Sensing Behavior of Metal-Free Porphyrin and Zinc Phthalocyanine Thin Film towards Xylene-Styrene and HCl Vapors in Planar Optical Waveguide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensing Film Fabrication
2.3. Gas Preparation
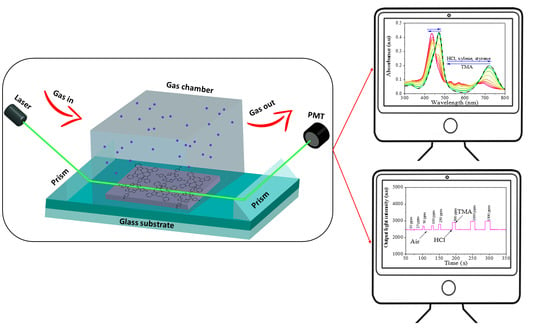
2.4. Planar Optical Waveguide Gas Detection System
3. Results and Discussion
3.1. Film Characterization
3.2. Optical Waveguide Analysis
3.3. Spectroscopic Analysis with Gas Exposure
3.4. Morphological Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujii, T.; Kawabe, S.; Horike, T.; Taguchi, T.; Ogata, M. Simultaneous determination of the urinary metabolites of toluene, xylene and styrene using high-performance capillary electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 1999, 730, 41–47. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. An e-nose made of carbon nanotube based quantum resistive sensors for the detection of eighteen polar/nonpolar VOC biomarkers of lung cancer. J. Mater. Chem. B 2013, 1, 4563–4575. [Google Scholar] [CrossRef]
- Xeringul, A.; Shawket, A.; Patime, Y.; Abliz, Y. V2O5 doped ZnFe2O4 composite thin film and it’s sensing properties. J. Funct. Mater. 2014, 45, 16048–16051. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, S.Y. High Sensitivity of HCl Gas Sensor Based on Pentacene Organic Field-Effect Transistor. Trans. Electr. Electron. Mater. 2021, 22, 140–145. [Google Scholar] [CrossRef]
- Feng, L.; Dong, C.; Li, M.; Li, L.; Jiang, X.; Gao, R.; Wang, R.; Zhang, L.; Ning, Z.; Gao, D.; et al. Terbium-based metal-organic frameworks: Highly selective and fast respond sensor for styrene detection and construction of molecular logic gate. J. Hazard. Mater. 2020, 388, 121816. [Google Scholar] [CrossRef]
- Kou, D.; Zhang, Y.; Zhang, S.; Wu, S.; Ma, W. High-sensitive and stable photonic crystal sensors for visual detection and discrimination of volatile aromatic hydrocarbon vapors. Chem. Eng. J. 2019, 375, 121987. [Google Scholar] [CrossRef]
- Kou, D.; Ma, W.; Zhang, S.; Li, R.; Zhang, Y. BTEX Vapor Detection with a Flexible MOF and Functional Polymer by Means of a Composite Photonic Crystal. ACS Appl. Mater. Interfaces 2020, 12, 11955–11964. [Google Scholar] [CrossRef]
- Mamtmin, G.; Abdurahman, R.; Yan, Y.; Nizamidin, P.; Yimit, A. A highly sensitive and selective optical waveguide sensor based on a porphyrin-coated ZnO film. Sens. Actuators A Phys. 2020, 309, 111918. [Google Scholar] [CrossRef]
- Yimit, A.; Rossberg, A.; Amemiya, T.; Itoh, K. Thin film composite optical waveguides for sensor applications: A review. Talanta 2005, 65, 1102–1109. [Google Scholar] [CrossRef]
- Tuerdi, G.; Nizamidin, P.; Kari, N.; Yimit, A.; Wang, F. Optochemical properties of gas-phase protonated tetraphenylporphyrin investigated using an optical waveguide NH3 sensor. RSC Adv. 2018, 8, 5614–5621. [Google Scholar] [CrossRef] [Green Version]
- Mamtmin, G.; Kari, N.; Abdurahman, R.; Nizamidin, P.; Yimit, A. 5, 10, 15, 20-tetrakis-(4-methoxyphenyl) porphyrin film/K+ ion-exchanged optical waveguide gas sensor. Opt. Laser Technol. 2020, 128, 106260. [Google Scholar] [CrossRef]
- Kari, N.; Koxmak, S.; Wumaier, K.; Nizamidin, P.; Abliz, S.; Yimit, A. Application of bromocresol purple nanofilm and laser light to detect mutton freshness. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 244, 118863. [Google Scholar] [CrossRef]
- Qi, Z.-M.; Honma, I.; Zhou, H. Chemical Gas Sensor Application of Open-Pore Mesoporous Thin Films Based on Integrated Optical Polarimetric Interferometry. Anal. Chem. 2006, 78, 1034–1041. [Google Scholar] [CrossRef]
- Calvete, M.; Yang, G.Y.; Hanack, M. Porphyrins and phthalocyanines as materials for optical limiting. Synth. Met. 2004, 141, 231–243. [Google Scholar] [CrossRef]
- Bommer, J.C.; Spikes, J.D. Phthalocyanines: Properties and Applications. Photochem. Photobiol. 1991, 53, 419. [Google Scholar] [CrossRef]
- Giovannetti, R.; Zannotti, M.; Alibabaei, L.; Ferraro, S. Equilibrium and Kinetic Aspects in the Sensitization of Monolayer Transparent TiO2Thin Films with Porphyrin Dyes for DSSC Applications. Int. J. Photoenergy 2014, 2014, 834269. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Sivanesan, A.; John, S.A.; Arumugam, S. Fabrication of optochemical and electrochemical sensors using thin films of porphyrin and phthalocyanine derivatives. J. Chem. Sci. 2012, 124, 1315–1325. [Google Scholar] [CrossRef]
- Martinez-Diaz, M.V.; De La Torre, G.; Torres, T. ChemInform Abstract: Lighting Porphyrins and Phthalocyanines for Molecular Photovoltaics. Cheminform 2010, 46, 7090–7108. [Google Scholar] [CrossRef]
- Senthilarasu, S.; Velumani, S.; Sathyamoorthy, R.; Subbarayan, A.; Ascencio, J.; Canizal, G.; Sebastian, P.; Chávez, J.; Pérez, R. Characterization of zinc phthalocyanine (ZnPc) for photovoltaic applications. Appl. Phys. A 2003, 77, 383–389. [Google Scholar] [CrossRef]
- Kruchinin, V.N.; Klyamer, D.D.; Spesivtsev, E.V.; Rykhlitskii, S.V.; Basova, T.V. Optical Properties of Thin Films of Zinc Phthalocyanines Determined by Spectroscopic Ellipsometry. Opt. Spectrosc. 2018, 125, 1019–1024. [Google Scholar] [CrossRef]
- Fan, F.-R.; Faulkner, L.R. Photovoltaic effects of metalfree and zinc phthalocyanines. II. Properties of illuminated thin-film cells. J. Chem. Phys. 1978, 69, 3341. [Google Scholar] [CrossRef]
- Zhou, Y.; Taima, T.; Miyadera, T.; Yamanari, T.; Yoshida, Y. Structural modifications of zinc phthalocyanine thin films for organic photovoltaic applications. J. Appl. Phys. 2012, 111, 103117. [Google Scholar] [CrossRef]
- Guo, T.; Zou, T.; Shi, P.; Song, Y.; Wu, M.; Xiao, F.; Zhang, J.; Wu, W.; Wang, H. A new polymorph of zinc-phthalocyanine and its optical properties. J. Cryst. Growth 2020, 546, 125760. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Ciccarella, G.; Siciliano, P.; Capone, S.; Rella, R. Spin-coated thin films of metal porphyrin–phthalocyanine blend for an optochemical sensor of alcohol vapours. Sens. Actuators B Chem. 2004, 100, 88–93. [Google Scholar] [CrossRef]
- Rella, R.; Capone, S.; Siciliano, P.; Spadavecchia, J.; Ciccarella, G. Spin-coated thin films of different metal phthalocyanines and porphyrin-phthalocyanine blend for optochemical sensors of volatile organic compounds. In Proceedings of the Second European Workshop on Optical Fibre Sensors, Santander, Spain, 9–11 June 2004; Volume 5502, pp. 435–438. [Google Scholar]
- Evyapan, M.; Hassan, A.; Dunbar, A. Understanding the gas adsorption kinetics of Langmuir-Schaefer porphyrin films using two comparative sensing systems. Sens. Actuators B Chem. 2018, 254, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.-M.; Honma, I.; Zhou, H. Tin-Diffused Glass Slab Waveguides Locally Covered with Tapered Thin TiO2 Films for Application as a Polarimetric Interference Sensor with an Improved Performance. Anal. Chem. 2005, 77, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Pasmooij, W.; Mandersloot, P.; Smit, M. Prism-coupling of light into narrow planar optical waveguides. J. Light. Technol. 1989, 7, 175–180. [Google Scholar] [CrossRef]
- National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 24 November 2020).
- Kaye and Laby Table of Physical and Chemical Constants. Available online: https://refractiveindex.info (accessed on 24 November 2020).
- Gouterman, M.; Wagnière, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Krichevsky, D.; Zasedatelev, A.V.; Tolbin, A.Y.; Luchkin, S.Y.; Karpo, A.B.; Krasovskii, V.I.; Tomilova, L.G. Highly transparent low-symmetry zinc phthalocyanine-based monolayers for NO2 gas detection. Thin Solid Films 2017, 642, 295–302. [Google Scholar] [CrossRef]
- Hamam, K.J.; AlOmari, M.I. A study of the optical band gap of zinc phthalocyanine nanoparticles using UV–Vis spectroscopy and DFT function. Appl. Nanosci. 2017, 7, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Makino, M.; Matsubayashi, K.; Kodama-Oda, Y.; Imawaka, N.; Mizuno, N.; Kumasaka, T.; Yoshino, K. Construction of a supramolecule comprising [2,3,9,10,16,17,23,24-octakis(2,6-dimethylphenoxy)phthalocyaninato]zinc(II) and (5,10,15,20-tetraphenylporphyrinato)zinc(II). IUCrData 2018, 3, x181741. [Google Scholar] [CrossRef]
- Senthilarasu, S.; Sathyamoorthy, R.; Kulkarni, S. Substrate temperature effects on structural orientations and optical properties of ZincPthalocyanine (ZnPc) thin films. Mater. Sci. Eng. B 2005, 122, 100–105. [Google Scholar] [CrossRef]
- Senthilarasu, S.; Hahn, Y.B.; Lee, S.-H. Nano structure formation in vacuum evaporated zinc phthalocyanine (ZnPc) thin films. J. Mater. Sci. Mater. Electron. 2008, 19, 482–486. [Google Scholar] [CrossRef]
- Zanfolim, A.A.; Volpati, D.; Olivati, C.A.; Job, A.; Constantino, C.J.L. Structural and Electric-Optical Properties of Zinc Phthalocyanine Evaporated Thin Films: Temperature and Thickness Effects. J. Phys. Chem. C 2010, 114, 12290–12299. [Google Scholar] [CrossRef]
- Wright, J.D. Phthalocyanines, Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001; pp. 6987–6991. [Google Scholar]
- Saini, R.; Mahajan, A.; Bedi, R.; Aswal, D.; Debnath, A. Solution processed films and nanobelts of substituted zinc phthalocyanine as room temperature ppb level Cl2 sensors. Sens. Actuators B Chem. 2014, 198, 164–172. [Google Scholar] [CrossRef]
- Xu, D.; Fan, D.; Shen, W. Catalyst-free direct vapor-phase growth of Zn1–xCuxO micro-cross structures and their optical properties. Nanoscale Res. Lett. 2013, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, G.; Fei, X.; Zhang, Y.; Tong, J.; Song, X.-M. Significant photoelectric conversion properties of multilayer films formed by a cationic zinc phthalocyanine complex and graphene oxide. RSC Adv. 2016, 6, 67017–67024. [Google Scholar] [CrossRef]
- Nilson, K.; Palmgren, P.; Åhlund, J.; Schiessling, J.; Gothelid, E.; Mårtensson, N.; Puglia, C.; Göthelid, M. STM and XPS characterization of zinc phthalocyanine on InSb(001). Surf. Sci. 2008, 602, 452–459. [Google Scholar] [CrossRef]
- Giovannetti, R.; Alibabaei, L.; Petetta, L. Aggregation behaviour of a tetracarboxylic porphyrin in aqueous solution. J. Photochem. Photobiol. A Chem. 2010, 211, 108–114. [Google Scholar] [CrossRef]
- De Luca, G.; Pollicino, G.; Romeo, A.; Patanè, S.; Scolaro, L.M. Control over the Optical and Morphological Properties of UV-Deposited Porphyrin Structures. Chem. Mater. 2006, 18, 5429–5436. [Google Scholar] [CrossRef]
- Zannotti, M.; Giovannetti, R.; Minofar, B.; Řeha, D.; Plackova, L.; D’Amato, C.A.; Rommozzi, E.; Dudko, H.V.; Kari, N.; Minicucci, M. Aggregation and metal-complexation behaviour of THPP porphyrin in ethanol/water solutions as function of pH. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Erokhin, V.; Ram, M.K.; Paddeu, S.; Valkova, L.; Nicolini, C. A physical insight into the gas-sensing properties of copper (II) tetra-(tert-butyl)-5,10,15,20-tetraazaporphyrin Langmuir–Blodgett films. Thin Solid Films 2000, 379, 279–286. [Google Scholar] [CrossRef]










| Chemicals | Refractive Index | Chemicals | Refractive Index |
|---|---|---|---|
| THPP | 1.75 | Air | 1.00 |
| ZnPc | 1.76 | HCl gas | 1.00 |
| Potassium ion-exchanged substrate | 1.52 | m-xylene | 1.50 |
| Glass prism | 1.78 | Styrene | 1.55 |
| Matching liquid | 1.74 |
| THPP | ZnPc | |||
|---|---|---|---|---|
| Maximum Bands | Wavelength (nm) | Molar Ext. Coef. (M−1 cm−1) | Wavelength (nm) | Molar Ext. Coef. (M−1 cm−1) |
| Soret band | 423 | 6.013 × 105 | 343 | 6.978 × 104 |
| Q bands | 520 | 1.886 × 105 | 604 | 1.154 × 104 |
| 558 | 1.667 × 105 | 669 | 2.766 × 104 | |
| 596 | 5.795 × 104 | |||
| 653 | 9.169 × 104 | |||
| Exposed Gases | Soret Band (nm) | Δλ (nm) | Q Band (nm) | ΔA at 532 nm (a.u) |
|---|---|---|---|---|
| HCl | 471 | 36 | 722 | 0.0837 |
| m-xylene | 467 | 28 | 715 | 0.0170 |
| Styrene | 471 | 34 | 721 | 0.0201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kari, N.; Zannotti, M.; Giovannetti, R.; Maimaiti, P.; Nizamidin, P.; Abliz, S.; Yimit, A. Sensing Behavior of Metal-Free Porphyrin and Zinc Phthalocyanine Thin Film towards Xylene-Styrene and HCl Vapors in Planar Optical Waveguide. Nanomaterials 2021, 11, 1634. https://doi.org/10.3390/nano11071634
Kari N, Zannotti M, Giovannetti R, Maimaiti P, Nizamidin P, Abliz S, Yimit A. Sensing Behavior of Metal-Free Porphyrin and Zinc Phthalocyanine Thin Film towards Xylene-Styrene and HCl Vapors in Planar Optical Waveguide. Nanomaterials. 2021; 11(7):1634. https://doi.org/10.3390/nano11071634
Chicago/Turabian StyleKari, Nuerguli, Marco Zannotti, Rita Giovannetti, Patigu Maimaiti, Patima Nizamidin, Shawket Abliz, and Abliz Yimit. 2021. "Sensing Behavior of Metal-Free Porphyrin and Zinc Phthalocyanine Thin Film towards Xylene-Styrene and HCl Vapors in Planar Optical Waveguide" Nanomaterials 11, no. 7: 1634. https://doi.org/10.3390/nano11071634
APA StyleKari, N., Zannotti, M., Giovannetti, R., Maimaiti, P., Nizamidin, P., Abliz, S., & Yimit, A. (2021). Sensing Behavior of Metal-Free Porphyrin and Zinc Phthalocyanine Thin Film towards Xylene-Styrene and HCl Vapors in Planar Optical Waveguide. Nanomaterials, 11(7), 1634. https://doi.org/10.3390/nano11071634