Highly Effective Self-Propagating Synthesis of Lamellar ZnO-Decorated MnO2 Nanocrystals with Improved Supercapacitive Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Layered MOx-δ-MnO2
2.2. Characterization of Synthesized Materials
2.3. Electrochemical Tests
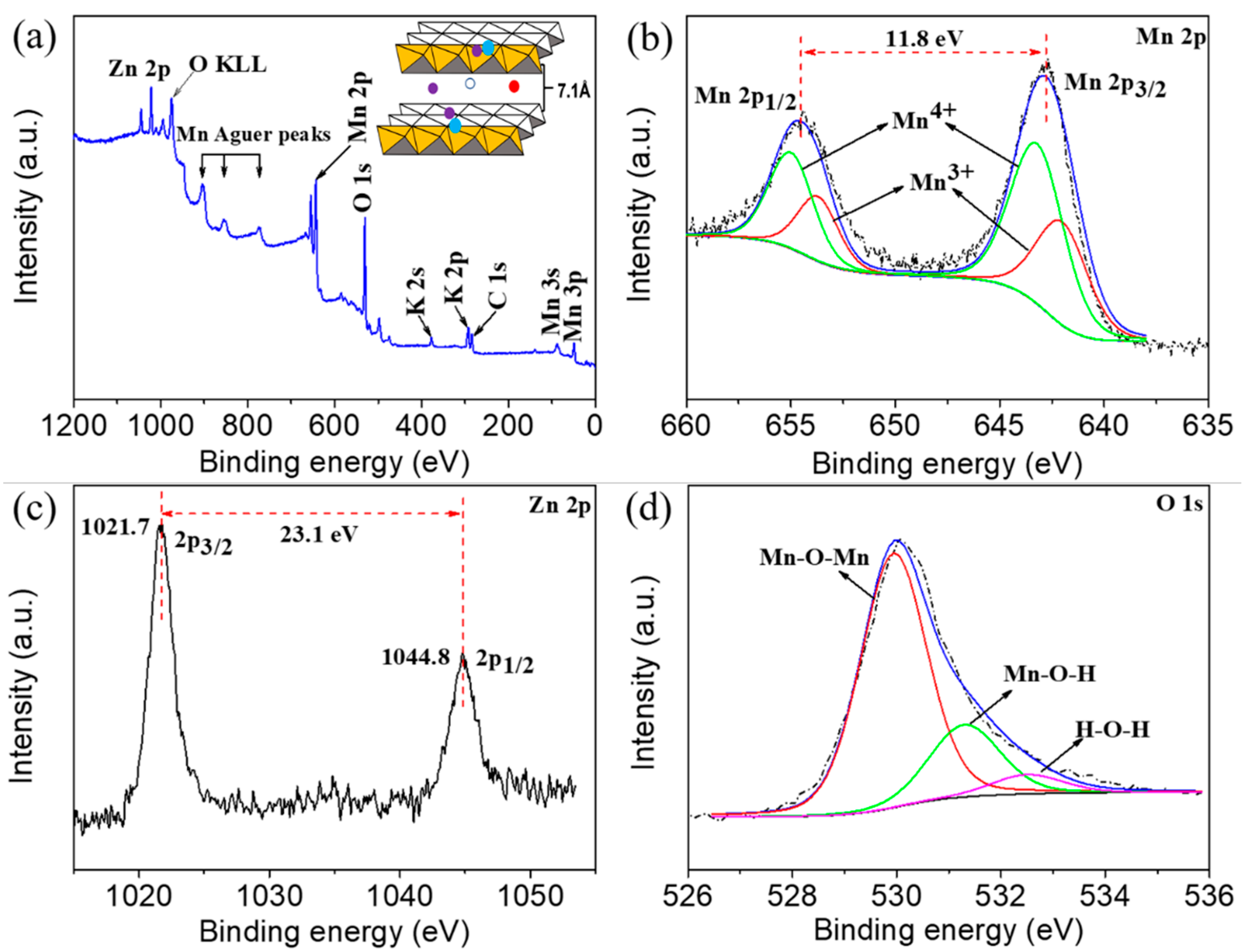
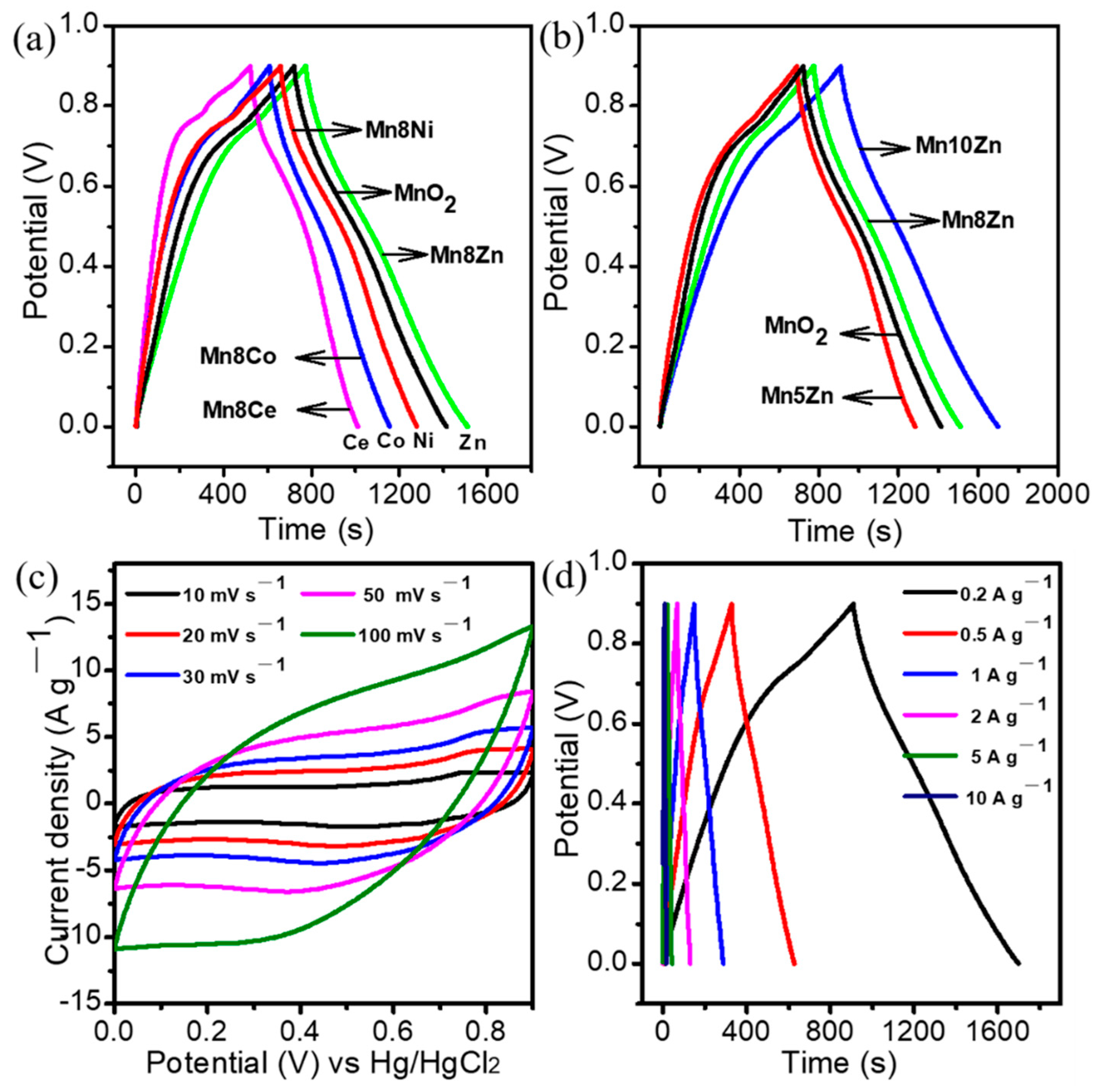
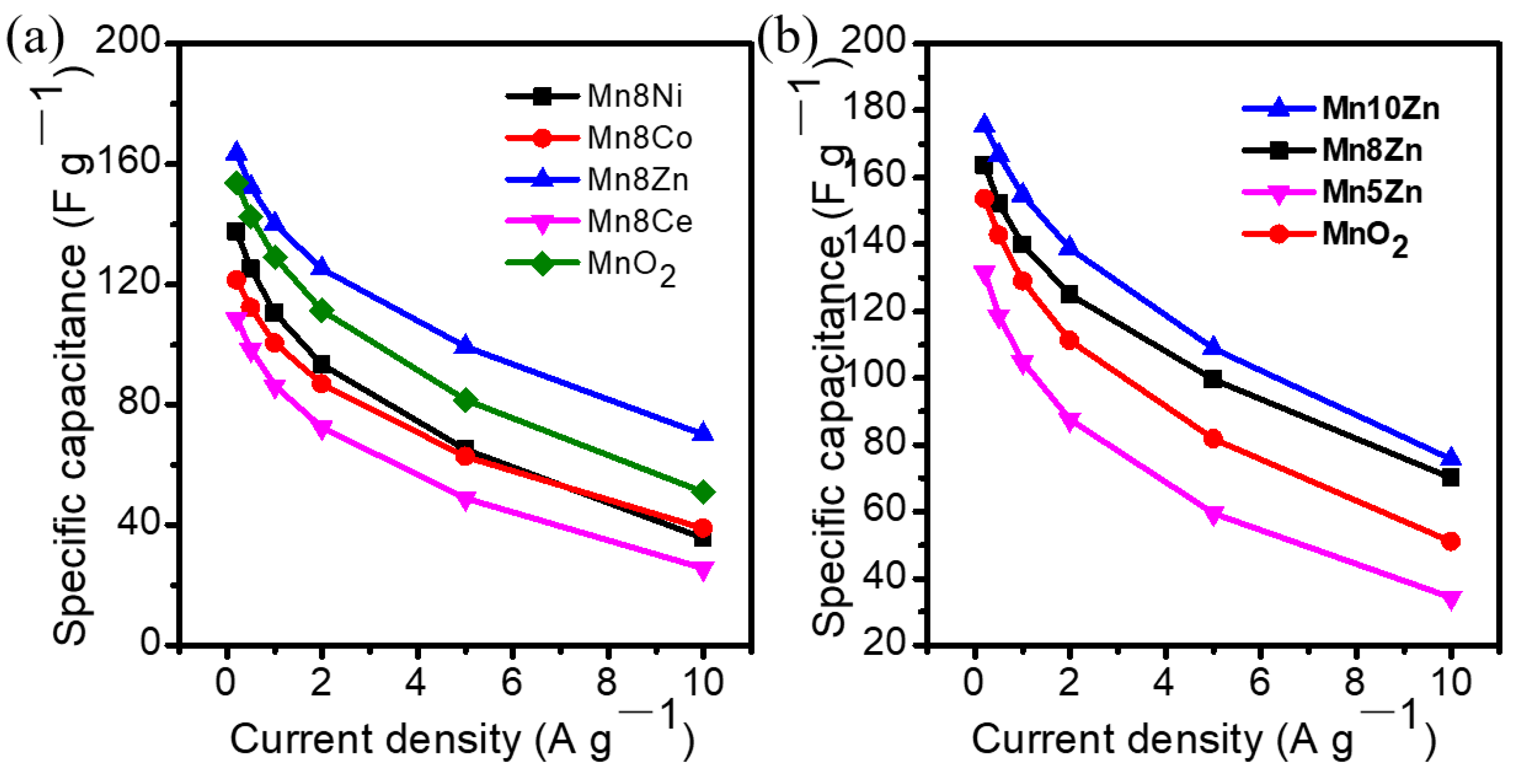
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Z.H.; Song, Y.; Feng, D.Y.; Sun, Z.; Sun, X.Q.; Liu, X.X. High Mass Loading MnO2 with Hierarchical Nanostructures for Supercapacitors. ACS Nano 2018, 12, 3557–3567. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Metz, P.; Hey, T.; Gong, Y.X.; Liu, D.W.; Edwards, D.D.; Howe, J.Y.; Huang, R.; Misture, S.T. The Critical Role of Point Defects in Improving the Specific Capacitance of δ-MnO2 Nanosheets. Nat. Commun. 2017, 8, 14559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, K.; Ali, I.; Hasnain, S.; Hussain, S.S.; Hussain, B.; Khan, M.S.; Ammar, S.M.; Hussain, B.; Hussain, A.; Javed, M.A.; et al. Reagents Assisted Mg-doped CeO2 for High-performance Energy-storage Applications. J. Electroanal. Chem. 2020, 873, 114401. [Google Scholar] [CrossRef]
- Subalakshmi, P.; Ganesan, M.; Sivashanmugam, A. Synthesis of 3D Architecture CuO Micro Balls and Nano Hexagons and its Electrochemical Capacitive Behavior. Mater. Design 2017, 119, 104–112. [Google Scholar] [CrossRef]
- Bounor, B.; Asbani, B.; Douard, C.; Favier, F.; Brousse, T.; Lethien, C. On Chip MnO2-based 3D Micro-supercapacitors with Ultra-high Areal Energy Density. Energy Storage Mater. 2021, 38, 520–527. [Google Scholar]
- Wang, Y.; Guo, J.; Wang, T.F.; Shao, J.F.; Wang, D.; Yang, Y.W. Mesoporous Transition Metal Oxides for Supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar]
- Tang, C.L.; Wei, X.; Jiang, Y.M.; Wu, X.Y.; Han, L.N.; Wang, K.X.; Chen, J.S. Cobalt-Doped MnO2 Hierarchical Yolk Shell Spheres with Improved Supercapacitive Performance. J. Phys. Chem. C 2015, 119, 8465–8471. [Google Scholar] [CrossRef]
- Nasser, R.; Zhang, G.F.; Song, J.M. Facile and Low-cost Synthesis of Cobalt-doped MnO2 Decorated with Graphene Oxide for High Performance 2.3V Aqueous Asymmetric Supercapacitors. Electrochim. Acta 2020, 345, 136198. [Google Scholar]
- Kang, J.; Hirata, A.; Kang, L.; Zhang, X.; Hou, Y.; Chen, L.; Li, C.; Fujita, T.; Akagi, K.; Chen, M. Enhanced Supercapacitor Performance of MnO2 by Atomic Doping. Angew. Chem. Int. Ed. 2013, 52, 1664–1667. [Google Scholar]
- Wang, J.; Tian, L.; Xie, W.L.; Wang, X.; Long, X.; Sun, K.; Emin, A.; Liu, D.Q.; Fu, Y.J.; Chen, Q.; et al. A Hierarchical Interconnected Nanosheet Structure of Porous δ-MnO2 on Graphite Paper as Cathode with a Broad Potential Window for NaNO3 Aqueous Electrolyte Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 2614–2622. [Google Scholar] [CrossRef]
- Chen, J.J.; Huang, Y.; Li, C.; Chen, X.F.; Zhang, X. Synthesis of NiO@MnO2 Core/shell Nanocomposites for Supercapacitor Application. Appl. Surf. Sci. 2016, 360, 534–539. [Google Scholar] [CrossRef]
- Lu, X.H.; Yu, M.H.; Wang, G.M.; Zhai, T.; Xie, S.L.; Ling, Y.C.; Tong, Y.X.; Li, Y. H-TiO2@MnO2//H-TiO2@C Core-shell Nanowires for High Performance and Flexible Asymmetric Supercapacitors. Adv. Mater. 2013, 25, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Jia, J.Q.; Wang, T.; Zhao, D.; Yang, J.; Dong, F.; Shang, Z.G.; Zhang, Y.X. Rational Design of Octahedron and Nanowire CeO2@MnO2 Core-shell Heterostructures with Outstanding Rate Capability for Asymmetric Supercapacitors. Chem. Commun. 2015, 51, 14840–14843. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Pan, W.; Xue, D.F. Phase Transformation of Ce3+-Doped MnO2 for Pseudocapacitive Electrode Materials. J. Phy. Chem. C 2016, 120, 20077–20081. [Google Scholar] [CrossRef]
- Peng, R.C.; Wu, N.; Zheng, Y.; Huang, Y.B.; Luo, Y.B.; Yu, P.; Zhuang, L. Large-Scale Synthesis of Metal-Ion-Doped Manganese Dioxide for Enhanced Electrochemical Performance. ACS Appl. Mater. Inter. 2016, 8, 8474–8480. [Google Scholar] [CrossRef]
- Li, L.M.; Luo, J.J.; Liu, Y.F.; Jing, F.L.; Su, D.S.; Chu, W. Self-Propagated Flaming Synthesis of Highly Active Layered CuO-δ-MnO2 Hybrid Composites for Catalytic Total Oxidation of Toluene Pollutant. ACS Appl Mater Interfaces 2017, 9, 21798–21808. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J.; Niu, L.Y.; Shen, C.; Zhang, Z.K.; Lin, J.H.; Shen, F.Y.; Gong, Y.Y.; Li, C.; Liu, X.J.; Xu, S.Q. Modulating the Electronic Structure and Pseudocapacitance of δ-MnO2 Through Transitional Metal M (M = Fe, Co and Ni) Doping. Electrochim. Acta 2019, 306, 529–540. [Google Scholar] [CrossRef]
- Xu, N.N.; Nie, Q.; Luo, Y.Q.; Yao, C.Z.; Gong, Q.J.; Liu, Y.Y.; Zhou, X.D.; Qiao, J.L. Controllable Hortensia-like MnO2 Synergized with Carbon Nanotubes as an Efficient Electrocatalyst for Long-Term Metal-Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 578–587. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Zhang, C.; He, K.; Chen, H.R.; Yao, W.T.; Sharifi-Asl, S.; Song, B.A.; Yang, Z.Z.; Nie, A.M.; Luo, X.Y.; et al. The Influence of Large Cations on the Electrochemical Properties of Tunnel-structured Metal Oxides. Nat. Commun. 2016, 7, 13374. [Google Scholar] [CrossRef]
- Li, L.M.; Chu, W.; Liu, Y. Insights into Key Parameters of MnO2 Catalyst toward High Catalytic Combustion Performance. J. Mater. Sci. 2021, 56, 6361–6373. [Google Scholar] [CrossRef]
- Bose, N.; Sundararajan, V.; Prasankumar, T.; Jose, S.P. α-MnO2 Coated Anion Intercalated Carbon Nanowires: A High Rate Capability Electrode Material for Supercapacitors. Mater. Lett. 2020, 278, 128457. [Google Scholar] [CrossRef]
- Radhamani, A.V.; Shareef, K.M.; Rao, M.S.R. ZnO@MnO2 Core-Shell Nanofiber Cathodes for High Performance Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30531–30542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yun, J.H.; Vishwa, B.; Singh, B.; Kim, J.; Kim, J.S.; Kim, B.S.; Lee, C.Y. Role of Ce3+ Valence State and Surface Oxygen Vacancies on Enhanced Electrochemical Performance of Single Step Solvothermally Synthesized CeO2 Nanoparticles. Electrochim. Acta 2018, 284, 709–720. [Google Scholar] [CrossRef]
- Qiao, Z.; Xia, C.; Cai, Y.X.; Afzal, M.; Wang, H.; Qiao, J.L.; Zhu, B. Electrochemical and Electrical Properties of Doped CeO2-ZnO Composite for Low-temperature Solid Oxide Fuel Cell Applications. J. Power Sources 2018, 392, 33–40. [Google Scholar] [CrossRef]
- Zhu, S.J.; Li, L.; Liu, J.B.; Wang, H.T.; Wang, T.; Zhang, Y.X.; Zhang, L.L.; Ruoff, R.S.; Dong, F. Structural Directed Growth of Ultrathin Parallel Birnessite on β-MnO2 for High-Performance Asymmetric Supercapacitors. ACS Nano 2018, 12, 1033–1042. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, Y.; Yang, D.; Li, J. High Performance MnO2 Supercapacitor Material Prepared by Modified Electrodeposition Method with Different Electrodeposition Voltages. J. Energy Storage 2020, 29, 101363. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, J.; Li, H.; Lan, L.; Deng, J. Highly Effective Self-Propagating Synthesis of Lamellar ZnO-Decorated MnO2 Nanocrystals with Improved Supercapacitive Performance. Nanomaterials 2021, 11, 1680. https://doi.org/10.3390/nano11071680
Li L, Li J, Li H, Lan L, Deng J. Highly Effective Self-Propagating Synthesis of Lamellar ZnO-Decorated MnO2 Nanocrystals with Improved Supercapacitive Performance. Nanomaterials. 2021; 11(7):1680. https://doi.org/10.3390/nano11071680
Chicago/Turabian StyleLi, Luming, Jing Li, Hongmei Li, Li Lan, and Jie Deng. 2021. "Highly Effective Self-Propagating Synthesis of Lamellar ZnO-Decorated MnO2 Nanocrystals with Improved Supercapacitive Performance" Nanomaterials 11, no. 7: 1680. https://doi.org/10.3390/nano11071680
APA StyleLi, L., Li, J., Li, H., Lan, L., & Deng, J. (2021). Highly Effective Self-Propagating Synthesis of Lamellar ZnO-Decorated MnO2 Nanocrystals with Improved Supercapacitive Performance. Nanomaterials, 11(7), 1680. https://doi.org/10.3390/nano11071680




