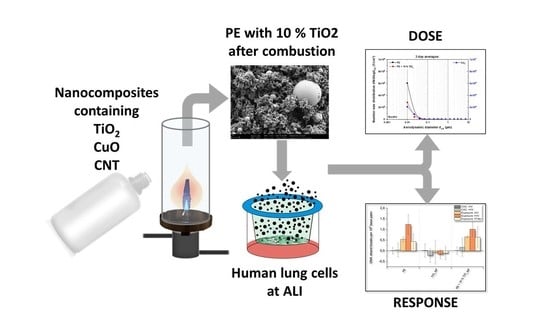
Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Combustion of Nanomaterials and Nanocomposites
2.1.1. Nanomaterials
2.1.2. Nanocomposites
2.1.3. Burner and Aerosol Conditioning
2.1.4. Characterization of Combustion Aerosols
2.1.5. Dose Determination
2.2. Cell Culture
2.2.1. Air-Liquid Interface Exposure
2.2.2. LDH Release
2.2.3. Alkaline Unwinding
2.2.4. Gene Expression Analysis
2.2.5. Inflammatory Response
2.3. Statistical Analysis
3. Results
3.1. Aerosol Characterization
3.1.1. Impact of Thermal Decomposition on the Aerosols Applied on A549 Cells
3.1.2. Impact of Thermal Decomposition on the Aerosols Applied on Reconstituted Tissue
3.2. Dosimetry of Deposited Particles on Test Systems
3.3. Characterization and Concentration of Combustion-Generated Volatile Organic Compounds
3.4. LDH Release by Combustion Aerosols
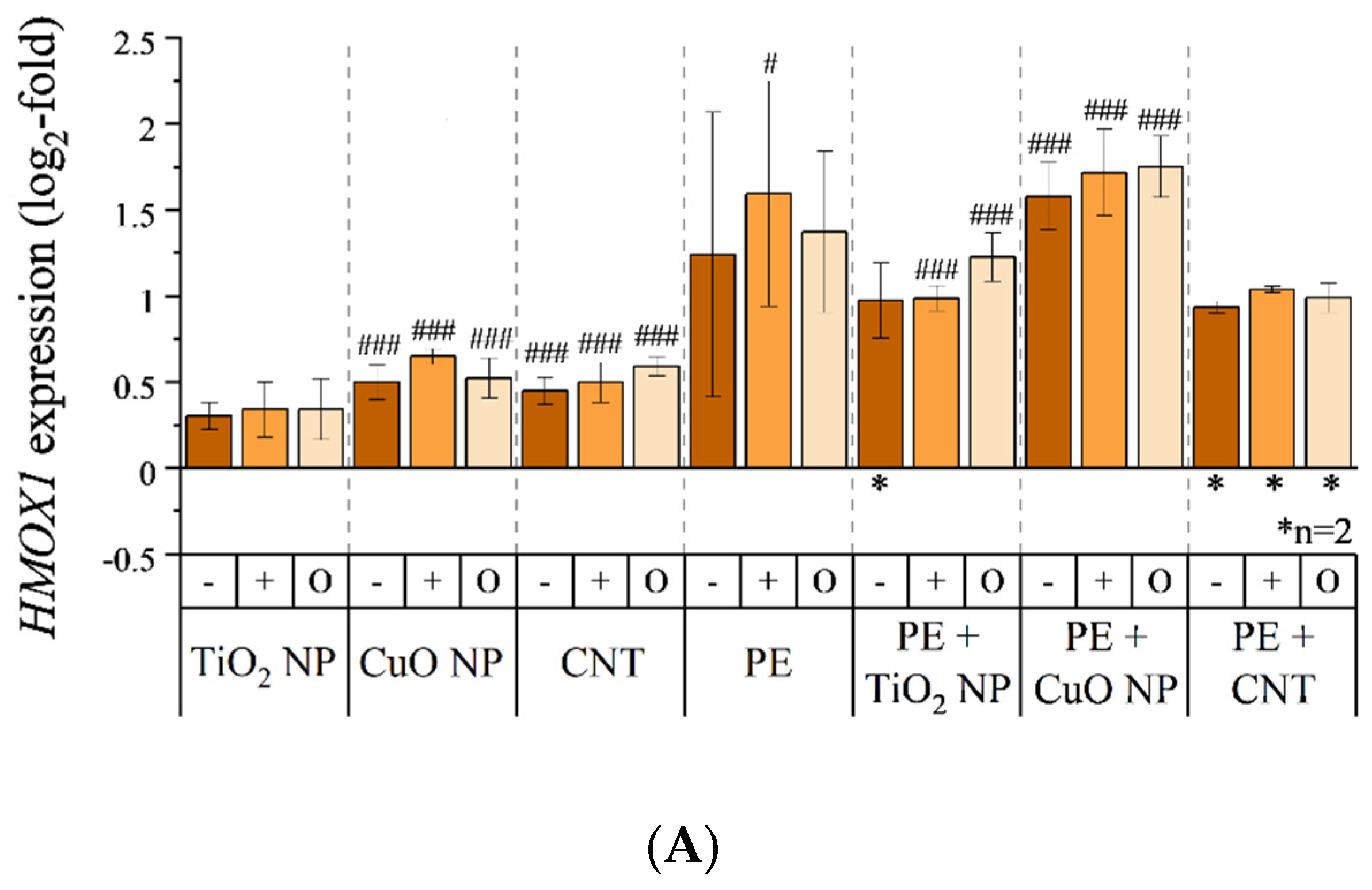
3.5. Transcriptional Toxicity Profile
3.6. Genotoxicity of Combustion Aerosols
3.7. Inflammatory Response on Combustion-Generated Aerosols
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson-Wright, C.; Singh, D.; Demokritou, P. Toxicological Implications of Released Particulate Matter during Thermal Decomposition of Nano-Enabled Thermoplastics. NanoImpact 2017, 5, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winey, K.I.; Vaia, R.A. Polymer Nanocomposites. MRS Bull. 2007, 32, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Schwirn, K.; Völker, D. Nanomaterials in the Environment—Current State of Knowledge and Regulations on Chemical Safety; German Environment Agency: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- Titandioxid in Kunststoffen—Kurzfassung der Ergebnisse aus der Analyse der Kunststoffverarbeitung, der Kunststoffabfallmengen und der Verwertung von Kunststoffabfällen in Deutschland 2017 (Titanium Dioxide in Plastics—Summary of Results from the Analysis of Plastics Processing, Plastic Waste Volumes and Plastic Waste Recycling in Germany 2017); Conversio Market & Strategy GmbH: Bavaria, Germany, 2019.
- Singh, D.; Sotiriou, G.A.; Zhang, F.; Mead, J.; Bello, D.; Wohlleben, W.; Demokritou, P. End-of-life thermal decomposition of nano-enabled polymers: Effect of nanofiller loading and polymer matrix on by-products. Environ. Sci. Nano 2016, 3, 1293–1305. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Singh, D.; Zhang, F.; Wohlleben, W.; Chalbot, M.G.; Kavouras, I.G.; Demokritou, P. An integrated methodology for the assessment of environmental health implications during thermal decomposition of nano-enabled products. Environ. Sci. Nano 2015, 2, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourchez, J.; Chivas-Joly, C.; Longuet, C.; Leclerc, L.; Sarry, G.; Lopez-Cuesta, J.M. End-of-life incineration of nanocomposites: New insights into nanofiller partitioning into by-products and biological outcomes of airborne emission and residual ash. Environ. Sci. Nano 2018, 5, 1951–1964. [Google Scholar] [CrossRef]
- Adam, V.; Nowack, B. European country-specific probabilistic assessment of nanomaterial flows towards landfilling, incineration and recycling. Environ. Sci. Nano 2017, 4, 1961–1973. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Singh, D.; Zhang, F.; Chalbot, M.G.; Spielman-Sun, E.; Hoering, L.; Kavouras, I.G.; Lowry, G.V.; Wohlleben, W.; Demokritou, P. Thermal decomposition of nano-enabled thermoplastics: Possible environmental health and safety implications. J. Hazard Mater. 2016, 305, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Vejerano, E.P.; Leon, E.C.; Holder, A.L.; Marr, L.C. Characterization of particle emissions and fate of nanomaterials during incineration. Environ. Sci. Nano 2014, 1, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Vejerano, E.P.; Holder, A.L.; Marr, L.C. Emissions of polycyclic aromatic hydrocarbons, polychlorinated dibenzo-p-dioxins, and dibenzofurans from incineration of nanomaterials. Environ. Sci. Technol. 2013, 47, 4866–4874. [Google Scholar] [CrossRef]
- Singh, D.; Schifman, L.A.; Watson-Wright, C.; Sotiriou, G.A.; Oyanedel-Craver, V.; Wohlleben, W.; Demokritou, P. Nanofiller Presence Enhances Polycyclic Aromatic Hydrocarbon (PAH) Profile on Nanoparticles Released during Thermal Decomposition of Nano-enabled Thermoplastics: Potential Environmental Health Implications. Environ. Sci. Technol. 2017, 51, 5222–5232. [Google Scholar] [CrossRef]
- Chivas-Joly, C.; Longuet, C.; Pourchez, J.; Leclerc, L.; Sarry, G.; Lopez-Cuesta, J.M. Physical, morphological and chemical modification of Al-based nanofillers in by-products of incinerated nanocomposites and related biological outcome. J. Hazard Mater. 2019, 365, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Part, F.; Berge, N.; Baran, P.; Stringfellow, A.; Sun, W.; Bartelt-Hunt, S.; Mitrano, D.; Li, L.; Hennebert, P.; Quicker, P.; et al. A review of the fate of engineered nanomaterials in municipal solid waste streams. J. Waste Manag. 2018, 75, 427–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panas, A.; Marquardt, C.; Nalcaci, O.; Bockhorn, H.; Baumann, W.; Paur, H.-R.; Mülhopt, S.; Diabaté, S.; Weiss, C. Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 2013, 7, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Guidance Document on Good In Vitro Method Practices (GIVIMP). In Series on Testing and Assessment; OECD: Paris, France, 2018.
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air–Liquid Interface In Vitro Models for Respiratory Toxicology Research. Appl. In Vitro Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Paur, H.-R.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In-vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung-A dialog between aerosol science and biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air-liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Mülhopt, S.; Diabaté, S.; Krebs, T.; Weiss, C.; Paur, H.R. Lung toxicity determination by in vitro exposure at the air liquid interface with an integrated online dose measurement. J. Phys. Conf. Ser. 2009, 170, 012008. [Google Scholar] [CrossRef] [Green Version]
- Mülhopt, S.; Paur, H.-R.; Schlager, C. Vorrichtung zur Messung von Feinstpartikelmassen; Karlsruher Inst Technologie: Karlsruhe, Germany, 2014. [Google Scholar]
- Mülhopt, S.; Schlager, C.; Paur, H.-R.; Berger, M. Vorrichtung für die Probenahme von Feinstpartikelmassen für Eine TEM-Untersuchung; Karlsruher Inst Technologie: Karlsruhe, Germany, 2018. [Google Scholar]
- Oeder, S.; Kanashova, T.; Sippula, O.; Sapcariu, S.C.; Streibel, T.; Arteaga-Salas, J.M.; Passig, J.; Dilger, M.; Paur, H.-R.; Schlager, C.; et al. Particulate Matter from Both Heavy Fuel Oil and Diesel Fuel Shipping Emissions Show Strong Biological Effects on Human Lung Cells at Realistic and Comparable In Vitro Exposure Conditions. PLoS ONE 2015, 10, e0126536. [Google Scholar] [CrossRef] [PubMed]
- Sapcariu, S.C.; Kanashova, T.; Dilger, M.; Diabate, S.; Oeder, S.; Passig, J.; Radischat, C.; Buters, J.; Sippula, O.; Streibel, T.; et al. Metabolic Profiling as Well as Stable Isotope Assisted Metabolic and Proteomic Analysis of RAW 264.7 Macrophages Exposed to Ship Engine Aerosol Emissions. PLoS ONE 2016, 11, e0157964. [Google Scholar] [CrossRef]
- Kanashova, T.; Sippula, O.; Oeder, S.; Streibel, T.; Passig, J.; Czech, H.; Kaoma, T.; Sapcariu, S.C.; Dilger, M.; Paur, H.-R.; et al. Emissions from a Modern Log wood Masonry Heater and Wood Pellet Boiler: Composition and Biological Impact on Air-Liquid Interface Exposed Human Lung Cancer Cells. JMCM 2018, 1, 23–35. [Google Scholar]
- Comouth, A.; Saathoff, H.; Naumann, K.-H.; Mülhopt, S.; Paur, H.-R.; Leisner, T. Modelling and measurement of particle deposition for cell exposure at the air liquid interface. J. Aerosol Sci. 2013, 63, 103–114. [Google Scholar] [CrossRef]
- Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.-E.; Biola-Clier, M.; Ambrose, S.; et al. Air–Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomaterials 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Strauch, B.M.; Niemand, R.K.; Winkelbeiner, N.L.; Hartwig, A. Comparison between micro-and nanosized copper oxide and water soluble copper chloride: Interrelationship between intracellular copper concentrations, oxidative stress and DNA damage response in human lung cells. Part. Fibre Toxicol. 2017, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Schoch, S.; Wall, J.; Strauch, B.M.; Hartwig, A. Toxicity and Gene Expression Profiling of Copper- and Titanium-Based Nanoparticles Using Air–Liquid Interface Exposure. Chem. Res. Toxicol. 2020, 33, 1237–1249. [Google Scholar] [CrossRef]
- Fischer, B.M.; Neumann, D.; Piberger, A.L.; Risnes, S.F.; Köberle, B.; Hartwig, A. Use of high-throughput RT-qPCR to assess modulations of gene expression profiles related to genomic stability and interactions by cadmium. Arch. Toxicol. 2016, 90, 2745–2761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskinen, J.; Pietarinen, K.; Lehtimäki, M. Electrical low pressure impactor. J. Aerosol Sci. 1992, 23, 353–360. [Google Scholar] [CrossRef]
- Kaur, K.; Overacker, D.; Ghandehari, H.; Reilly, C.; Paine, R.; Kelly, K.E. Determining real-time mass deposition with a quartz crystal microbalance in an electrostatic, parallel-flow, air-liquid interface exposure system. J. Aerosol Sci. 2021, 151, 105653. [Google Scholar] [CrossRef] [PubMed]
- Tippe, A.; Heinzmann, U.; Roth, C. Deposition of fine and ultrafine aerosol particles during exposure at the air/cell interface. J. Aerosol Sci. 2002, 33, 207–218. [Google Scholar] [CrossRef]
- Hartwig, A.; Klyszcz-Nasko, H.; Schlepegrell, R.; Beyersmann, D. Cellular damage by ferric nitrilotriacetate and ferric citrate in V79 cells: Interrelationship between lipid peroxidation, DNA strand breaks and sister chromatid exchanges. Carcinogenesis 1993, 14, 107–112. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Baumann, W.; Teuscher, N.; Hauser, M.; Gehrmann, J.; Paur, H.-R.; Stapf, D. Behaviour of engineered nanoparticles in a lab-scale flame and combustion chamber. Energy Procedia 2017, 120, 705–712. [Google Scholar] [CrossRef]
- Paur, H.-R.; Baumann, W.; Hauser, M.; Lang, I.; Teuscher, N.; Seifert, H.; Stapf, D. Thermal Stability and Material Balance of Nanomaterials in Waste Incineration. J. Phys. Conf. Ser. 2017, 838, 012012. [Google Scholar] [CrossRef] [Green Version]
- Ihalainen, M.; Lind, T.; Arffman, A.; Torvela, T.; Jokiniemi, J. Break-Up and Bounce of TiO2 Agglomerates by Impaction. Aerosol Sci. Technol. 2013, 48, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Mülhopt, S.; Paur, H.-R.; Diabaté, S.; Krug, H.F. In Vitro Testing of Inhalable Fly Ash at the Air Liquid Interface. In Advanced Environmental Monitoring; Kim, Y.J., Platt, U., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 402–414. [Google Scholar]
- Mülhopt, S.; Schlager, C.; Berger, M.; Murugadoss, S.; Hoet, P.H.; Krebs, T.; Paur, H.-R.; Stapf, D. A novel TEM grid sampler for airborne particles to measure the cell culture surface dose. Sci. Rep. 2020, 10, 8401. [Google Scholar] [CrossRef] [PubMed]
- Jugan, M.-L.; Barillet, S.; Simon-Deckers, A.; Herlin-Boime, N.; Sauvaigo, S.; Douki, T.; Carriere, M. Titanium dioxide nanoparticles exhibit genotoxicity and impair DNA repair activity in A549 cells. Nanotoxicology 2012, 6, 501–513. [Google Scholar] [CrossRef]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carriere, M. In vitro investigation of oxide nanoparticle and carbon nanotube toxicity and intracellular accumulation in A549 human pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Tedja, R.; Marquis, C.; Lim, M.; Amal, R. Biological impacts of TiO2 on human lung cell lines A549 and H1299: Particle size distribution effects. J. Nanopart. Res. 2011, 13, 3801–3813. [Google Scholar] [CrossRef]
- Loret, T.; Peyret, E.; Dubreuil, M.; Aguerre-Chariol, O.; Bressot, C.; le Bihan, O.; Amodeo, T.; Trouiller, B.; Braun, A.; Egles, C.; et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part. Fibre Toxicol. 2016, 13, 58. [Google Scholar] [CrossRef] [Green Version]
- Rach, J.; Budde, J.; Möhle, N.; Aufderheide, M. Direct exposure at the air-liquid interface: Evaluation of an in vitro approach for simulating inhalation of airborne substances. J. Appl. Toxicol. 2014, 34, 506–515. [Google Scholar] [CrossRef]
- Steinritz, D.; Möhle, N.; Pohl, C.; Papritz, M.; Stenger, B.; Schmidt, A.; Kirkpatrick, C.J.; Thiermann, H.; Vogel, R.; Hoffmann, S.; et al. Use of the Cultex® Radial Flow System as an in vitro exposure method to assess acute pulmonary toxicity of fine dusts and nanoparticles with special focus on the intra- and inter-laboratory reproducibility. Chem. Biol. Interact. 2013, 206, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Kooter, I.; Ilves, M.; Gröllers-Mulderij, M.; Duistermaat, E.; Tromp, P.C.; Kuper, F.; Kinaret, P.; Savolainen, K.; Greco, D.; Karisola, P.; et al. Molecular signature of asthma-enhanced sensitivity to CuO nanoparticle aerosols from 3D cell model. ACS Nano 2019, 13, 6932–6946. [Google Scholar] [CrossRef] [Green Version]
- Kooter, I.M.; Gröllers-Mulderij, M.; Steenhof, M.; Duistermaat, E.; van Acker, F.A.A.; Staal, Y.C.M.; Tromp, P.C.; Schoen, E.; Kuper, C.F.; van Someren, E. Cellular Effects in an In Vitro Human 3D Cellular Airway Model and A549/BEAS-2B In Vitro Cell Cultures Following Air Exposure to Cerium Oxide Particles at an Air–Liquid Interface. Appl. In Vitro Toxicol. 2016, 2, 56–66. [Google Scholar] [CrossRef]
- Kooter, I.M.; Gröllers-Mulderij, M.; Duistermaat, E.; Kuper, F.; Schoen, E.D. Factors of concern in a human 3D cellular airway model exposed to aerosols of nanoparticles. Toxicol. In Vitro 2017, 44, 339–348. [Google Scholar] [CrossRef]
- Semisch, A.; Ohle, J.; Witt, B.; Hartwig, A. Cytotoxicity and genotoxicity of nano-and microparticulate copper oxide: Role of solubility and intracellular bioavailability. Part. Fibre Toxicol. 2014, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Cronholm, P.; Karlsson, H.L.; Hedberg, J.; Lowe, T.A.; Winnberg, L.; Elihn, K.; Wallinder, I.O.; Moller, L. Intracellular uptake and toxicity of Ag and CuO nanoparticles: A comparison between nanoparticles and their corresponding metal ions. Small 2013, 9, 970–982. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Moller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef]
- Aufderheide, M.; Halter, B.; Mohle, N.; Hochrainer, D. The CULTEX RFS: A comprehensive technical approach for the in vitro exposure of airway epithelial cells to the particulate matter at the air-liquid interface. Biomed. Res. Int. 2013, 2013, 734137. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.; Park, J.H.; Peters, T.M.; Thorne, P.S. Toxicity of copper oxide nanoparticles in lung epithelial cells exposed at the air--liquid interface compared with in vivo assessment. Toxicol. In Vitro 2015, 29, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Uddin, N.; Nyden, M.; Davis, R. Characterization of Nanoparticle Release from Polymer Nanocomposites Due to Fire. In Proceedings of the Nano-Tech 2011 Conference, Boston, MA, USA, 13–16 June 2011. [Google Scholar]
- Chortarea, S.; Barosova, H.; Clift, M.J.D.; Wick, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Human Asthmatic Bronchial Cells Are More Susceptible to Subchronic Repeated Exposures of Aerosolized Carbon Nanotubes at Occupationally Relevant Doses Than Healthy Cells. ACS Nano 2017, 11, 7615–7625. [Google Scholar] [CrossRef] [PubMed]
- Mascelloni, M.; Delgado-Saborit, J.M.; Hodges, N.J.; Harrison, R.M. Study of gaseous benzene effects upon A549 lung epithelial cells using a novel exposure system. Toxicol. Lett. 2015, 237, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Dilger, M.; Orasche, J.; Zimmermann, R.; Paur, H.-R.; Diabaté, S.; Weiss, C. Toxicity of wood smoke particles in human A549 lung epithelial cells: The role of PAHs, soot and zinc. Arch. Toxicol. 2016, 90, 3029–3044. [Google Scholar] [CrossRef] [PubMed]








| Material | Mass Concentration (µg/cm³) | Dose (ng/cm²) | Dose HV (ng/cm²) | Cell System |
|---|---|---|---|---|
| TiO2 | 137 ± 20 | 15 ± 2 | 73 ± 11 | A549 |
| CuO | 256 ± 151 | 27 ± 16 | 136 ± 80 | A549 |
| CNT (+ Gum arabic) | 44 ± 7 | 5 ± 1 | 23 ± 4 | A549 |
| Gum arabic | 34 ± 4 | 4 ± 0,4 | 18 ± 2 | A549 |
| PE | 505 ± 8 | 54 ± 1 | 268 ± 4 | A549 |
| PE + TiO2 | 527 ± 317 | 56 ± 34 | 280 ± 168 | A549 |
| PE + CuO | 235 ± 18 | 25 ± 2 | 125 ± 10 | A549 |
| PE + CNTs | 106 ± 16 | 11 ± 2 | 54 ± 8 | A549 |
| PE | 465 ± 120 | 48 ± 12 | 238 ± 58 | MucilAir™ |
| PE + TiO2 | 209 ± 27 | 22 ± 3 | 111 ± 14 | MucilAir™ |
| TiO2 | 20,610 ± 801 | 2186 ± 361 | 10,932 ± 1803 | MucilAir™ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hufnagel, M.; May, N.; Wall, J.; Wingert, N.; Garcia-Käufer, M.; Arif, A.; Hübner, C.; Berger, M.; Mülhopt, S.; Baumann, W.; et al. Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model. Nanomaterials 2021, 11, 1685. https://doi.org/10.3390/nano11071685
Hufnagel M, May N, Wall J, Wingert N, Garcia-Käufer M, Arif A, Hübner C, Berger M, Mülhopt S, Baumann W, et al. Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model. Nanomaterials. 2021; 11(7):1685. https://doi.org/10.3390/nano11071685
Chicago/Turabian StyleHufnagel, Matthias, Nadine May, Johanna Wall, Nadja Wingert, Manuel Garcia-Käufer, Ali Arif, Christof Hübner, Markus Berger, Sonja Mülhopt, Werner Baumann, and et al. 2021. "Impact of Nanocomposite Combustion Aerosols on A549 Cells and a 3D Airway Model" Nanomaterials 11, no. 7: 1685. https://doi.org/10.3390/nano11071685