigeration cycle. Heat Mass Transf. 2020, 56, 2303–2311. [Google Scholar] [CrossRef]
- Krishna Sabareesh, R.; Gobinath, N.; Sajith, V.; Das, S.; Sobhan, C.B. Application of TiO2 nanoparticles as a lubricant-additive for vapor compression refrigeration systems—An experimental investigation. Int. J. Refrig. 2012, 35, 1989–1996. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Q.; Wu, Y. Use of nanoparticles to make mineral oil lubricants feasible for use in a residential air conditioner employing hydro-fluorocarbons refrigerants. Energy Build. 2010, 42, 2111–2117. [Google Scholar] [CrossRef]
- Coumaressin, T.; Palaniradja, K. Performance analysis of a refrigeration system using nano fluid. Int. J. Adv. Mech. Eng. 2014, 4, 459–470. [Google Scholar]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Babarinde, T.O.; Akinlabi, S.A.; Madyira, D.M. Enhancing the Performance of Vapour Compression Refrigeration System using Nano Refrigerants: A review. IOP Conf. Series: Mater. Sci. Eng. 2018, 413, 012068. [Google Scholar] [CrossRef] [Green Version]
- Akhavan-Behabadi, M.A.; Sadoughi, M.K.; Darzi, M.; Fakoor-Pakdaman, M. Experimental study on heat transfer characteristics of R600a/POE/CuO nano-refrigerant flow condensation. Exp. Therm. Fluid Sci. 2015, 66, 46–52. [Google Scholar] [CrossRef]
- Ajayi, O.O.; Useh, O.O.; Banjo, S.O.; Oweoye, F.T.; Attabo, A.; Ogbonnaya, M.; Okokpujie, I.P.; Salawu, E.Y. Investigation of the heat transfer effect of Ni/R134a nanorefrigerant in a mobile hybrid powered vapour compression refrigerator. IOP Conf. Series: Mater. Sci. Eng. 2018, 391, 012001. [Google Scholar] [CrossRef]
- Bhutta, M.U.; Khan, Z.A. Wear and friction performance evaluation of nickel based nanocomposite coatings under refrigerant lubrication. Tribol. Int. 2020, 148, 106312. [Google Scholar] [CrossRef]
- Park, K.-J.; Jung, D. Boiling heat transfer enhancement with carbon nanotubes for refrigerants used in building air-conditioning. Energy Build. 2007, 39, 1061–1064. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Ge, Z.; Wang, H.T. Characteristics of Flow Boiling Heat Transfer and Pressure Drop of MWCNT–R123 Nanorefrigerant: Experimental Investigations and Correlations. Nanoscale Microscale Thermophys. Eng. 2016, 20, 97–120. [Google Scholar] [CrossRef]
- Sun, B.; Wang, H.; Yang, D. Effects of surface functionalization on the flow boiling heat transfer characteristics of MWCNT/R141b nanorefrigerants in smooth tube. Exp. Therm. Fluid Sci. 2018, 92, 162–173. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, G.; Peng, H. Measurement and model on thermal conductivities of carbon nanotube nanorefrigerants. Int. J. Therm. Sci. 2009, 48, 1108–1115. [Google Scholar] [CrossRef]
- Peng, H.; Ding, G.; Hu, H.; Jiang, W. Influence of carbon nanotubes on nucleate pool boiling heat transfer characteristics of refrigerant–oil mixture. Int. J. Therm. Sci. 2010, 49, 2428–2438. [Google Scholar] [CrossRef]
- Ahmadpour, M.; Akhavan-Behabadi, M.A.; Sajadi, B.; Salehi-Kohestani, A. Experimental study of R600a/oil/MWCNT nano-refrigerant condensing flow inside micro-fin tubes. Heat Mass Transf. 2020, 56, 749–757. [Google Scholar] [CrossRef]
- Kumaresan, V.; Velraj, R.; Das, S.K. Convective heat transfer characteristics of secondary refrigerant based CNT nanofluids in a tubular heat exchanger. Int. J. Refrig. 2012, 35, 2287–2296. [Google Scholar] [CrossRef]
- Asadi, M.; Asadi, A.; Aberoumand, S. An experimental and theoretical investigation on the effects of adding hybrid nanoparticles on heat transfer efficiency and pumping power of an oil-based nanofluid as a coolant fluid. Int. J. Refrig. 2018, 89, 83–92. [Google Scholar] [CrossRef] [Green Version]
- Baskar, S.; Chandrasekaran, M.; Kumar, T.V.; Vivek, P.; Ramasubramanian, S. Experimental studies on flow and heat transfer characteristics of secondary refrigerant-based CNT nanofluids for cooling applications. Int. J. Ambient. Energy 2018, 41, 285–288. [Google Scholar] [CrossRef]
- Wang, Z.; Han, F.; Ji, Y.; Li, W. Performance and Exergy Transfer Analysis of Heat Exchangers with Graphene Nanofluids in Seawater Source Marine Heat Pump System. Energies 2020, 13, 1762. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Peng, H.; Ding, G. Dispersion stability of multi-walled carbon nanotubes in refrigerant with addition of surfactant. Appl. Therm. Eng. 2015, 91, 163–171. [Google Scholar] [CrossRef]
- Alawi, O.A.; Sidik, N.A.C. The effect of temperature and particles concentration on the determination of thermo and physical properties of SWCNT-nanorefrigerant. Int. Commun. Heat Mass Transf. 2015, 67, 8–13. [Google Scholar] [CrossRef]
- Dalkilic, A.S.; Mahian, O.; Kucukyildirim, B.O.; Eker, A.A.; Ozturk, T.H.; Jumpholkul, C.; Wongwises, S. Experimental Study on the Stability and Viscosity for the Blends of Functionalized MWCNTs with Refrigeration Compressor Oils. Curr. Nanosci. 2018, 14, 216–226. [Google Scholar] [CrossRef]
- Patil, M.S.; Kim, S.C.; Seo, J.-H.; Lee, M.-Y. Review of the Thermo-Physical Properties and Performance Characteristics of a Refrigeration System Using Refrigerant-Based Nanofluids. Energies 2016, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Walvekar, R.G.; Hajibeigy, M.T.; Javadi, F.S. Efficient air-condition unit by using nano-refrigerant. In Proceedings of the 1st Engineering Undergraduate Research Catalyst Conference, Selangor, Malaysia, 1–2 July 2013. [Google Scholar]
- Jalili, B.; Ghafoori, H.; Jalili, P. Investigation of carbon nano-tube (CNT) particles effect on the performance of a refrigeration cycle. Int. J. Mater. Sci. Innov. 2014, 2, 8–17. [Google Scholar]
- Kruse, H.; Schroeder, M. Fundamentals of lubrication in refrigerating systems and heat pumps. Int. J. Refrig. 1985, 8, 347–355. [Google Scholar] [CrossRef]
- Cremaschi, L. A fundamental view of the flow boiling heat transfer characteristics of nano-refrigerants. In ASME International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers: Houston, TX, USA, 2012; pp. 2779–2792. [Google Scholar]
- Vasconcelos, A.A.; Gómez, A.O.C.; Filho, E.P.B.; Parise, J.A.R. Experimental evaluation of SWCNT-water nanofluid as a secondary fluid in a refrigeration system. Appl. Therm. Eng. 2017, 111, 1487–1492. [Google Scholar] [CrossRef]
- Kamaraj, N.; Manoj Babu, A. Experimental analysis of Vapour Compression Refrigeration System using the refrigerant with Nano particles. In International Conference on Engineering Innovations and Solutions (ICEIS) 2016; Seventh Sense Research Group: Tamilnadu, India, 2016; pp. 16–25. [Google Scholar]
- Yang, S.; Cui, X.; Zhou, Y.; Chen, C. Study on the effect of graphene nanosheets refrigerant oil on domestic refrigerator performance. Int. J. Refrig. 2020, 110, 187–195. [Google Scholar] [CrossRef]
- Pico, D.F.M.; da Silva, L.R.R.; Schneider, P.; Filho, E.P.B. Performance evaluation of diamond nanolubricants applied to a refrigeration system. Int. J. Refrig. 2019, 100, 104–112. [Google Scholar] [CrossRef]
- Pico, D.F.M.; da Silva, L.R.R.; Mendoza, O.S.H.; Filho, E.P.B. Experimental study on thermal and tribological performance of diamond nanolubricants applied to a refrigeration system using R32. Int. J. Heat Mass Transf. 2020, 152, 119493. [Google Scholar] [CrossRef]
- Rahman, S.; Issa, S.; Said, Z.; El Haj Assad, M.; Zadeh, R.; Barani, Y. Performance enhancement of a solar powered air conditioning system using passive techniques and SWCNT /R-407c nano refrigerant. Case Stud. Therm. Eng. 2019, 16, 100565. [Google Scholar] [CrossRef]
- Mahian, O.; Bellos, E.; Markides, C.N.; Taylor, R.A.; Alagumalai, A.; Yang, L.; Qin, C.; Lee, B.J.; Ahmadi, G.; Safaei, M.R.; et al. Recent advances in using nanofluids in renewable energy systems and the environmental implications of their uptake. Nano Energy 2021, 86, 106069. [Google Scholar] [CrossRef]
- Meyer, L.; Tsatsaronis, G.; Buchgeister, J.; Schebek, L. Exergoenvironmental analysis for evaluation of the environmental impact of energy conversion systems. Energy 2009, 34, 75–89. [Google Scholar] [CrossRef]
- Bertoldo, R.; Mays, C.; Poumadère, M.; Schneider, N.; Svendsen, C. Great deeds or great risks? Scientists’ social representations of nanotechnology. J. Risk Res. 2016, 19, 760–779. [Google Scholar]
- Card, J.W.; Magnuson, B.A. A method to assess the quality of studies that examine the toxicity of engineered nanomaterials. Int. J. Toxicol. 2010, 29, 402–410. [Google Scholar] [CrossRef]
- Faizal, M.; Saidur, R.; Mekhilef, S.; Alim, M. Energy, economic and environmental analysis of metal oxides nanofluid for flat-plate solar collector. Energy Convers. Manag. 2013, 76, 162–168. [Google Scholar] [CrossRef]
- Michael Joseph Stalin, P.; Arjunan, T.V.; Matheswaran, M.M.; Dolli, H.; Sadanandam, N. Energy, economic and environmental investigation of a flat plate solar collector with CeO2/water nanofluid. J. Therm. Anal. Calorim. 2020, 139, 3219–3233. [Google Scholar] [CrossRef]
- Hassani, S.; Saidur, R.; Mekhilef, S.; Taylor, R.A. Environmental and exergy benefit of nanofluid-based hybrid PV/T systems. Energy Convers. Manag. 2016, 123, 431–444. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Gróf, G.; Abu-Nada, E.; Mahian, O. Evacuated tube solar collector performance using copper nanofluid: Energy and environmental analysis. Appl. Therm. Eng. 2019, 162, 114205. [Google Scholar] [CrossRef]
- Boyaghchi, F.A.; Chavoshi, M.; Sabeti, V. Optimization of a novel combined cooling, heating and power cycle driven by geothermal and solar energies using the water/CuO (copper oxide) nanofluid. Energy 2015, 91, 685–699. [Google Scholar] [CrossRef]
- Boyaghchi, F.A.; Chavoshi, M. Multi-criteria optimization of a micro solar-geothermal CCHP system applying water/CuO nanofluid based on exergy, exergoeconomic and exergoenvironmental concepts. Appl. Therm. Eng. 2017, 112, 660–675. [Google Scholar] [CrossRef]
- Sahota, L.; Shyam; Tiwari, G. Energy matrices, enviroeconomic and exergoeconomic analysis of passive double slope solar still with water based nanofluids. Desalination 2017, 409, 66–79. [Google Scholar] [CrossRef]
- Grosu, Y.; Anagnostopoulos, A.; Balakin, B.; Krupanek, J.; Navarro, M.E.; González-Fernández, L.; Ding, Y.; Faik, A. Nanofluids based on molten carbonate salts for high-temperature thermal energy storage: Thermophysical properties, stability, compatibility and life cycle analysis. Sol. Energy Mater. Sol. Cells 2021, 220, 110838. [Google Scholar] [CrossRef]
- Sahota, L.; Tiwari, G. Exergoeconomic and enviroeconomic analyses of hybrid double slope solar still loaded with nanofluids. Energy Convers. Manag. 2017, 148, 413–430. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Bahadar, H.; Maqbool, F.; Niaz, K.; Abdollahi, M. Toxicity of Nanoparticles and an Overview of Current Experimental Models. Iran. Biomed. J. 2016, 20, 1–11. [Google Scholar]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Khlebtsov, N.G.; Dykman, L.A. Optical properties and biomedical applications of plasmonic nanoparticles. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1–35. [Google Scholar] [CrossRef]
- Ali, N. Assessment of Using 99Mo and 99mTc Isotopes in Kuwait Medical Sector. Heal. Phys. 2016, 110, 387–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, V.; Samal, S.S.; Manoharan, N. Safety and Risk Associated with Nanoparticles—A Review. J. Miner. Mater. Charact. Eng. 2010, 9, 455–459. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Schaeublin, N.M.; Braydich-Stolle, L.K.; Maurer, E.I.; Park, K.; MacCuspie, R.; Afrooz, A.; Vaia, R.A.; Saleh, N.B.; Hussain, S.M. Does Shape Matter? Bioeffects of Gold Nanomaterials in a Human Skin Cell Model. Langmuir 2012, 28, 3248–3258. [Google Scholar] [CrossRef]
- Cancino-Bernardi, J.; Paino, I.; Souza, J.; Marangoni, V.; Nogueira, P.; Zucolotto, V. Current Challenges in the Commercialization of Nanocolloids: Toxicology and Environmental Issues. In Nanocolloids; Elsevier: Amsterdam, The Netherlands, 2016; pp. 427–463. [Google Scholar]
- Ferreira, A.; Cemlyn-Jones, J.; Cordeiro, C.R. Nanoparticles, Nanotechnology and Pulmonary Nanotoxicology. Rev. Port. de Pneumol. (Engl. Ed.) 2013, 19, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Jabbal, M.; Yan, Y.; Reay, D. A review on graphene based nanofluids: Preparation, characterization and applications. J. Mol. Liq. 2019, 279, 444–484. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.-Q.; Zhao, M.-Q.; Qian, W.-Z.; Wei, F. Carbon Nanotube Mass Production: Principles and Processes. ChemSusChem 2011, 4, 864–889. [Google Scholar] [CrossRef]
- Syam Sundar, L.; Sousa, A.; Singh, M.K. Heat transfer enhancement of low volume concentration of carbon nanotube-Fe3O4/water hybrid nanofluids in a tube with twisted tape inserts under turbulent flow. J. Therm. Sci. Eng. Appl. 2015, 7. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A. Enhanced heat transfer and friction factor of MWCNT–Fe3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Z.; Sunden, B. Effects of hybrid nanofluid mixture in plate heat exchangers. Exp. Therm. Fluid Sci. 2016, 72, 190–196. [Google Scholar] [CrossRef]
- Leong, K.; Ahmad, K.Z.K.; Ong, H.C.; Ghazali, M.; Baharum, A. Synthesis and thermal conductivity characteristic of hybrid nanofluids—A review. Renew. Sustain. Energy Rev. 2017, 75, 868–878. [Google Scholar] [CrossRef]
- Maddah, H.; Aghayari, R.; Ahmadi, M.H.; Rahimzadeh, M.; Ghasemi, N. Prediction and modeling of MWCNT/Carbon (60/40)/SAE 10 W 40/SAE 85 W 90(50/50) nanofluid viscosity using artificial neural network (ANN) and self-organizing map (SOM). J. Therm. Anal. Calorim. 2018, 134, 2275–2286. [Google Scholar] [CrossRef]
- Bakthavatchalam, B.; Shaik, N.B.; Hussain, P.B. An Artificial Intelligence Approach to Predict the Thermophysical Properties of MWCNT Nanofluids. Processes 2020, 8, 693. [Google Scholar] [CrossRef]
- Afrand, M.; Nadooshan, A.A.; Hassani, M.; Yarmand, H.; Dahari, M. Predicting the viscosity of multi-walled carbon nanotubes/water nanofluid by developing an optimal artificial neural network based on experimental data. Int. Commun. Heat Mass Transf. 2016, 77, 49–53. [Google Scholar] [CrossRef]
- Alrashed, A.A.A.A.; Gharibdousti, M.S.; Goodarzi, M.; de Oliveira, L.R.; Safaei, M.R.; Bandarra Filho, E.P. Effects on thermophysical properties of carbon based nanofluids: Experimental data, modelling using regression, ANFIS and ANN. Int. J. Heat Mass Transf. 2018, 125, 920–932. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Saeed, M.; Al-Zubi, F.; Sedaghat, A.; Bahzad, H. Deposition of Stainless Steel Thin Films: An Electron Beam Physical Vapour Deposition Approach. Materials 2019, 12, 571. [Google Scholar] [CrossRef] [Green Version]
- Ali, N.; Teixeira, J.A.; Addali, A. Effect of Water Temperature, pH Value, and Film Thickness on the Wettability Behaviour of Copper Surfaces Coated with Copper Using EB-PVD Technique. J. Nano Res. 2019, 60, 124–141. [Google Scholar] [CrossRef]
- Ali, N.; Teixeira, J.A.; Addali, A.; Al-Zubi, F.; Shaban, E.; Behbehani, I. The effect of aluminium nanocoating and water pH value on the wettability behavior of an aluminium surface. Appl. Surf. Sci. 2018, 443, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Buongiorno, J.; Hu, L.-W.; Kim, S.J.; Hannink, R.; Truong, B.; Forrest, E. Nanofluids for Enhanced Economics and Safety of Nuclear Reactors: An Evaluation of the Potential Features, Issues, and Research Gaps. Nucl. Technol. 2008, 162, 80–91. [Google Scholar] [CrossRef]
- Mignacca, B.; Locatelli, G. Economics and finance of Small Modular Reactors: A systematic review and research agenda. Renew. Sustain. Energy Rev. 2020, 118, 109519. [Google Scholar] [CrossRef]
- Hernandez, R.; Folsom, C.P.; Woolstenhulme, N.E.; Jensen, C.B.; Bess, J.D.; Gorton, J.P.; Brown, N.R. Review of pool boiling critical heat flux (CHF) and heater rod design for CHF experiments in TREAT. Prog. Nucl. Energy 2020, 123, 103303. [Google Scholar] [CrossRef]
- Hu, H.; Peng, H.; Ding, G. Nucleate pool boiling heat transfer characteristics of refrigerant/nanolubricant mixture with surfactant. Int. J. Refrig. 2013, 36, 1045–1055. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, L. Boiling and two-phase flow phenomena of refrigerant-based nanofluids: Fundamentals, applications and challenges. Int. J. Refrig. 2013, 36, 421–446. [Google Scholar] [CrossRef]
- Bahiraei, M.; Salmi, H.K.; Safaei, M.R. Effect of employing a new biological nanofluid containing functionalized graphene nanoplatelets on thermal and hydraulic characteristics of a spiral heat exchanger. Energy Convers. Manag. 2019, 180, 72–82. [Google Scholar] [CrossRef]
- Dalkılıç, A.S.; Mercan, H.; Özçelik, G.; Wongwises, S. Optimization of the finned double-pipe heat exchanger using nanofluids as working fluids. J. Therm. Anal. Calorim. 2020, 143, 859–878. [Google Scholar] [CrossRef]
- Darzi, M.; Sadoughi, M.; Sheikholeslami, M. Condensation of nano-refrigerant inside a horizontal tube. Phys. B Condens. Matter 2018, 537, 33–39. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Darzi, M.; Sadoughi, M. Heat transfer improvement and pressure drop during condensation of refrigerant-based nanofluid; an experimental procedure. Int. J. Heat Mass Transf. 2018, 122, 643–650. [Google Scholar] [CrossRef]
- Sunardi, C.; Markus; Setyawan, A. The effects of the condenser pressure drop on the cooling performance of an air conditioning unit using R-410A. AIP Conf. Proc. 2001, 2018, 020006. [Google Scholar]
- Tashtoush, B.M.; Al-Nimr, M.d.A.; Khasawneh, M.A. Investigation of the use of nano-refrigerants to enhance the performance of an ejector refrigeration system. Appl. Energy 2017, 206, 1446–1463. [Google Scholar] [CrossRef]
- Ambreen, T.; Kim, M.-H. Heat transfer and pressure drop correlations of nanofluids: A state of art review. Renew. Sustain. Energy Rev. 2018, 91, 564–583. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, G.; Peng, H.; Gao, Y.; Wang, K. Experimental and Model Research on Nanorefrigerant Thermal Conductivity. HVACR Res. 2009, 15, 651–669. [Google Scholar] [CrossRef]
- Sidik, N.C.; Alawi, O.A. Computational investigations on heat transfer enhancement using nanorefrigerants. J. Adv. Res. Des. 2014, 1, 35–41. [Google Scholar]
- Ndoye, F.T.; Schalbart, P.; Leducq, D.; Alvarez, G. Numerical study of energy performance of nanofluids used in secondary loops of refrigeration systems. Int. J. Refrig. 2015, 52, 122–132. [Google Scholar] [CrossRef]
- Bahman, A.M.; Ziviani, D.; Groll, E.A. Vapor injected compression with economizing in packaged air conditioning systems for high temperature climate. Int. J. Refrig. 2018, 94, 136–150. [Google Scholar] [CrossRef]
- Loaiza, J.C.V.; Pruzaesky, F.C.; Parise, J.A.R. A numerical study on the application of nanofluids in refrigeration systems. In International Refrigeration and Air Conditioning Conference at Purdue; Purdue University: Purdue, IN, USA, 2010; p. 1495. [Google Scholar]
- Mahbubul, I.M.; Fadhilah, S.A.; Saidur, R.; Leong, K.Y.; Amalina, M.A. Thermophysical properties and heat transfer performance of Al2O3/R-134a nanorefrigerants. Int. J. Heat Mass Transf. 2013, 57, 100–108. [Google Scholar] [CrossRef]
- Mahbubul, I.; Saidur, R.; Amalina, M. Influence of particle concentration and temperature on thermal conductivity and viscosity of Al2O3/R141b nanorefrigerant. Int. Commun. Heat Mass Transf. 2013, 43, 100–104. [Google Scholar] [CrossRef]
- Mahbubul, I.; Saadah, A.; Saidur, R.; Khairul, M.; Kamyar, A. Thermal performance analysis of Al2O3/R-134a nanorefrigerant. Int. J. Heat Mass Transf. 2015, 85, 1034–1040. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Renault, F.L.; Gómez, A.O.C.; Sarafraz, M.; Khan, H.; Safaei, M.R.; Filho, E.P.B. Nanofluids as secondary fluid in the refrigeration system: Experimental data, regression, ANFIS, and NN modeling. Int. J. Heat Mass Transf. 2019, 144, 118635. [Google Scholar] [CrossRef]
- Bahman, A.M.; Groll, E.A. Application of Second-Law Analysis for the Environmental Control Unit at High Ambient Temperature. Energies 2020, 13, 3274. [Google Scholar] [CrossRef]
- Bhattad, A.; Sarkar, J.; Ghosh, P. Energy-Economic Analysis of Plate Evaporator using Brine-based Hybrid Nanofluids as Secondary Refrigerant. Int. J. Air. Cond. Refrig. 2018, 26, 1850003. [Google Scholar] [CrossRef]
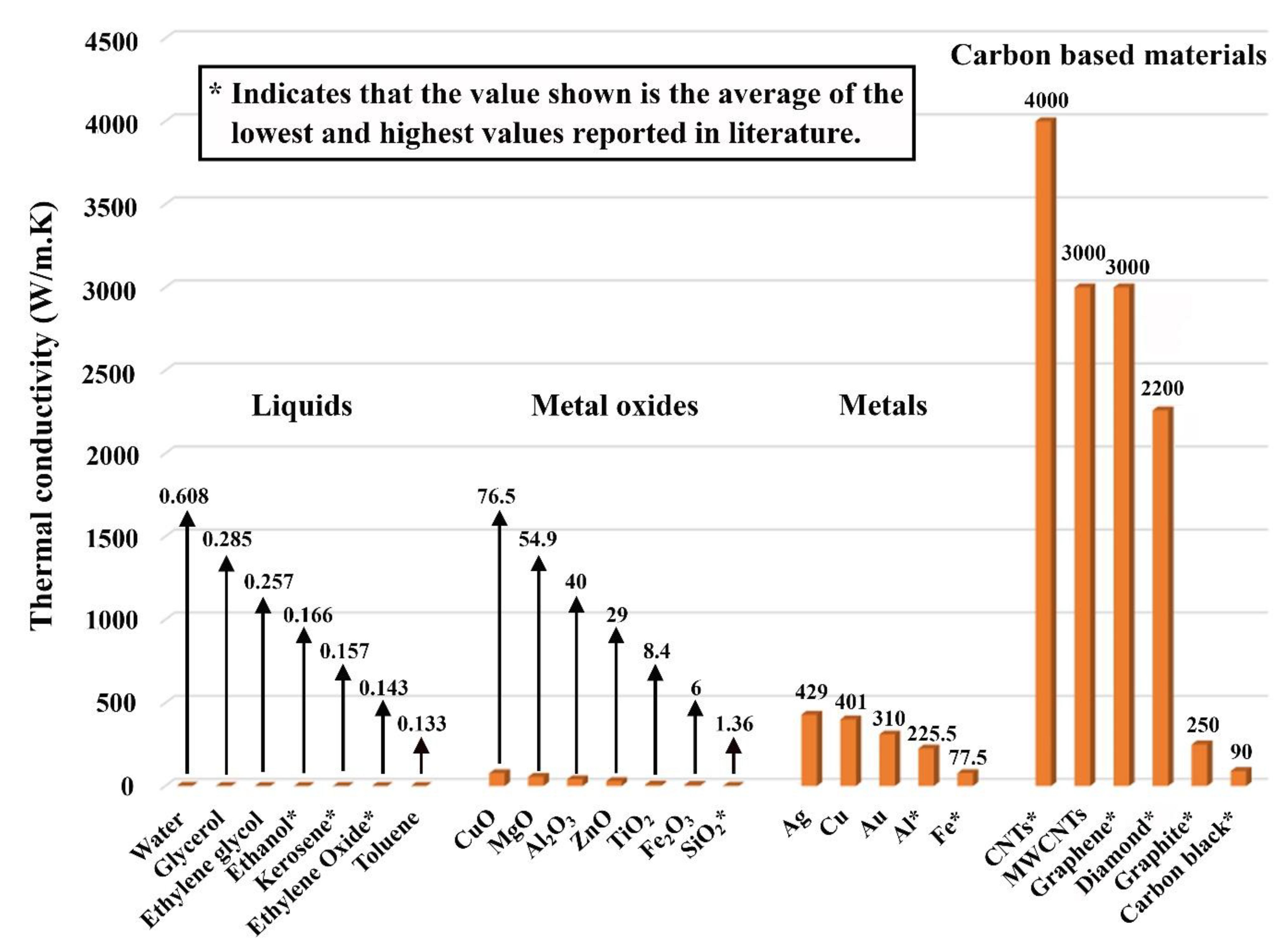
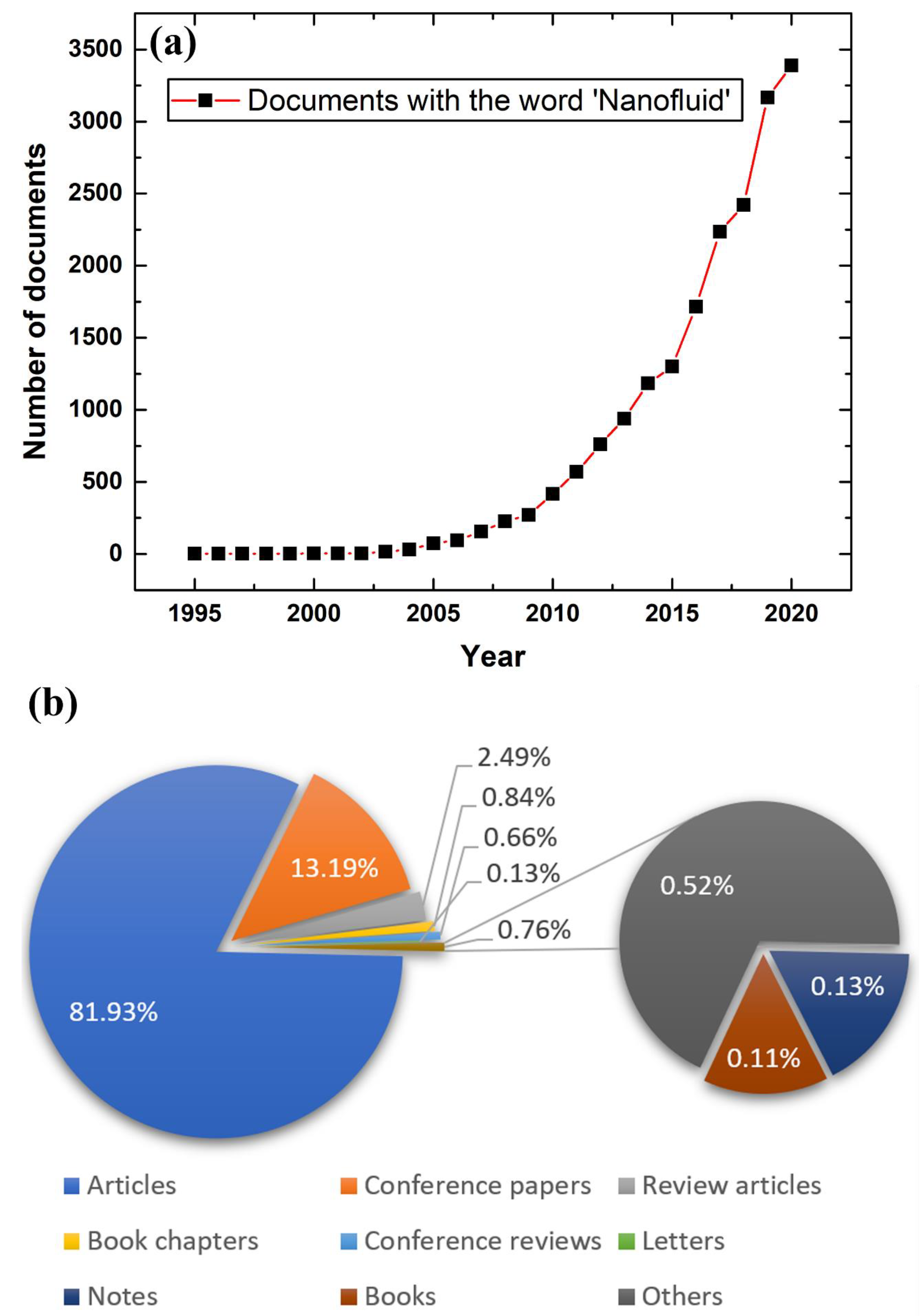
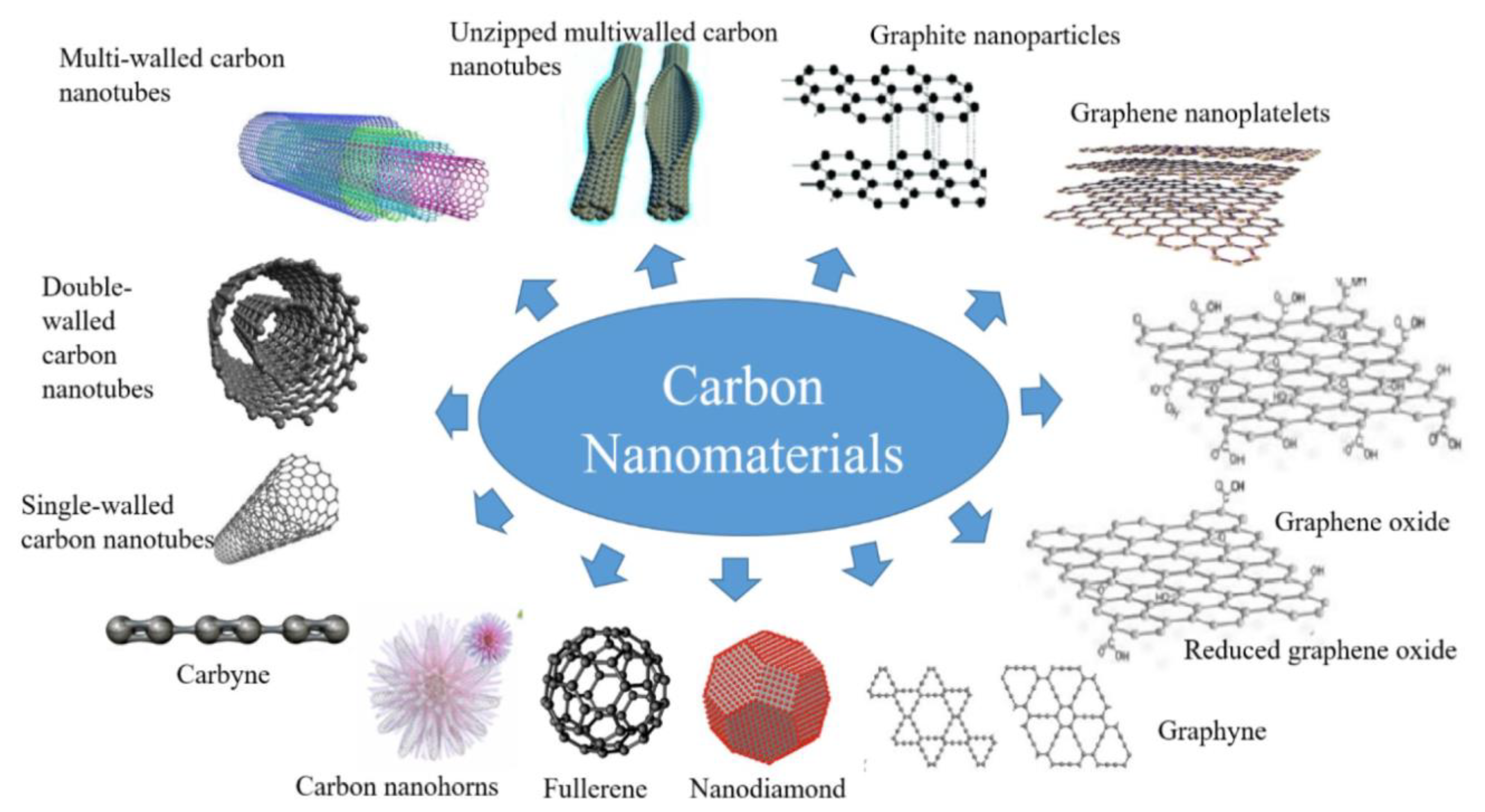
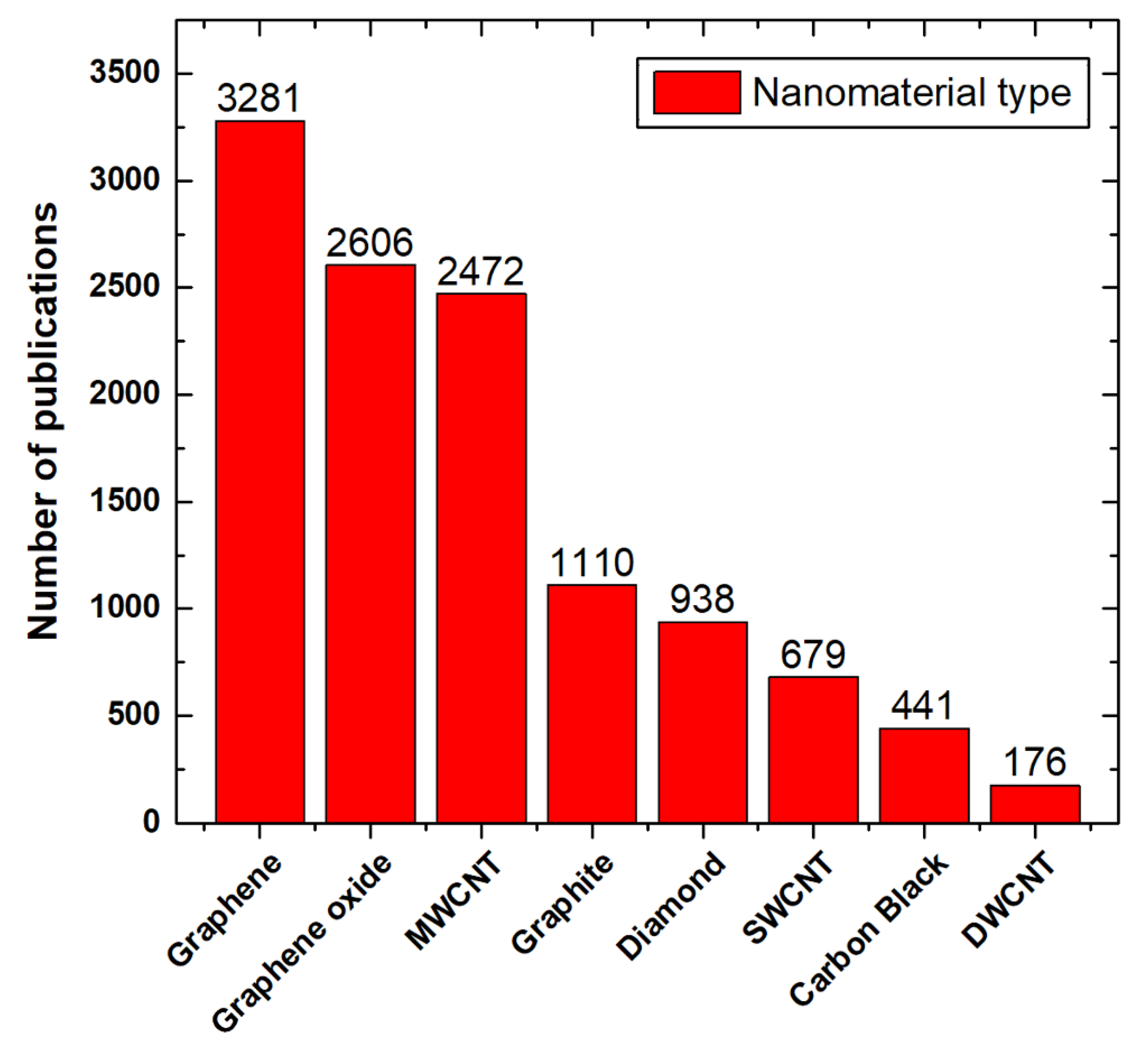
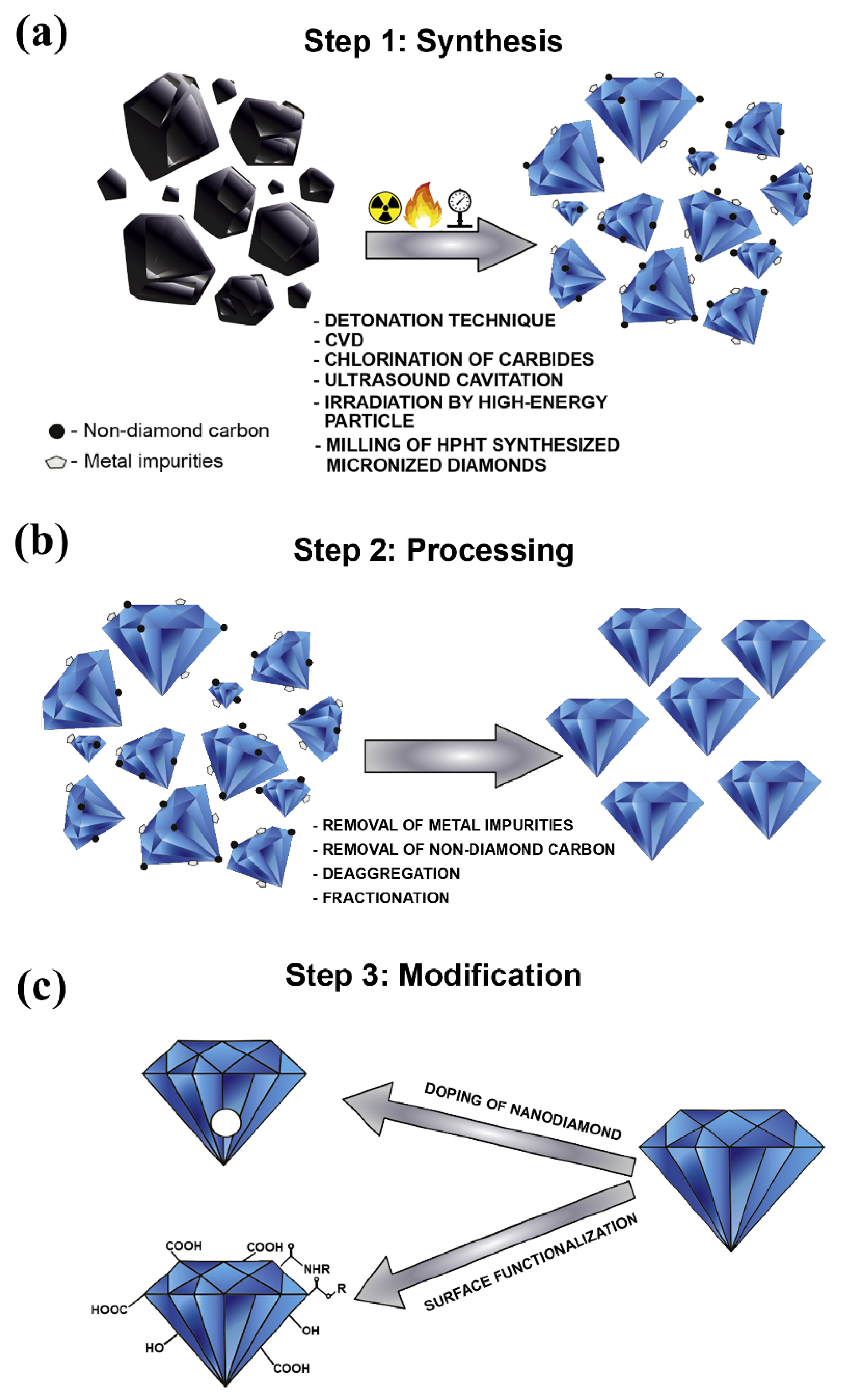

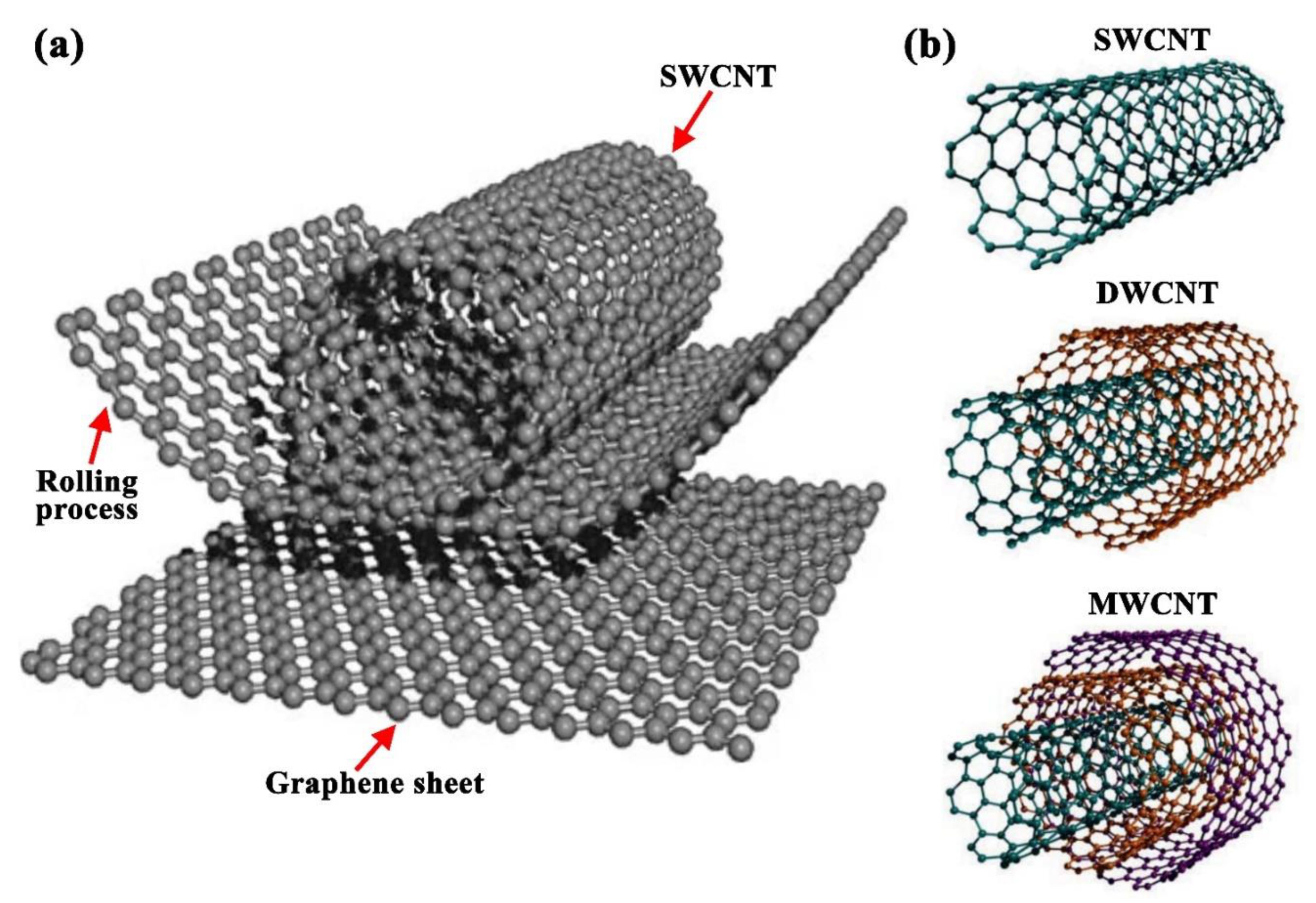
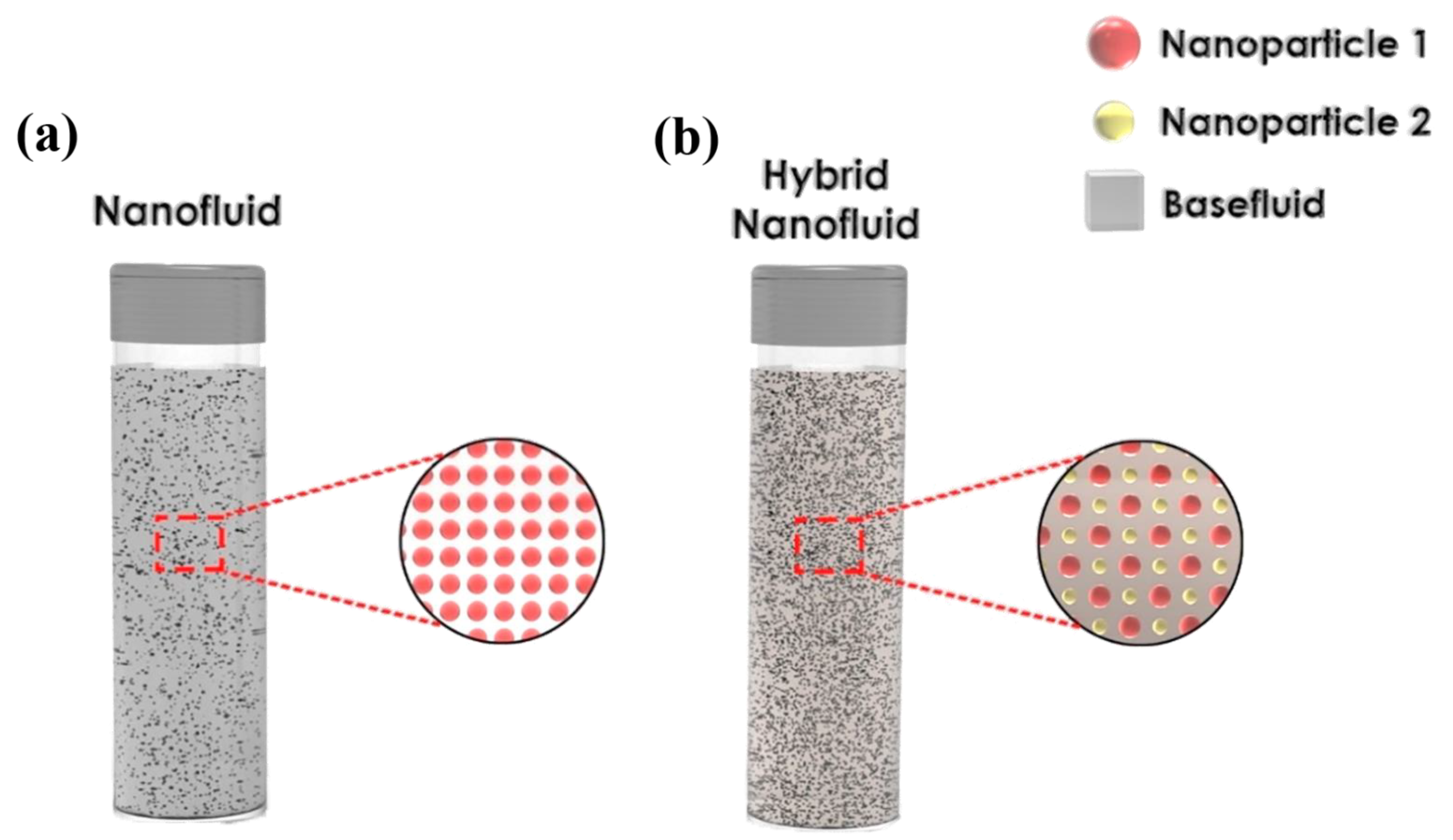

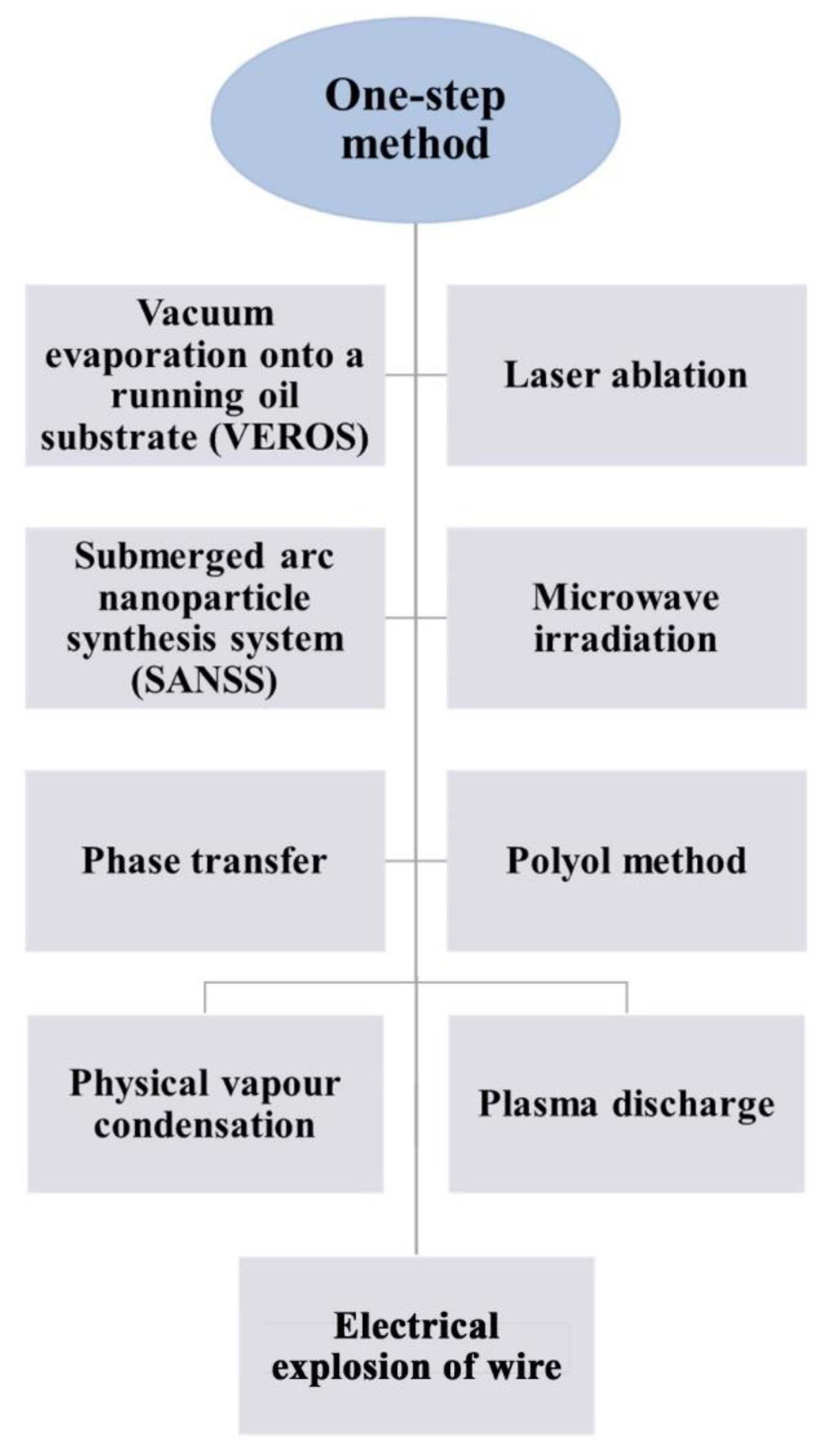
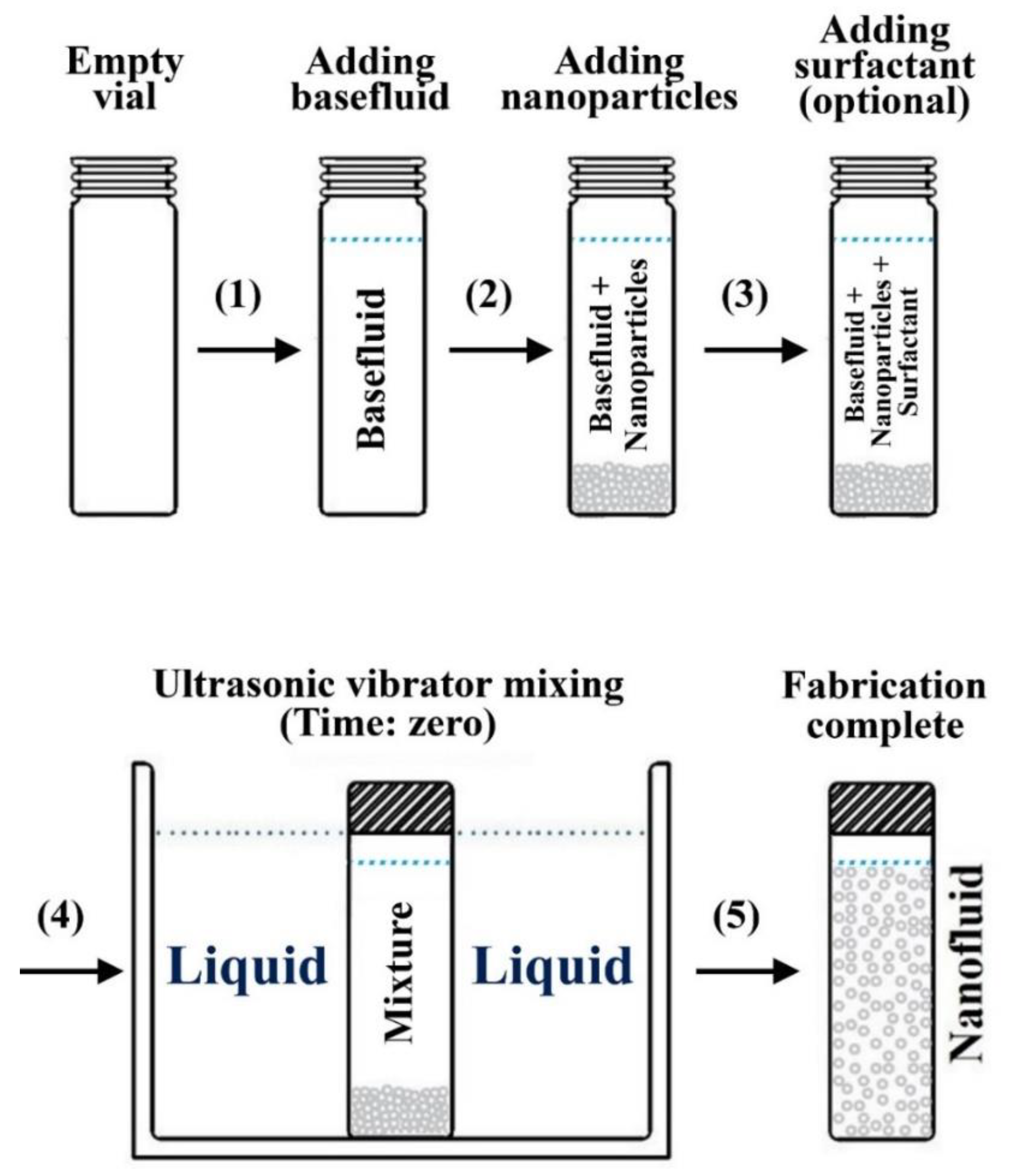
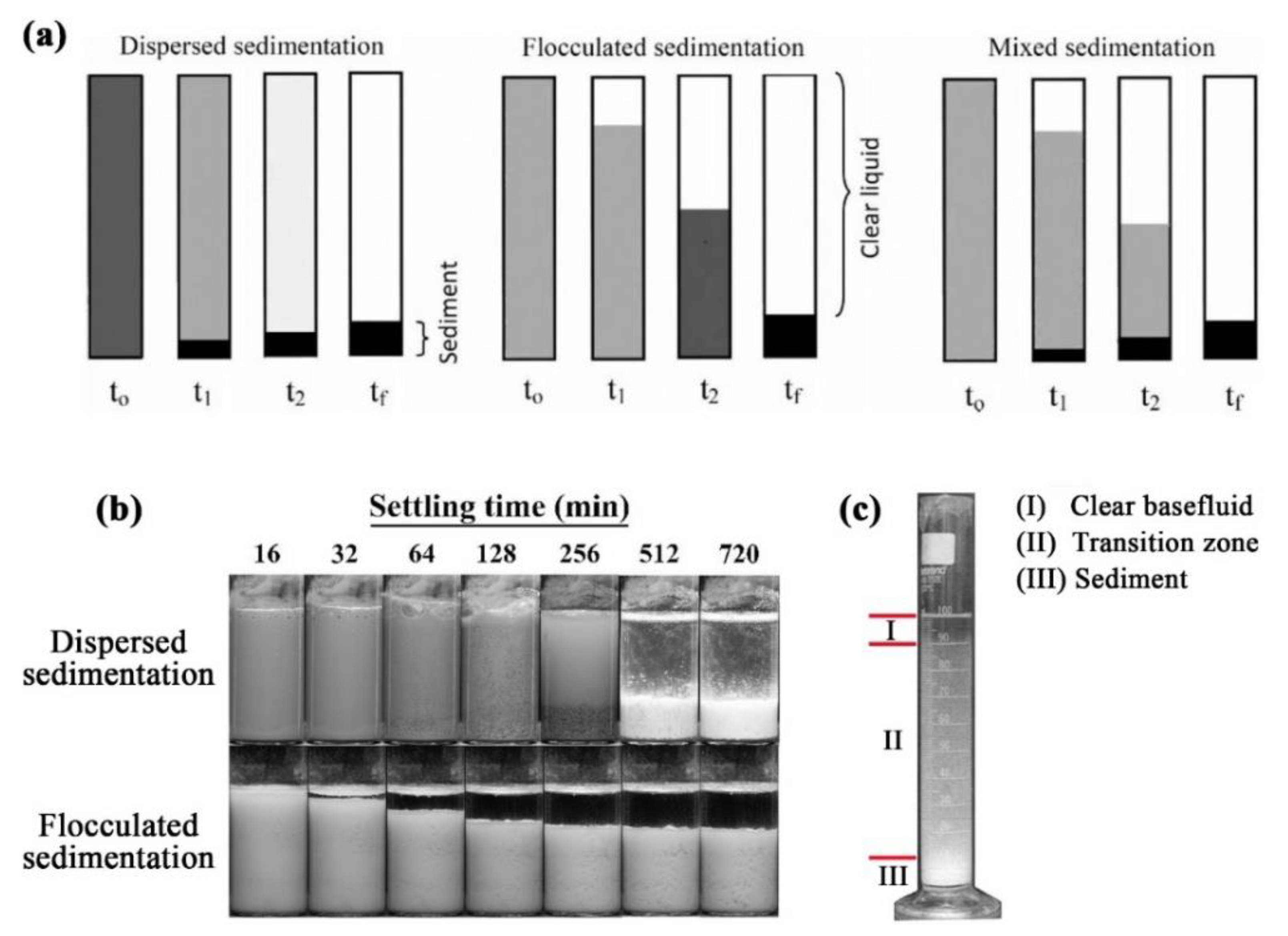
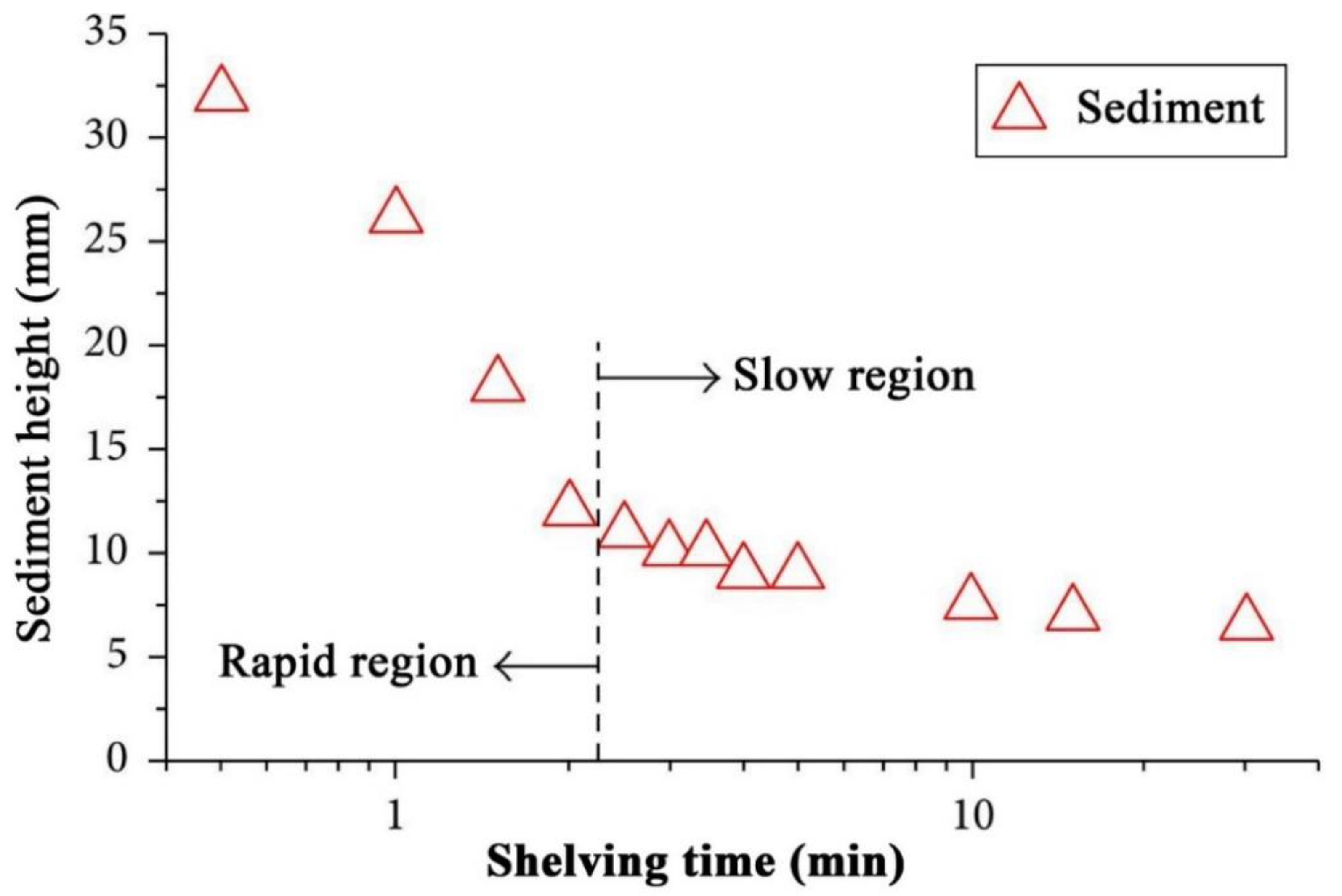

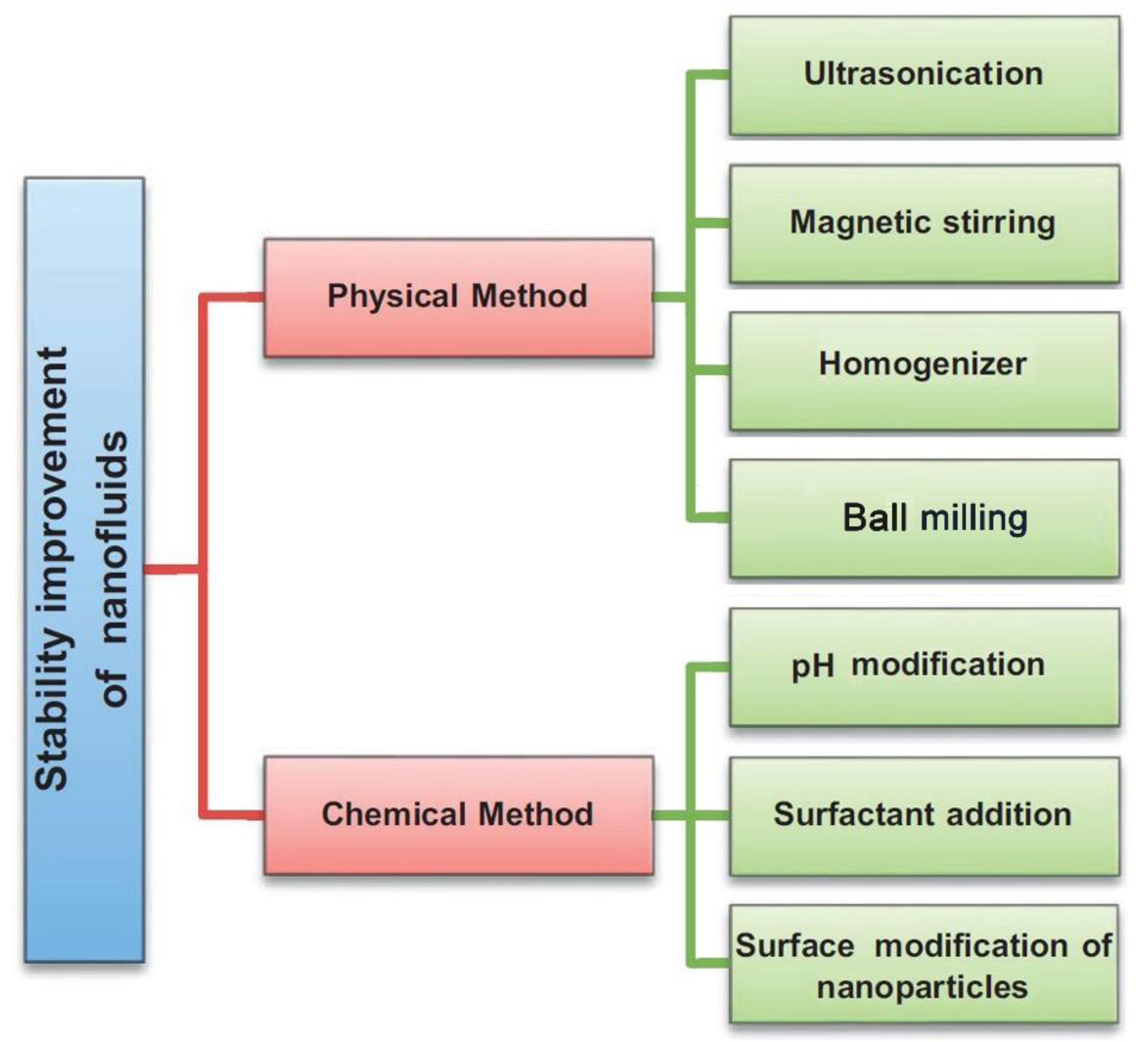
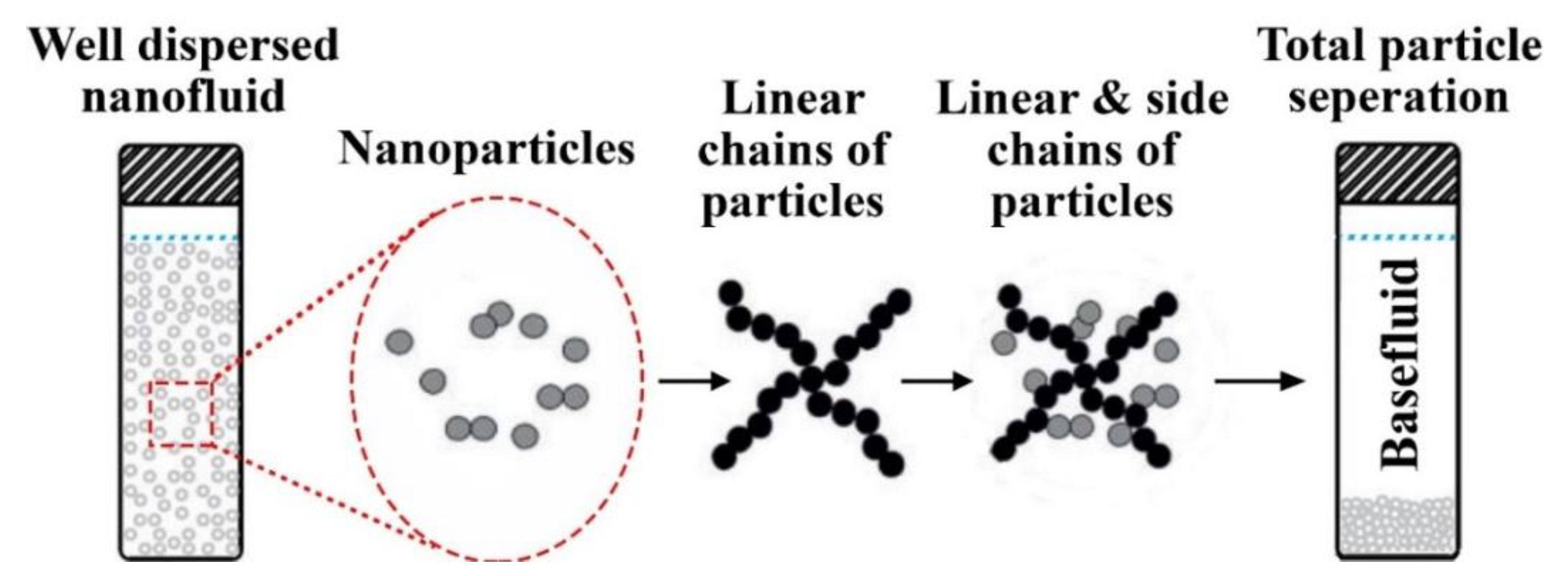
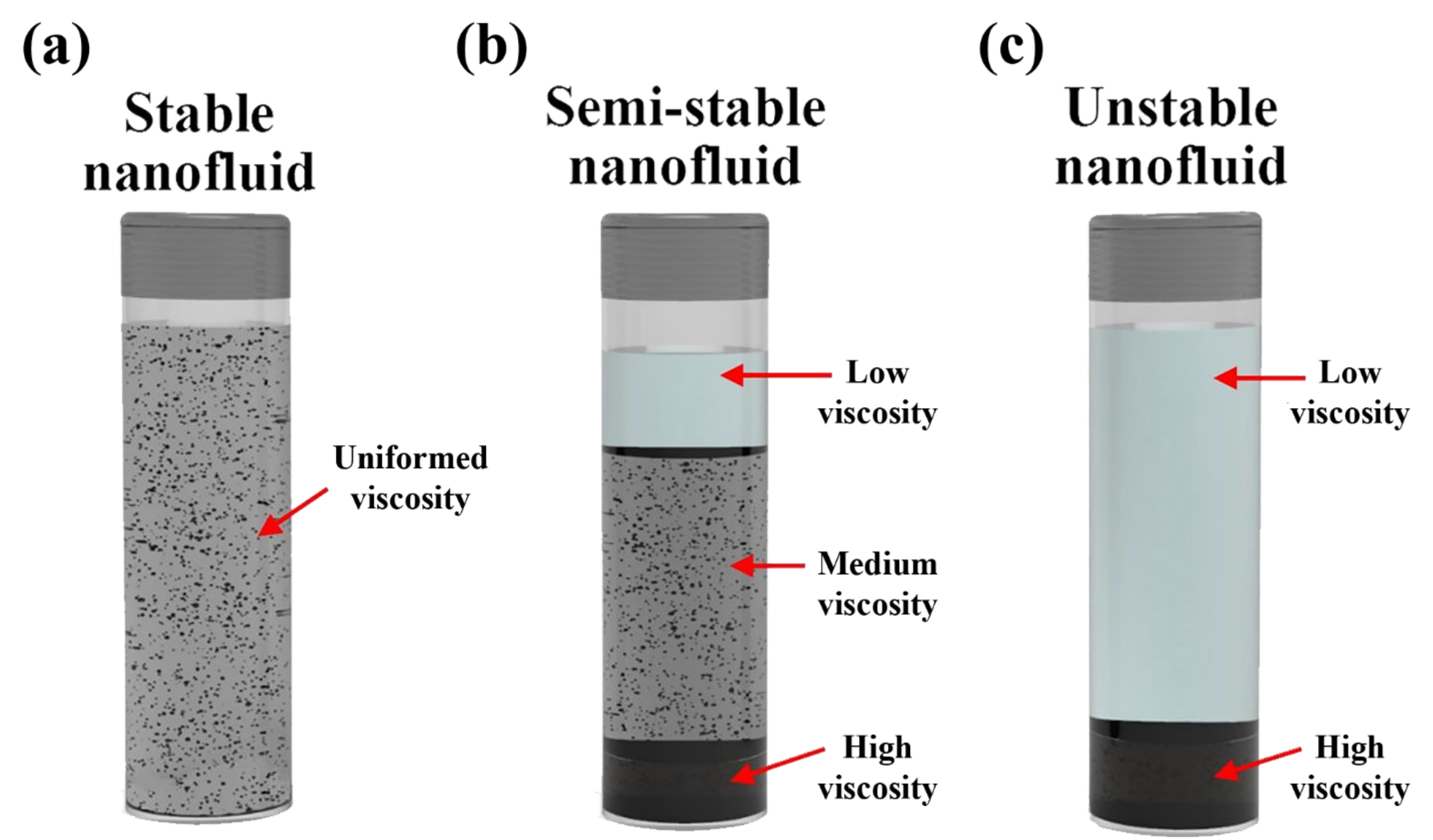
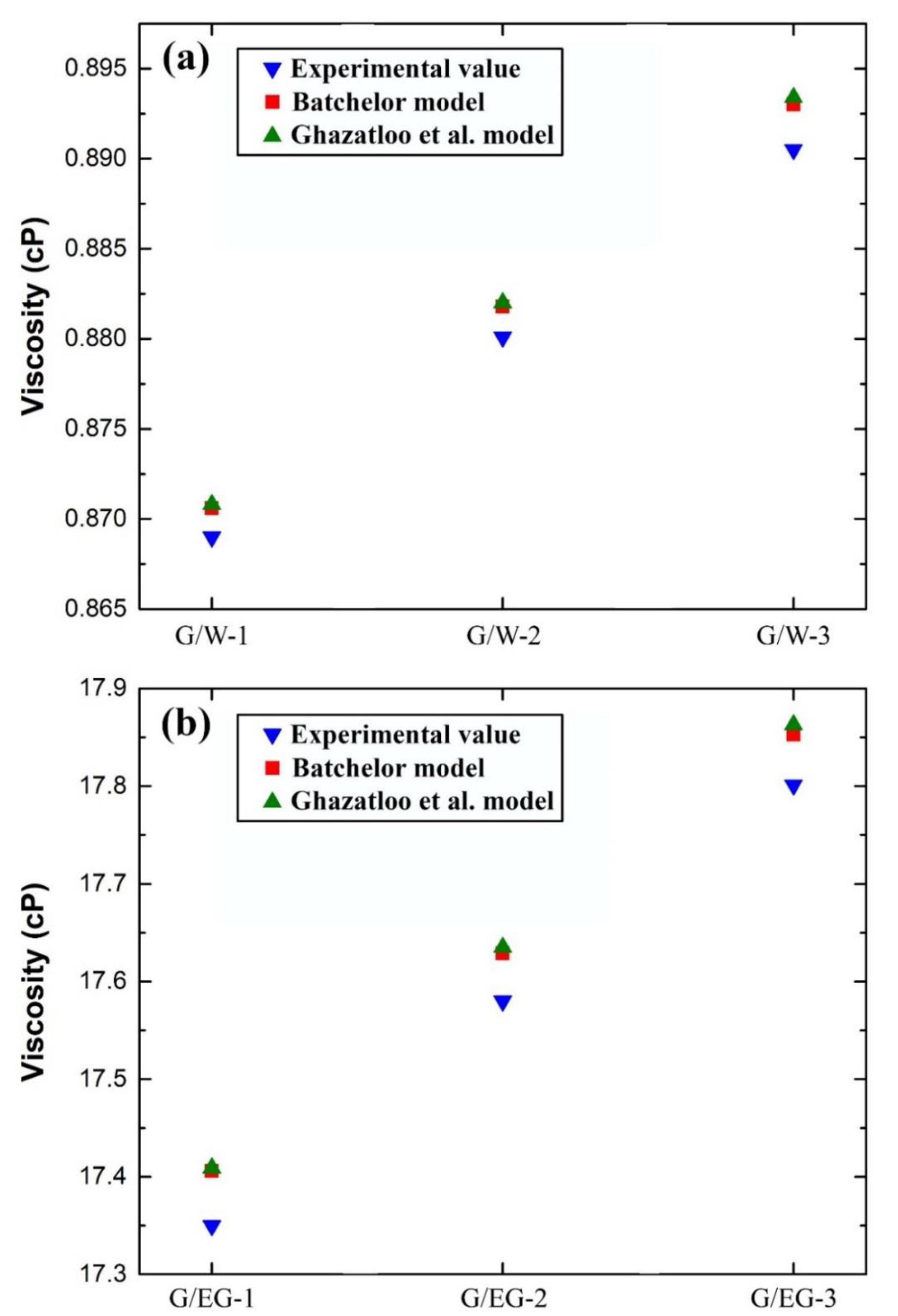
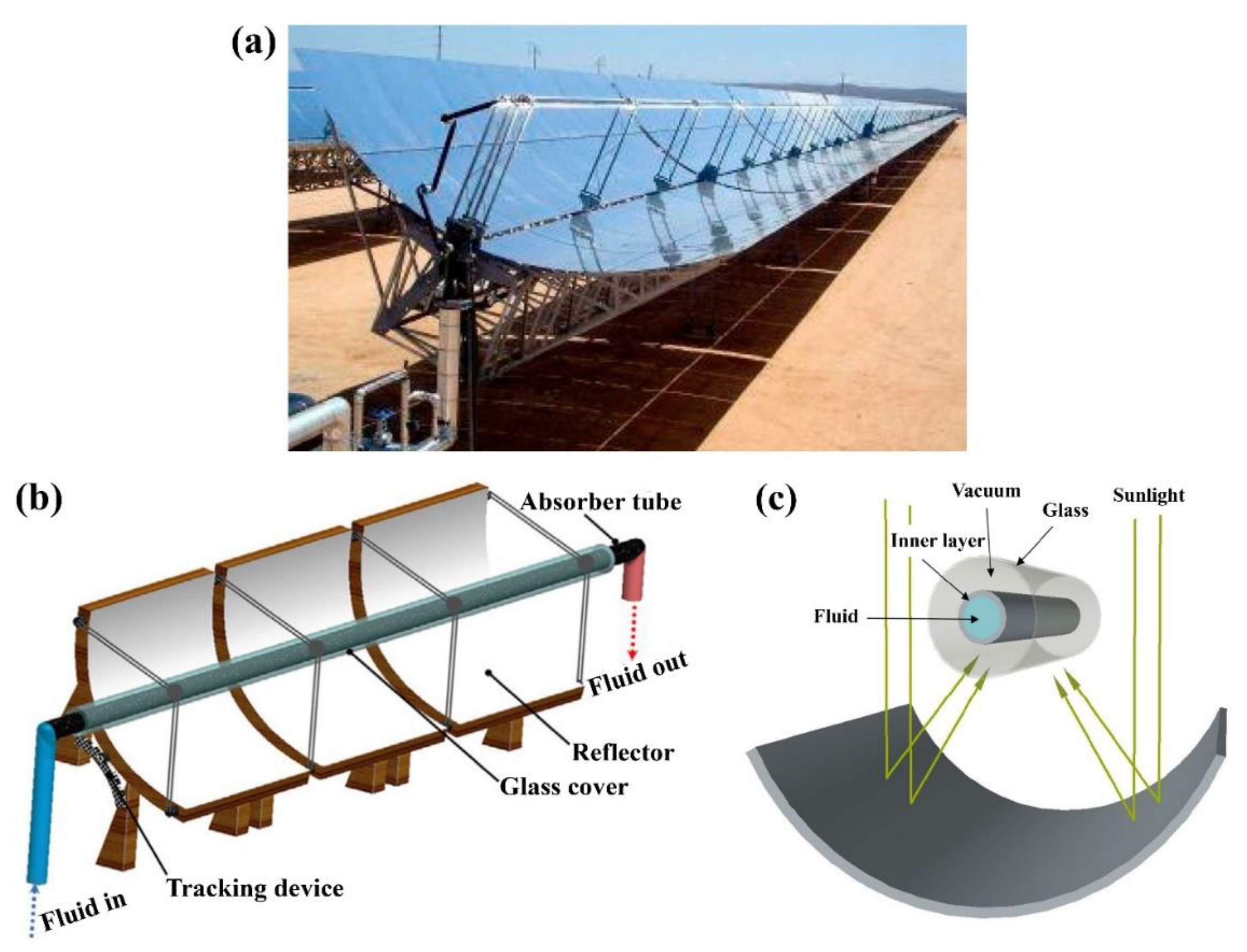
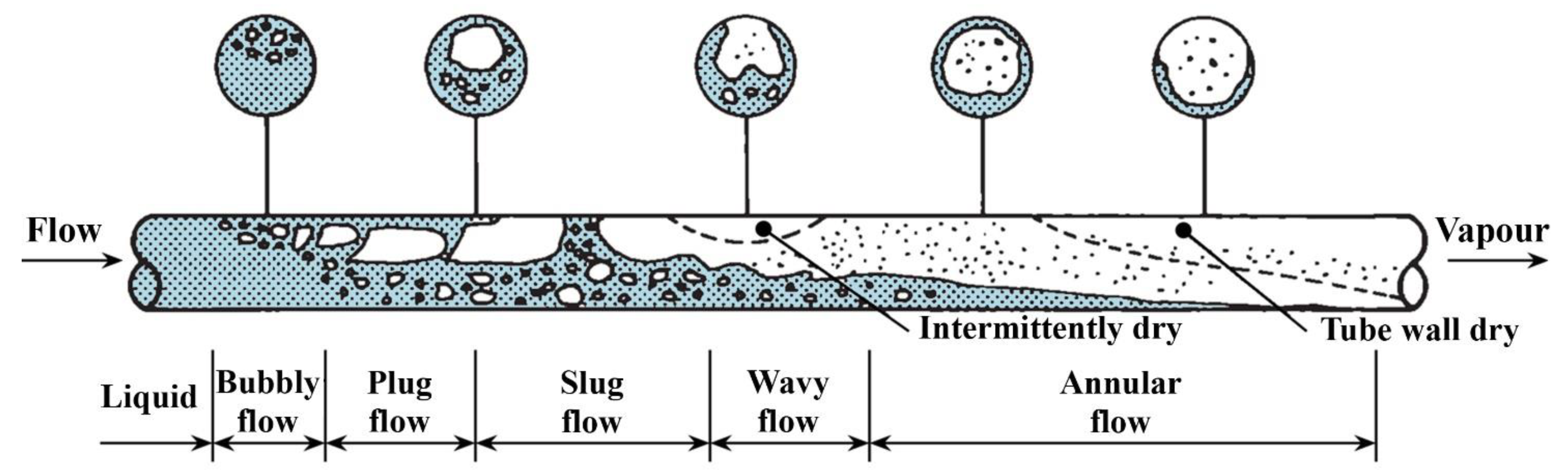
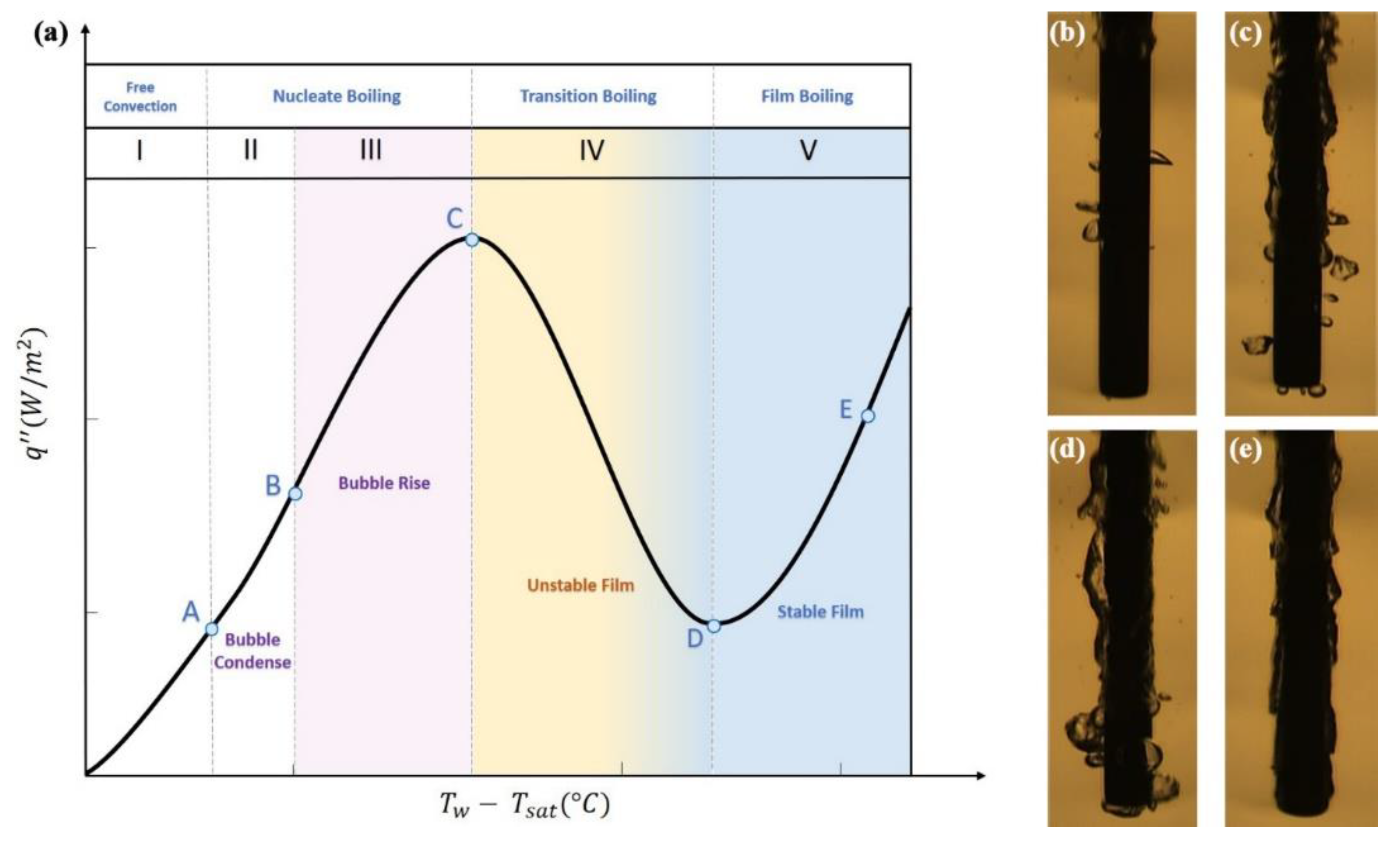
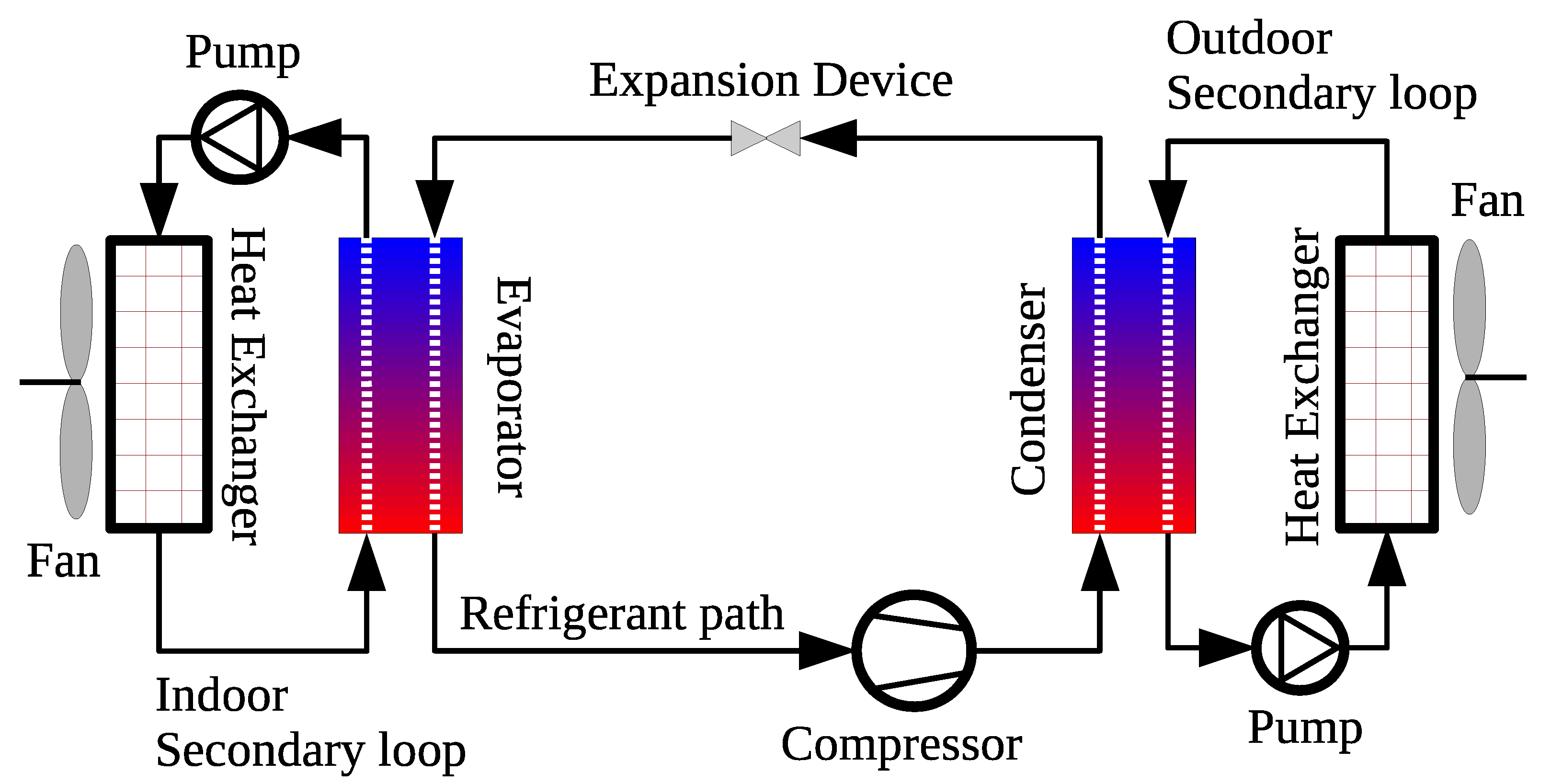

| Type of Particles | Type of Base-Fluid | Fraction (%) | Formulae | Ref. | Eq. |
|---|---|---|---|---|---|
| Single type | Single type | vol. | | [13,37] | (1) |
| Single type | Two type | vol. | have equal volume ratio | [141] | (2) |
| Two type | Single type | vol. | have equal volume ratio | [142,143] | (3) |
| Two type | Two type | vol. | [144] | (4) |
| Material | Base Fluid | Particles Dimensions (nm) | Particles Concentration | Additional Information | Ref. |
|---|---|---|---|---|---|
| ND | EG | 30–50 | <1.4 vol. % |
| [149] |
| EG | 5–10 | 0.25–5.0 vol. % |
| [150] | |
| EG | 5–10 | 0.25–1.0 vol. % |
| [151] | |
| EG—water | 30–50 | 0. 5–2.0 vol. % |
| [152] | |
| EG and mineral oil | 5 | 2.0 g |
| [153] | |
| Highly refined thermal oil | 3–10 | 0.25–1.0 wt % |
| [154] | |
| Naphthenic transformer oil (NTO) | 10 | 1.0 g |
| [155] | |
| propylene glycol (PG)—water | 5–10 | 0.2–1.0 vol. % |
| [156] | |
| Graphene | Water | 2–5 * | 10 mg/mL |
| [157] |
| Water | 6000–8000 * | 0.001–0.01 vol. % |
| [158] | |
| Water | 2 * | 0.025–0.1 wt % |
| [159] | |
| EG and water | – | 0.005–0.056 vol. % |
| [160] | |
| Glycerol | 15–50 * | 13 wt % |
| [161] | |
| CNTs | Water | 9–15 ^ | 0.5 wt % |
| [162] |
| Vegetable cutting oil | 10–20 ^ | 0.6 vol. % |
| [163] | |
| Turbine meter oil | 5–16.1 ^ | 0.05–0.4 wt % |
| [164] | |
| Water | 2–4 ^ | 0.01–0.5 vol. % |
| [165] | |
| EG | 2–4 ^ | 0.02–0.6 vol. % |
| [166] | |
| Water | 1–2 ^ | 0.1–0.5 vol. % |
| [167] | |
| Water | 0.8–1.6 ^ | 0.3 vol. % |
| [168] |
| Surfactant Classification | Head Group Charge | Example(s) |
|---|---|---|
| Cationic | +ve | Cetyltrimethyl ammonium bromide (CTAB), distearyl dimethyl ammonium chloride (DSDMAC), and benzalkonium chloride (BAC). |
| Non-ionic | neutral or uncharged | Oleic acid, polyvinylpyrrolidone (PVP), Arabic gum (AG), Tween 80, and oleylamine. |
| Anionic | −ve | Sodium dodecyl benzenesulfonate (SDBS), and SDS. |
| Amphoteric | +ve and −ve | lecithin. |
| Developer/s | Year | Formula | Dependent Parameter | Limitations |
|---|---|---|---|---|
| Maxwell [231] | 1890 | are the effective thermal conductivity of the nanofluid, base fluid thermal conductivity, and nanoparticles thermal conductivity, respectively. | Suited for spherical shaped particles. | |
| Jefferson et al. [232] | 1958 | The model is used for spherical particles but always underestimate the effective thermal conductivity by 25%. | ||
| Hamilton and Crosser [233] | 1962 | Preferred for spherical and cylindrical shaped particles with n = 3/ψ, where n and ψ are the empirical shape factor and particle sphericity, respectively. For perfectly spherical particles ψ = 1. | ||
| Wasp et al. [234] | 1977 | Particles should have a sphericity of ≤1. | ||
| Yu and Choi [235] | 2003 | ; where is the ratio of the nanolayer thickness to the particle radius. | , interfacial particle layer, and radius | Modified version of the Maxwell [231] model for spherical particles. The main problem is that it is inadequate the non-linear trend of thermal conductivity. |
| Xuan et al. [236] | 2003 | ; where is the Boltzmann constant (1.381 × 10−23 J/K), T is the temperature of the mixture, is the particle apparent radius, and is the kinematic viscosity of the liquid. | , , , , , and | Hard to predict the thermal conductivity for linear temperatures. |
| Nan et al. [237] | 2003 | Can only be used with CNTs nanofluids. | ||
| Kumar et al. [218] | 2004 | ;
| dimensions of the particles, , and ν | The Brownian motion has the dominative effect on the thermal conductivity prediction over all other factors. |
| Jang and Choi [213] | 2004 |
| dimensions of the particles, , ν, and | Both conduction and convection heat transfer are accounted for, while the heating duration is much higher. |
| Yu and Choi [238] | 2004 |
| , and interfacial resistance | This is a renovated Hamilton and Crosser [233] model with n = 3/ψ−α, where α is an empirical parameter that depends on both particle sphericity and the particle to liquid thermal conductivity ratio. In addition, this model includes the interface layer between the particles and the surrounding liquid but cannot predict the nonlinear behaviour of the thermal conductivity. |
| Prasher et al. [239] | 2005 |
| , , and | Only considers the dispersed particles convection effect. |
| Xue [240] | 2005 | Suitable for nanofluids made of dispersed CNTs. | ||
| Murshed et al. [241] | 2006 | The particles need to be uniformly dispersed in the suspension for appropriate effective thermal conductivity prediction. | ||
| Vajjha et al. [242] | 2010 |
| , particles type, and base fluid temperature | Limited to nanofluids of temperatures between 295 and 363 K. |
| Xing et al. [243] | 2016 |
| , T, and aspect ratio | Can only be used for CNTs suspensions. Furthermore, not all of the parameters are accounted in the correlation, while the effect of the micro-motion is the most significant parameter. |
| Gao et al. [244] | 2018 | where is the length of the nanoplatelet, t is the nanoplatelet thickness, and is the average flatness ratio of the graphene nanoplatelet. | , , , , and . | This model is designed for suspensions of water, as the base fluid, and graphene nanoplatelet. |
| Li et al. [245] | 2019 | where is the equivalent particle thermal conductivity, and is the thickness of the nanolayer surrounding the particle. | , , , and fluid temperature | This model is a modified form of the Yu and Choi model with the nanolayer constant value changed to quadratic. |
| Jóźwiak et al. [246] | 2020 |
| , and particles morphology | This is a modified version of the Murshed et al. [241] model, which is suitable for ionic liquid nanofluids (also known as ionanofluids) with dispersed CNTs. |
| Developer/s | Year | Formula | Dependent Parameter | Limitations |
|---|---|---|---|---|
| Einstein [271] | 1906 | Suited for suspensions of <0.02 vol. % and spherical shaped particles. | ||
| Hatschek [272] | 1913 | Designed for suspensions with up to 40 vol. % of spherical particles but does not account for the size of the dispersed particle. The formula also showed very large deviation from the actual viscosity value. | ||
| Saitô [273] | 1950 | Preferred for dispersions of small spherical particles and is affected by the Brownian motion of the particles. | ||
| Mooney [274] | 1951 | where is the self-crowding factor. | , and | This is an extended version of the Einstein’s [271] formula that can be used for suspensions of spherical particles with any concentration. The downside is that the modeled suspension needs to meet the functional equation so that the can be independent of the stepwise sequence of adding further particles concentrations. |
| Brinkman [275] | 1952 | Enhanced form of the previous Einstein [271] formula, where it can be used for particles concentrations of up to 4 vol. %. | ||
| Roscoe [276] | 1952 | ; where is a constant that is equal to 1 (for very diverse particles sizes), −2.5 (for similar particles sizes and <0.05 vol. %), and 1.35 (for higher vol. %); and is a constant that is equal to −2.5 (for the very diverse particles sizes case and the >0.05 vol. % suspension) and 1 (for the <0.05 vol. % of similar sized particles). | Can be used with any dispersion concentration but the particles need to be of spherical shape. | |
| Maron and Pierce [277] | 1956 | where is the packing fraction of the particles. | , and | Suitable for suspensions of small spherical particles and of similar sizes. |
| Krieger and Dougherty [278] | 1959 | ; | , and | For dispersed spherical particles of ≤0.2 vol. %, but the model does not account for the particle’s interfacial layers and their aggregation. |
| Frankel and Acrivos [279] | 1967 | ; where is the maximum attainable concentration. | Employed for uniform spherical particles and assumes that the rise in viscosity with the increase in particles concentration is due to their hydrodynamic interactions. | |
| Nielson [280] | 1970 | , and | This is a modified form of the Einstein’s [271] formula but it lacks accurate suspension viscosity prediction. | |
| Brenner and Condiff [281] | 1974 | where is the axis aspect ratio of the dispersed particle. | , aspect ratio, and shear rate | Shows good prediction capability for dispersed particles of rod shape but less effective for other shapes. |
| Jeffrey and Acrivos [282] | 1976 | , and aspect ratio | Designed for suspensions of rod-shaped particles. | |
| Batchelor [270] | 1977 | , and Brownian motion | The model includes the interaction between the particles but fails to provide good prediction agreement. | |
| Graham [283] | 1981 | where is the minimum separation distance between the surface of two spherical particles. | , , and | Suitable for spherical particles only and has good prediction agreement with Einstein [271] formula when very low particles concentrations are used or when is very close to that of . |
| Kitano et al. [284] | 1981 | , and | Similar to the Maron and Pierce [277] formula but the value is preliminarily defined numerically and is better suited for two phase mixtures. | |
| Bicerano et al. [285] | 1999 | where is the intrinsic viscosity, and is the Huggins coefficient. | , , and | More determined towards analyzing the relation between particles concentration and . |
| Wang et al. [286] | 1999 | Simple model that was formed from a set of experimental results obtained from modifying the suspension particles size and concentration. | ||
| Masoumi et al. [248] | 2009 |
| , T, , particle size, and Brownian motion | The formula is bound by the experimental conditions that were used in its development. |
| Chevalier et al. [250] | 2009 | where is the average diameter of the aggregates, and is the fractal dimension, which depends on the shape of the dispersed particles, the type of agglomeration, and the shear flow. and are usually set to 0.65, for random packing of spheres, and 1.8, respectively. | , , , and | This model depends on the agglomerate size, and thus it is not optimum for determining the for stabile suspensions. |
| Chandrasekar et al. [190] | 2010 | where and are regression coefficients that can be obtained from preliminary experimental results. | Specific area, , , and sphericity of the particles | Depends on preliminary experimental results to set-up the unknown coefficients. |
| Bobbo et al. [287] | 2012 | , and | Developed for single-walled carbon nanohorn (SWCNH) and TiO2 nanofluids based on the Batchelor formula and experimental measurements of the at a range of temperatures from 283.2 to 353.2 K, and concentrations from 0.01 to 1 wt %. | |
| Esfe et al. [288] | 2014 | , and | Limited for water based MWCNTs nanofluids of 0–1 vol. %. | |
| Aberoumand et al. [289] | 2016 | Used for low temperature oil based suspensions. | ||
| Akbari et al. [290] | 2017 | , and | Suitable for nanofluids of <3 vol. % and of temperature ≤50 °C. | |
| Esfe et al. [291] | 2019 | , and | Suitable for MWCNTs and TiO2 hybrid nanofluids of between 0.05 and 0.85 vol. %. | |
| Ansón-Casaos et al. [292] | 2020 | where is equal to 2.5 for spherical particles or can be replaced by a function, , to determine the suspension property containing 1D and 2D dispersed solids. | , and | Suitable for SWCNTs and graphene oxide. |
| Ilyas et al. [154] | 2020 | where , , and are the temperature fitting parameters in Kelvin, whereas and are the dynamic viscosity fitting parameters in Pa.s. The values of these parameters (i.e., to ) can be found in the published source. | , and | Suitable for ND dispersed in thermal oil and is valid for the range of 0 ≤ ≤ 1 and 298.65 ≤ T (K) ≤ 338.15. |
| Industry | Process | Required Temperature Range (°C) |
|---|---|---|
| Dairy | Boiler feed water | 60–90 |
| Agricultural products | Drying | 80–200 |
| Textile | Drying | 100–130 |
| Chemistry | Petroleum | 100–150 |
| Desalinization | Heat transfer fluid | 100–250 |
| Ref. | Nanofluid | Concentration/Particle Size | Heating Surface | CHF Enhancement% |
|---|---|---|---|---|
| [370] | CNT | 0.1–0.3 wt % | – | Enhanced |
| [371] | CNT | 0.01–0.05 vol. % | Cu block | 38.2 |
| [372] | CNT | 0.5–4 wt % | Cu plate | 60–130 |
| [361] | CNT | 0.05 vol. % | SS foil | 108 122 |
| [373] | CNT | 1.0 vol. % | SS tube | 29 |
| [374] | MWCNT | 0.01–0.02 wt % | SS cylinder | Enhanced |
| [371] | MWCNT | 0.0001–0.05 vol. % | Cu block | 200 |
| [375] | MWCNT | 0.1–0.3 wt % | Microfin Cu disk | 95 |
| [372] | f-MWCNT | 0.5–4 wt % | Cu plate | 200 |
| [162] | f-MWCNT | 0.25–1 wt % | SS tube | 37.5 |
| [376] | f-MWCNT | 0.01–0.1 wt % | Cu disk | 271.9 |
| [377] | f-MWCNT | 0.01 vol. % | Cu block | 98.2 |
| [378] | f-SWCNT | 2.0 wt | Ni-Cr wire | 300 |
| [379] | GO | ≤0.001 wt % | Copper plate | Enhanced |
| [380] | GO | 0.0005 wt % | Ni-Cr wire | 320 |
| [381] | GO | 0.001 vol. % | – | 179 |
| [382] | GO | 0.0001, 0.0005, 0.0010, and 0.005 wt % | Ni wire | Enhanced |
| [383] | GO | 0.01 vol. % | Ni-Cr wire | – |
| [366] | ND | 1 g/L | Cu plate | Enhanced |
| [366] | ND | <1 g/L | Cu plate | Deterioration |
| [384] | ND | 0.01–0.1 vol. % | SS plate | Unchanged |
| [384] | ND | 0.01 vol. % | SS plate | 11 |
| Ref. | Nanofluid | Concentration | Heating Surface | CHF Enhancement% |
|---|---|---|---|---|
| [373] | MWCNT | 1.0 vol. % | Cu block | 28.7 |
| [371] | MWCNT | 0.0001–0.05 vol. % | Cu block | 38.2 |
| [385] | MWCNT | 0.25%, 0.5%, and 1.0 vol. % | Ni-Cr wire | 320 |
| [375] | MWCNT | 0.1–0.3 wt % | Microfin Cu disk | 77 |
| [372] | f-MWCNT | 0.5–4 wt % | Cu plate | 130 |
| [162] | f-MWCNT | 0.25–1 wt % | SS tube | 66 |
| [376] | f-MWCNT | 0.01–0.1 wt % | Cu disk | 38.5 |
| [377] | f-MWCNT | 0.01 vol. % | Cu block | 10.15 |
| [386] | Graphene | 0.1 and 0.3 wt % | Cu | 96 |
| Ref. | Nanomaterial(s) | Heating Surface | ||
|---|---|---|---|---|
| [387] | ND (0.01 vol. %) | ITO | 230 | 260 |
| [388] | CNT-1 CNT-2 CNT-3 CNT-4 (0.5 wt.%) | 316L SS sphere | 215 218 218 219 | 241, 294, 303, 328, 335 211, 229, 277, 281, 287 228, 246, 254, 262, 264 231, 238, 243, 254, 256 |
| [389] | GO (0.0001 wt %) | SS sphere | 230 | 236.1 |
| GO (0.001 wt %) | 239.6 | |||
| GO (0.005 wt %) | 235.7 | |||
| GO (0.01 wt %) | 235.6 | |||
| GO (0.05 wt %) | 233.1 | |||
| GO (0.1 wt %) | 235.9 | |||
| [390] | Al2O3 SiO2 ND (0.1 vol. %) | SS | 249, 247, 249, 247, 250, 250, 251 | 244, 343, 345, 394, 348, 399, 389 251, 330, 368, 368, 377, 389, 397 252, 252, 250, 253, 255, 264, 279 |
| Al2O3 SiO2 ND (0.1 vol. %) | Zr | 267, 272, 253, 272, 260, 266, 253 | 287, 347, 354, 400, 401, 411, 412 282, 323, 362, 372, 415 278, 275, 269, 269, 274, 283, 272 |
| Reference | Nanofluid | Test Conditions | Nanoparticle | ||
|---|---|---|---|---|---|
| Concentration | Diameter (nm) | Length (µm) | |||
| Park and Jung [435] | CNT–R-123 CNT–R-134a | Heat Flux 20–60 kW/m2 | 1.0 vol. % | 20 | 1 |
| Zhang et al. [436] | MWCNT–R-123 | Heat Flux – | 0.02–0.20 vol. % | 30–70 | 2–10 |
| Sun et al. [437] | MWCNT–R-141b | Mass flux 100 to 350 kg/(m2s) | 0.059, 0.117 and 0.176 vol. % | 8 | 10–30 |
| Jiang et al. [438] | CNT–R-113 | Temperature 303 K | 0.2–1.0 vol. % | 15–80 | 1.5–10 |
| Peng et al. [439] | CNT–POE–R-113 | Heat Flux 10–80 kW/m2 | 0.1–1 wt % | 15–80 | 1.5–10 |
| Ahmadpour et al. [440] | MWCNT–mineral oil–R-600A | Heat Flux – | 0.1-.3 wt % | 5–15 | 50 |
| Kumaresan et al. [441] | MWCNT–EG–water | Temperature 273–313K | 0.15–0.45 vol. % | 30–50 | 10–20 |
| Baskar et al. [443] | MWCNT–propanol + isopropyl alcohol | Temperature 273–303K | 0.15–0.3 vol. % | – | – |
| Wang et al. [444] | Graphene–EG | Temperature 328–333K | 0.01–1 wt % | – | 5–15 |
| Lin et al. [445] | MWCNT–R-141b | – | 250–750 mg/L | 15–80 | 1.5–10 |
| Alawi and Sidik [446] | SWCNT–R-134a | Temperature 300–320 K | 1.0–5.0 vol. % | 20 | – |
| Dalkilic et al. [447] | MWCNT–POE | Temperature 288–323 K | 0.01–0.1 wt % | 10–30 | – |
| Ref. | Nanofluid | Nanoparticle | Compressor Discharge Temperature | Compressor Power | Cooling Capacity | COP | ||
|---|---|---|---|---|---|---|---|---|
| Concentration | Diameter (nm) | Length (µm) | ||||||
| Abbas et al. [449] | CNT–POE–R-134a | 0.01–0.1 wt % | – | – | – | Reduced by 2.2% | – | Improved by 4.2% |
| Jalili et al. [450] | MWCNT–water | 0–2000 ppm | 10–20 | 5–15 | – | – | – | – |
| Kamaraj and Manoj Babu [454] | CNT–POE–mineral oil–R-134a | 0.1 and 0.2 g/L | 13 | – | Negligible reduction | Negligible reduction | Improved by 16.7% | Improved by 16.7% |
| Vasconcelos et al. [453] | MWCNT–water–R-22 | 0.035–0.212 vol. % | 1–2 | 5–30 | – | Negligible reduction | Improved by 22.2% | Improved by 27.3–33.3% |
| Pico et al. [456] | ND–POE–R-410A | 0.1 and 0.5 mass % | 3–6 | – | Reduced by 3–4 °C | Negligible reduction | Improved by 4.2–7% | Improved by 4–8% |
| Pico et al. [457] | ND–POE–R-32 | 0.1 and 0.5 mass % | 3–6 | – | Reduced by 1.2–2 °C | Negligible reduction | Improved by 1–2.4% | Improved by 1–3.2% |
| Yang et al. [455] | Graphene–SUNISO 3GS–R-600a | 10, 20, and 30 mg/L | 100–3000 | – | Reduced by 2.5–4.6% | Reduced by 14.8–20.4% | Improved by 5.6% | – |
| Rahman et al. [458] | SWCNT–R-407c | 5 vol. % | – | – | – | Reduced by 4% | – | Improved by 4.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.; Bahman, A.M.; Aljuwayhel, N.F.; Ebrahim, S.A.; Mukherjee, S.; Alsayegh, A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials 2021, 11, 1628. https://doi.org/10.3390/nano11061628
Ali N, Bahman AM, Aljuwayhel NF, Ebrahim SA, Mukherjee S, Alsayegh A. Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials. 2021; 11(6):1628. https://doi.org/10.3390/nano11061628
Chicago/Turabian StyleAli, Naser, Ammar M. Bahman, Nawaf F. Aljuwayhel, Shikha A. Ebrahim, Sayantan Mukherjee, and Ali Alsayegh. 2021. "Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review" Nanomaterials 11, no. 6: 1628. https://doi.org/10.3390/nano11061628
APA StyleAli, N., Bahman, A. M., Aljuwayhel, N. F., Ebrahim, S. A., Mukherjee, S., & Alsayegh, A. (2021). Carbon-Based Nanofluids and Their Advances towards Heat Transfer Applications—A Review. Nanomaterials, 11(6), 1628. https://doi.org/10.3390/nano11061628