Ag-Based Synergistic Antimicrobial Composites. A Critical Review
Abstract
:1. Introduction
1.1. Antimicrobial Resistance as Unnoticed Threat
1.2. Nanotechnology and Silver
1.3. Compounds of Natural Origin That Exhibit a Wide Range Antimicrobial Action and Do Not Cause Development of AMR
1.4. Novel Approaches for the Creation of Organic/Inorganic Composites and Nanofabrication of Synergistic Antimicrobials, Combination with Drugs
1.5. Aim of the Review
2. General Synthetic Methods of AgNPs
2.1. Conventional Methods—Chemical Synthesis
2.2. Electrochemical Synthesis and Electrospinning Methods
2.3. Physical Methods
2.4. Bioinspired Methods
2.5. Development of Complex Composite Systems by Various Synthetic Routes
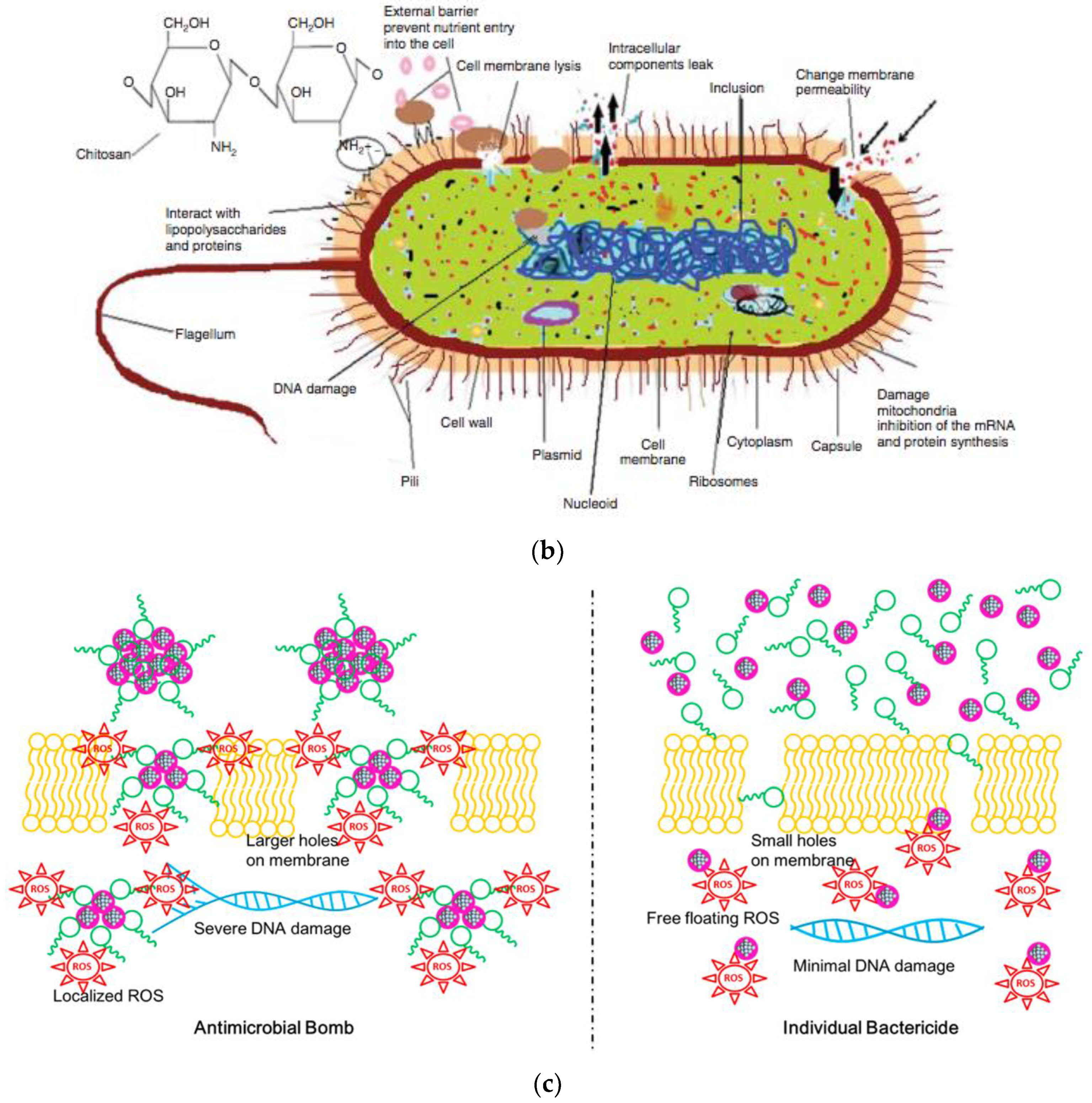
3. Mechanisms of Antimicrobial Action
4. Silver-Based Composites with Synergistic Antimicrobial Activity: Organic Parts of the Composite with Intrinsic AM Action
4.1. Polysaccharides and Derivatives
4.2. Phenolic Compounds
4.3. Organic Acids
4.4. Peptides
5. Overview on Pros and Cons of Ag-Based NAMs Application
6. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADI | Acceptance Daily Intake |
| Ag–pep | Silver-Peptide Conjugate |
| Ag/AgNPs/NPs | Silver/Silver Nanoparticles |
| AgNCs | Silver Nanoclusters |
| AMPs | Antimicrobial Peptides |
| AMR | Antimicrobial Resistance |
| ARBs | Antibiotic Resistant Breakers |
| CA | Citric Acid |
| CAUITI | Catheter-Associated Urinary Tract Infection—Relevant Microbes |
| CCM | Curcumin |
| CD-MOF | Cyclodextrin-Based Metal–Organic Framework |
| CFR | Case Fatality Rate |
| CFs | Carboxymethylated Cellulose Fibers |
| CFU | Colony Forming Units |
| CNFS/CNFs | Cellulose Nanofibrils Films |
| COVID-19 | Corona Virus Disease 19 |
| CS | Chitosan |
| DNA | Deoxyribonucleic Acid |
| E330 | Citric Acid |
| EC50 | The 50% Effective Concentration |
| EC90 | The 90% Effective Concentration |
| ECM | Extracellular Matrix |
| ESKAPE | Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, And Enterobacter Spp. |
| EVD | Ebola Virus Disease |
| FDA | Food And Drug Administration |
| FT-IR | Fourier-Transform Infrared Spectroscopy |
| GA | Gallic Acid |
| Gm− | Gram-Negative Bacteria |
| Gm+ | Gram-Positive Bacteria |
| GRGDS | (Gly-Arg-Gly-Asp-Ser) Peptide |
| GSH | Glutathione |
| H1N1 | Influenza A Virus |
| HA | Hydroxyapatite |
| HAIs | Hospital Acquired Infections |
| HDP | Host Defense Peptides |
| HKUST-1 | MOF, Hong Kong University of Science And Technology |
| HSV-2 | Herpex Simplex Virus 2 |
| HY | Hyaluronic Acid |
| ICP-AES/ICP-OES | Inductively Coupled Plasma Atomic Emission Spectroscopy/Inductively Coupled Plasma Optical Emission Spectrometry |
| LbL | Layer-by-Layer |
| MBC | Minimum Bactericidal Concentration |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus Infection |
| MIC | Minimum Inhibitory Concentration |
| MOF | Metal-Organic Framework |
| MRSA | Methicillin Resistant Streptococcus Aureus |
| NAM/NAMs | Nanoantimicrobial(s) |
| PCA | Polycaffeic Acid |
| PEG | Polyethylene Glycol |
| PEI | Polyethyleimine |
| PET | Polyethylene Terephtalate |
| PPA | Phenylpropanolamine |
| PVA | Polyvinyl Alcohol |
| PVP | Polyvinylpyrrolidone |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-Related Coronavirus 2 |
| SDS | Sodium Dodecyl Sulphate |
| SEM | Scanning Electron Microscopy |
| TE | Green Tea Extract |
| TEM | Transmission Electron Microscopy |
| WHO | World Health Organization |
References
- Bakis, C.E.; Bank, L.C.; Brown, V.L.; Cosenza, E.; Davalos, J.F.; Lesko, J.J.; Machida, A.; Rizkalla, S.H.; Triantafillou, T.C. Fiber-Reinforced Polymer Composites for Construction—State-of-the-Art Review. J. Compos. Constr. 2002, 6, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Tjong, S.C. Structural and Mechanical Properties of Polymer Nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Hinrichsen, G. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Hagrman, P.J.; Hagrman, D.; Zubieta, J. Organic-Inorganic Hybrid Materials: From “Simple” Coordination Polymers to Organodiamine-Templated Molybdenum Oxides. Angew. Chem. Int. Ed. 1999, 38, 2638–2684. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C. Nanocomposites for Food Packaging Applications. Food Res. Int. 2009, 42, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Egbo, M.K. A Fundamental Review on Composite Materials and Some of Their Applications in Biomedical Engineering. J. King Saud Univ. Eng. Sci. 2020. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoš, A.; Kovács, Á.T. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol. 2017, 25, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, L.; Singh, P.K.; Hartmann, R.; Nadell, C.D.; Drescher, K. Dynamic Biofilm Architecture Confers Individual and Collective Mechanisms of Viral Protection. Nat. Microbiol. 2018, 3, 26–31. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Bialecka-Fornal, M.; Weatherwax, C.; Larkin, J.W.; Prindle, A.; Liu, J.; Garcia-Ojalvo, J.; Süel, G.M. Encoding Membrane-Potential-Based Memory within a Microbial Community. Cell Syst. 2020, 10. [Google Scholar] [CrossRef]
- Eggensperger, C.G.; Giagnorio, M.; Holland, M.C.; Dobosz, K.M.; Schiffman, J.D.; Tiraferri, A.; Zodrow, K.R. Sustainable Living Filtration Membranes. Environ. Sci. Technol. Lett. 2020, 7, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, Y.; Gupta, A.; Todi, S.; Myatra, S.; Samaddar, D.P.; Patil, V.; Bhattacharya, P.K.; Ramasubban, S. Guidelines for Prevention of Hospital Acquired Infections. Indian J. Crit. Care Med. Peer Rev. Off. Publ. Indian Soc. Crit. Care Med. 2014, 18, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Miller, W.R.; Arias, C.A. Mechanisms of Antimicrobial Resistance among Hospital-Associated Pathogens. Expert Rev. Anti Infect. Ther. 2018, 16, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.; García, S. Animals as Sources of Food-Borne Pathogens: A Review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm Formation and Control Strategies of Foodborne Pathogens: Food Safety Perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef] [Green Version]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; DePaola, A. Seafood Pathogens and Information on Antimicrobial Resistance: A Review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce from 2010 to 2017. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Günther, S.; van Griensven, J. Ebola Virus Disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef] [Green Version]
- Oh, M.-D.; Park, W.B.; Park, S.-W.; Choe, P.G.; Bang, J.H.; Song, K.-H.; Kim, E.S.; Kim, H.B.; Kim, N.J. Middle East Respiratory Syndrome: What We Learned from the 2015 Outbreak in the Republic of Korea. Korean J. Intern. Med. 2018, 33, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders-Hastings, P.R.; Krewski, D. Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission. Pathogens 2016, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Munster, V.J.; Koopmans, M.; van Doremalen, N.; van Riel, D.; de Wit, E. A Novel Coronavirus Emerging in China—Key Questions for Impact Assessment. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, G. Case Fatality Rate Analysis of Italian COVID-19 Outbreak. J. Med. Virol. 2020, 92, 919–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livingston, E.; Bucher, K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef] [Green Version]
- De Natale, G.; Ricciardi, V.; De Luca, G.; De Natale, D.; Di Meglio, G.; Ferragamo, A.; Marchitelli, V.; Piccolo, A.; Scala, A.; Somma, R.; et al. The COVID-19 Infection in Italy: A Statistical Study of an Abnormally Severe Disease. J. Clin. Med. 2020, 9, 1564. [Google Scholar] [CrossRef]
- Donà, D.; Di Chiara, C.; Sharland, M. Multi-Drug-Resistant Infections in the COVID-19 Era: A Framework for Considering the Potential Impact. J. Hosp. Infect. 2020, 106, 198–199. [Google Scholar] [CrossRef]
- Monnet, D.L.; Harbarth, S. Will Coronavirus Disease (COVID-19) Have an Impact on Antimicrobial Resistance? Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and Differences between Silver Ions and Silver in Nanoforms as Antibacterial Agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Lim, D.W.; Choi, J. Assessment of Size-Dependent Antimicrobial and Cytotoxic Properties of Silver Nanoparticles. Available online: https://www.hindawi.com/journals/amse/2014/763807/ (accessed on 15 June 2020).
- Ul-Islam, S.; Butola, B.S. Advanced Textile Engineering Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 9781119488071. [Google Scholar]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yin, Y.; Liu, J. Silver Nanoparticles in the Environment. Environ. Sci. Process. Impacts 2012, 15, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food Applications of Natural Antimicrobial Compounds. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Dahl, J.A.; Maddux, B.L.S.; Hutchison, J.E. Toward Greener Nanosynthesis. Chem. Rev. 2007, 107, 2228–2269. [Google Scholar] [CrossRef] [Green Version]
- Gour, A.; Jain, N.K. Advances in Green Synthesis of Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Vermerris, W. Antimicrobial Nanomaterials Derived from Natural Products—A Review. Materials 2016, 9, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-Based Nanosystems and Their Exploited Antimicrobial Activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.A.; Bizerra, F.C.; Da Silva, P.I. Antimicrobial Compounds from Natural Sources. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. The Science of Antibiotic Discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Abo-Shama, U.H.; El-Gendy, H.; Mousa, W.S.; Hamouda, R.A.; Yousuf, W.E.; Hetta, H.F.; Abdeen, E.E. Synergistic and Antagonistic Effects of Metal Nanoparticles in Combination with Antibiotics against Some Reference Strains of Pathogenic Microorganisms. Infect. Drug Resist. 2020, 13, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.-Y.; Ko, W.-C.; Hsueh, P.-R. Nanoparticles in the Treatment of Infections Caused by Multidrug-Resistant Organisms. Front. Pharmacol. 2019, 10, 1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzammil, S.; Hayat, S.; Fakhar-E-Alam, M.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A.; et al. Nanoantibiotics: Future Nanotechnologies to Combat Antibiotic Resistance. Front. Biosci. Elite Ed. 2018, 10, 352–374. [Google Scholar] [CrossRef] [Green Version]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic Resistance Breakers: Current Approaches and Future Directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent Advances in Synthetic Methods and Applications of Silver Nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.J.; Gole, A.M.; Hunyadi, S.E.; Orendorff, C.J. One-Dimensional Colloidal Gold and Silver Nanostructures. Inorg. Chem. 2006, 45, 7544–7554. [Google Scholar] [CrossRef] [PubMed]
- Graff, A.; Wagner, D.; Ditlbacher, H.; Kreibig, U. Silver Nanowires. Eur. Phys. J. At. Mol. Opt. Plasma Phys. 2005, 34, 263–269. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Zhang, X.; Wang, J.; Guo, M.; Wiley, B.J.; Li, C.; Hu, C. Carbamide Promoted Polyol Synthesis and Transmittance Properties of Silver Nanocubes. Inorg. Chem. Front. 2016, 3, 547–555. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Gilroy, K.D.; Tao, J.; Zhu, C.; Yang, X.; Sun, X.; Xia, Y. Facile Synthesis of Silver Nanocubes with Sharp Corners and Edges in an Aqueous Solution. ACS Nano 2016, 10, 9861–9870. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, W.; Huang, Y.; Zhang, S.; Wei, H.; Xu, H. Controlled Synthesis of Uniform Silver Nanospheres. J. Phys. Chem. C 2010, 114, 7427–7431. [Google Scholar] [CrossRef]
- Lin, X.; Lin, S.; Liu, Y.; Gao, M.; Zhao, H.; Liu, B.; Hasi, W.; Wang, L. Facile Synthesis of Monodisperse Silver Nanospheres in Aqueous Solution via Seed-Mediated Growth Coupled with Oxidative Etching. Langmuir 2018, 34, 6077–6084. [Google Scholar] [CrossRef]
- Xue, B.; Wang, D.; Zuo, J.; Kong, X.; Zhang, Y.; Liu, X.; Tu, L.; Chang, Y.; Li, C.; Wu, F.; et al. Towards High Quality Triangular Silver Nanoprisms: Improved Synthesis, Six-Tip Based Hot Spots and Ultra-High Local Surface Plasmon Resonance Sensitivity. Nanoscale 2015, 7, 8048–8057. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Xia, Y. Synthesis of Silver Nanostructures with Controlled Shapes and Properties. Acc. Chem. Res. 2007, 40, 1067–1076. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver Nanoparticles: Various Methods of Synthesis, Size Affecting Factors and Their Potential Applications–a Review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Nurunnabi, M.; Shahriar, S.M.; Ahammad, A.J.S.; Shim, Y.Y.; Reaney, M.J.T.; Cho, J.Y. Green Chemistry Synthesis of Silver Nanoparticles and Their Potential Anticancer Effects. Cancers 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slepička, P.; Slepičková Kasálková, N.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2020, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Roldán, M.V.; Pellegri, N.; de Sanctis, O. Electrochemical Method for Ag-PEG Nanoparticles Synthesis. Available online: https://www.hindawi.com/journals/jnp/2013/524150/ (accessed on 22 February 2021).
- Kim, K.T.; Park, D.-S. Simple Electrochemical Synthesis of Polyethylenimine-Encapsulated Ag Nanoparticles from Solid AgCl Applied in Catalytic Reduction of H2O2. Catalysts 2020, 10, 1416. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. One-Step Synthesis of Silver Nanoparticles Embedded Polyurethane Nano-Fiber/Net Structured Membrane as an Effective Antibacterial Medium. Polymers 2019, 11, 1185. [Google Scholar] [CrossRef] [Green Version]
- De Matteis, V.; Cascione, M.; Toma, C.C.; Leporatti, S. Silver Nanoparticles: Synthetic Routes, In Vitro Toxicity and Theranostic Applications for Cancer Disease. Nanomaterials 2018, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally Stable Silver Nanoparticles Synthesized by Laser Ablation in Alcoholic Organic Solvent. Colloids Surf. Physicochem. Eng. Asp. 2018, 559, 148–158. [Google Scholar] [CrossRef]
- Mirza, I.; O’Connell, G.; Wang, J.J.; Lunney, J.G. Comparison of Nanosecond and Femtosecond Pulsed Laser Deposition of Silver Nanoparticle Films. Nanotechnology 2014, 25, 265301. [Google Scholar] [CrossRef] [PubMed]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and Applications of Silver Nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Natsuki, J.; Natsuki, T.; Hashimoto, Y. A Review of Silver Nanoparticles: Synthesis Methods, Properties and Applications. Int. J. Mater. Sci. Appl. 2015, 4, 325. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J. Nanobiotechnol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Jorge de Souza, T.A.; Rosa Souza, L.R.; Franchi, L.P. Silver Nanoparticles: An Integrated View of Green Synthesis Methods, Transformation in the Environment, and Toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Ramos, N.; Miranda, M.S.; Franco, A.R.; Silva, S.S.; Azevedo, J.; Dias, I.R.; Reis, R.L.; Viegas, C.; Gomes, M.E. Toward Spinning Greener Advanced Silk Fibers by Feeding Silkworms with Nanomaterials. ACS Sustain. Chem. Eng. 2020, 8, 11872–11887. [Google Scholar] [CrossRef]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Bruening, M.L. Catalytic Nanoparticles Formed by Reduction of Metal Ions in Multilayered Polyelectrolyte Films. Nano Lett. 2002, 2, 497–501. [Google Scholar] [CrossRef]
- Shakya, S.; He, Y.; Ren, X.; Guo, T.; Maharjan, A.; Luo, T.; Wang, T.; Dhakhwa, R.; Regmi, B.; Li, H.; et al. Ultrafine Silver Nanoparticles Embedded in Cyclodextrin Metal-Organic Frameworks with GRGDS Functionalization to Promote Antibacterial and Wound Healing Application. Small 2019, 15, 1901065. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial Wound Dressing Nanofiber Mats from Multicomponent (Chitosan/Silver-NPs/Polyvinyl Alcohol) Systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Rujitanaroj, P.; Pimpha, N.; Supaphol, P. Wound-Dressing Materials with Antibacterial Activity from Electrospun Gelatin Fiber Mats Containing Silver Nanoparticles. Polymer 2008, 49, 4723–4732. [Google Scholar] [CrossRef]
- Vimala, K.; Mohan, Y.M.; Sivudu, K.S.; Varaprasad, K.; Ravindra, S.; Reddy, N.N.; Padma, Y.; Sreedhar, B.; MohanaRaju, K. Fabrication of Porous Chitosan Films Impregnated with Silver Nanoparticles: A Facile Approach for Superior Antibacterial Application. Colloids Surf. B Biointerfaces 2010, 76, 248–258. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella Pneumoniae Infection Biology: Living to Counteract Host Defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, C.; Meng, J.; Wang, X.; Meng, X.; Sun, X.; Xu, Y.; Zhao, W.; Ni, Y. Synthesis of Novel Cellulose-Based Antibacterial Composites of Ag Nanoparticles@ Metal-Organic Frameworks@ Carboxymethylated Fibers. Carbohydr. Polym. 2018, 193, 82–88. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia Coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Salas-Orozco, M.; Niño-Martínez, N.; Martínez-Castañón, G.-A.; Méndez, F.T.; Jasso, M.E.C.; Ruiz, F. Mechanisms of Resistance to Silver Nanoparticles in Endodontic Bacteria: A Literature Review. Available online: https://www.hindawi.com/journals/jnm/2019/7630316/ (accessed on 31 May 2020).
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yao, Q.; Zhang, T.; Chen, X.; Wu, Z.; Zhang, N.; Shao, Y.; Cheng, Y. Antibacterial Activity and Mechanism of Green Tea Polysaccharide Conjugates against Escherichia coli. Ind. Crop. Prod. 2020, 152. [Google Scholar] [CrossRef]
- Li, D.; Zhou, B.; Lv, B. Antibacterial Therapeutic Agents Composed of Functional Biological Molecules. J. Chem. 2020, 2020, e6578579. [Google Scholar] [CrossRef] [Green Version]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential Antibacterial Mechanism of Silver Nanoparticles and the Optimization of Orthopedic Implants by Advanced Modification Technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Gafri, H.F.S.; Zuki, F.M.; Aroua, M.K.; Hashim, N.A. Mechanism of Bacterial Adhesion on Ultrafiltration Membrane Modified by Natural Antimicrobial Polymers (Chitosan) and Combination with Activated Carbon (PAC). Rev. Chem. Eng. 2019, 35, 421–443. [Google Scholar] [CrossRef]
- Chirkov, S.N. The Antiviral Activity of Chitosan (Review). Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Potara, M.; Jakab, E.; Damert, A.; Popescu, O.; Canpean, V.; Astilean, S. Synergistic Antibacterial Activity of Chitosan-Silver Nanocomposites on Staphylococcus aureus. Nanotechnology 2011, 22, 135101. [Google Scholar] [CrossRef] [PubMed]
- González-Campos, J.B.; Mota-Morales, J.D.; Kumar, S.; Zárate-Triviño, D.; Hernández-Iturriaga, M.; Prokhorov, Y.; Lepe, M.V.; García-Carvajal, Z.Y.; Sanchez, I.C.; Luna-Bárcenas, G. New Insights into the Bactericidal Activity of Chitosan-Ag Bionanocomposite: The Role of the Electrical Conductivity. Colloids Surf. B Biointerfaces 2013, 111, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Lim, T.-P.; Leong, D.T.; Xie, J. Antimicrobial Cluster Bombs: Silver Nanoclusters Packed with Daptomycin. ACS Nano 2016, 10, 7934–7942. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood Waste: A Source for Preparation of Commercially Employable Chitin/Chitosan Materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and Chitosans for the Repair of Wounded Skin, Nerve, Cartilage and Bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [Green Version]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [Green Version]
- Mohandas, A.; Deepthi, S.; Biswas, R.; Jayakumar, R. Chitosan Based Metallic Nanocomposite Scaffolds as Antimicrobial Wound Dressings. Bioact. Mater. 2018, 3, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.K.H.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobialwound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Rhim, J.-W.; Hong, S.-I.; Park, H.-M.; Ng, P.K.W. Preparation and Characterization of Chitosan-Based Nanocomposite Films with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A “green” Chitosan-Silver Nanoparticle Composite as a Heterogeneous as Well as Micro-Heterogeneous Catalyst. Nanotechnology 2008, 19. [Google Scholar] [CrossRef]
- Sanpui, P.; Murugadoss, A.; Prasad, P.V.D.; Ghosh, S.S.; Chattopadhyay, A. The Antibacterial Properties of a Novel Chitosan–Ag-Nanoparticle Composite. Int. J. Food Microbiol. 2008, 124, 142–146. [Google Scholar] [CrossRef]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The Synthesis of Chitosan-Based Silver Nanoparticles and Their Antibacterial Activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/Silver Nanocomposites: Synergistic Antibacterial Action of Silver Nanoparticles and Silver Ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Wang, L.-S.; Wang, C.-Y.; Yang, C.-H.; Hsieh, C.-L.; Chen, S.-Y.; Shen, C.-Y.; Wang, J.-J.; Huang, K.-S. Synthesis and Anti-Fungal Effect of Silver Nanoparticles–Chitosan Composite Particles. Int. J. Nanomedicine 2015, 10, 2685–2696. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal Uses of the Mushroom Cordyceps Militaris: Current State and Prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Agrawal, D.C.; Tsay, H.-S.; Shyur, L.-F.; Wu, Y.-C.; Wang, S.-Y. Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9789811059780. [Google Scholar]
- Latif, U.; Al-Rubeaan, K.; Saeb, A.T.M. A Review on Antimicrobial Chitosan-Silver Nanocomposites: A Roadmap toward Pathogen Targeted Synthesis. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 448–458. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. Fabrication, Characterization of Chitosan/Nanosilver Film and Its Potential Antibacterial Application. J. Biomater. Sci. Polym. Ed. 2009, 20, 2129–2144. [Google Scholar] [CrossRef]
- Chen, P.; Song, L.; Liu, Y.; Fang, Y. Synthesis of Silver Nanoparticles by γ-Ray Irradiation in Acetic Water Solution Containing Chitosan. Radiat. Phys. Chem. 2007, 76, 1165–1168. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan–Hyaluronic Acid/Nano Silver Composite Sponges for Drug Resistant Bacteria Infected Diabetic Wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Tamara, F.R.; Lin, C.; Mi, F.-L.; Ho, Y.-C. Antibacterial Effects of Chitosan/Cationic Peptide Nanoparticles. Nanomaterials 2018, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Ono, T.; Miyahira, Y.; Nguyen, V.Q.; Matsui, T.; Ishihara, M. Antiviral Activity of Silver Nanoparticle/Chitosan Composites against H1N1 Influenza A Virus. Nanoscale Res. Lett. 2013, 8, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.; Qu, D.; Wang, H.; Sun, Z.; Liu, X.; Chen, J.; Li, C.; Li, X.; Chen, Z. Intranasal Administration of Chitosan Against Influenza A (H7N9) Virus Infection in a Mouse Model. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The Improved Antiviral Activities of Amino-Modified Chitosan Derivatives on Newcastle Virus. Drug Chem. Toxicol. 2019, 44, 1–6. [Google Scholar] [CrossRef]
- Davydova, V.N.; Nagorskaya, V.P.; Gorbach, V.I.; Kalitnik, A.A.; Reunov, A.V.; Solov’eva, T.F.; Ermak, I.M. Chitosan Antiviral Activity: Dependence on Structure and Depolymerization Method. Appl. Biochem. Microbiol. 2011, 47, 103–108. [Google Scholar] [CrossRef]
- Sinclair, T.R.; van den Hengel, S.K.; Raza, B.G.; Rutjes, S.A.; de Roda Husman, A.M.; Peijnenburg, W.J.G.M.; Roesink, H.D.W.; de Vos, W.M. Surface Chemistry-Dependent Antiviral Activity of Silver Nanoparticles. Nanotechnology 2021, 32, 365101. [Google Scholar] [CrossRef]
- Dung, T.T.N.; Nam, V.N.; Nhan, T.T.; Ngoc, T.T.B.; Minh, L.Q.; Nga, B.T.T.; Le, V.P.; Quang, D.V. Silver Nanoparticles as Potential Antiviral Agents against African Swine Fever Virus. Mater. Res. Express 2020, 6, 1250g9. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, K.; Okido, M. Hydroxyapatite Coating of Titanium Implants Using Hydroprocessing and Evaluation of Their Osteoconductivity. Bioinorg. Chem. Appl. 2012, 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. 7—Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (Hap) for Biomedical Applications; Mucalo, M., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2015; pp. 143–157. ISBN 9781782420330. [Google Scholar]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Marsich, E.; Bellomo, F.; Turco, G.; Travan, A.; Donati, I.; Paoletti, S. Nano-Composite Scaffolds for Bone Tissue Engineering Containing Silver Nanoparticles: Preparation, Characterization and Biological Properties. J. Mater. Sci. Mater. Med. 2013, 24, 1799–1807. [Google Scholar] [CrossRef]
- Tavakol, S.; Nikpour, M.R.; Hoveizi, E.; Tavakol, B.; Rezayat, S.M.; Adabi, M.; Shajari Abokheili, S.; Jahanshahi, M. Investigating the Effects of Particle Size and Chemical Structure on Cytotoxicity and Bacteriostatic Potential of Nano Hydroxyapatite/Chitosan/Silica and Nano Hydroxyapatite/Chitosan/Silver; as Antibacterial Bone Substitutes. J. Nanopart. Res. 2014, 16, 2622. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q.; Pang, X. Preparation and Characterization of Chitosan-Silver/Hydroxyapatite Composite Coatings OnTiO2 Nanotube for Biomedical Applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Andrade, F.A.C.; de Oliveira Vercik, L.C.; Monteiro, F.J.; da Silva Rigo, E.C. Preparation, Characterization and Antibacterial Properties of Silver Nanoparticles–Hydroxyapatite Composites by a Simple and Eco-Friendly Method. Ceram. Int. 2016, 42, 2271–2280. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of Hydroxyapatite–Silver–Chitosan Nanocomposite Coatings. Surf. Coat. Technol. 2008, 202, 3815–3821. [Google Scholar] [CrossRef]
- Saravanan, S.; Nethala, S.; Pattnaik, S.; Tripathi, A.; Moorthi, A.; Selvamurugan, N. Preparation, Characterization and Antimicrobial Activity of a Bio-Composite Scaffold Containing Chitosan/Nano-Hydroxyapatite/Nano-Silver for Bone Tissue Engineering. Int. J. Biol. Macromol. 2011, 49, 188–193. [Google Scholar] [CrossRef]
- Martins, A.F.; Facchi, S.P.; Follmann, H.D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Antimicrobial Activity of Chitosan Derivatives Containing N-Quaternized Moieties in Its Backbone: A Review. Int. J. Mol. Sci. 2014, 15, 20800–20832. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a Chitosan-Based Wound Dressing with Improved Hemostatic and Antimicrobial Properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Park, J.-H.; Lee, J.-Y. Antibacterial Action of Polyphosphate on Porphyromonas Gingivalis. Antimicrob. Agents Chemother. 2011, 55, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennaro, R.; Paoletti, S. Non-Cytotoxic Silver Nanoparticle-Polysaccharide Nanocomposites with Antimicrobial Activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus epidermidis Pathogenesis. Methods Mol. Biol. Clifton NJ 2014, 1106, 17–31. [Google Scholar] [CrossRef]
- Banerjee, M.; Mallick, S.; Paul, A.; Chattopadhyay, A.; Ghosh, S.S. Heightened Reactive Oxygen Species Generation in the Antimicrobial Activity of a Three Component Iodinated Chitosan−Silver Nanoparticle Composite. Langmuir 2010, 26, 5901–5908. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic Combination of Chitosan Acetate with Nanoparticle Silver as a Topical Antimicrobial: Efficacy against Bacterial Burn Infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of Silver Nanoparticles onto Different Surface Structures of Chitin/Chitosan and Correlations with Antimicrobial Activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Chen, L.; Huang, L.; Cao, S.; Luo, X.; Liu, K. Novel Antimicrobial Chitosan–Cellulose Composite Films Bioconjugated with Silver Nanoparticles. Ind. Crop. Prod. 2015, 70, 395–403. [Google Scholar] [CrossRef]
- Díez, I.; Eronen, P.; Österberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H.A. Functionalization of Nanofibrillated Cellulose with Silver Nanoclusters: Fluorescence and Antibacterial Activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Slepička, P.; Kasálková, N.S.; Pišlová, M.; Kotrba, K.; Švorčík, V. Antibacterial Properties of Silver Coated Regenerated Cellulose. Micro Nano Lett. 2020, 15, 159–162. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, X.; Ni, L.; Tang, Z.; Zhang, Y.; Zhang, Y.; Zhang, W. Antibacterial Cellulose Membrane via One-Step Covalent Immobilization of Ammonium/Amine Groups. Desalination 2015, 359, 156–166. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Hassabo, A.G.; Shaarawy, S.; Hebeish, A. Benign Development of Cotton with Antibacterial Activity and Metal Sorpability through Introduction Amino Triazole Moieties and AgNPs in Cotton Structure Pre-Treated with Periodate. Carbohydr. Polym. 2017, 178, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; De Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, H.; Wei, Y.; Cong, F. Hyaluronan-Inorganic Nanohybrid Materials for Biomedical Applications. Biomacromolecules 2017, 18, 1677–1696. [Google Scholar] [CrossRef]
- Cárdenas-Triviño, G.; Ruiz-Parra, M.; Vergara-González, L.; Ojeda-Oyarzún, J.; Solorzano, G. Synthesis and Bactericidal Properties of Hyaluronic Acid Doped with Metal Nanoparticles. Available online: https://www.hindawi.com/journals/jnm/2017/9573869/ (accessed on 13 May 2020).
- Abdel-Mohsen, A.M.; Hrdina, R.; Burgert, L.; Abdel-Rahman, R.M.; Hašová, M.; Šmejkalová, D.; Kolář, M.; Pekar, M.; Aly, A.S. Antibacterial Activity and Cell Viability of Hyaluronan Fiber with Silver Nanoparticles. Carbohydr. Polym. 2013, 92, 1177–1187. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of Natural Phenolic Antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Lin, Y.; Jain, R.; Yan, Y. Microbial Production of Antioxidant Food Ingredients via Metabolic Engineering. Curr. Opin. Biotechnol. 2014, 26, 71–78. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis Vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef] [PubMed]
- Almajano, M.P.; Carbó, R.; Jiménez, J.A.L.; Gordon, M.H. Antioxidant and Antimicrobial Activities of Tea Infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and Curcumin: Biological Actions and Medicinal Applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef]
- Tyagi, P.; Singh, M.; Kumari, H.; Kumari, A.; Mukhopadhyay, K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE 2015, 10, e0121313. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of Curcumin, a Component of Golden Spice, and Its Miraculous Biological Activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, T.; Bais, H.P. Curcumin, a Known Phenolic from Curcuma Longa, Attenuates the Virulence of Pseudomonas aeruginosa PAO1 in Whole Plant and Animal Pathogenicity Models. J. Agric. Food Chem. 2008, 56, 1955–1962. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Ali, S.A. Synergistic Antibacterial Activity of Curcumin with Antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic Antibacterial Effect of Curcumin against Methicillin-Resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial Action of Curcumin against Staphylococcus aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [Green Version]
- Vaughn, A.R.; Haas, K.N.; Burney, W.; Andersen, E.; Clark, A.K.; Crawford, R.; Sivamani, R.K. Potential Role of Curcumin Against Biofilm-Producing Organisms on the Skin: A Review. Phytother. Res. PTR 2017, 31, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Kali, A.; Bhuvaneshwar, D.; Charles, P.M.V.; Seetha, K.S. Antibacterial Synergy of Curcumin with Antibiotics against Biofilm Producing Clinical Bacterial Isolates. J. Basic Clin. Pharm. 2016, 7, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoury, E.; Abiad, M.; Kassaify, Z.G.; Patra, D. Green Synthesis of Curcumin Conjugated Nanosilver for the Applications in Nucleic Acid Sensing and Anti-Bacterial Activity. Colloids Surf. B Biointerfaces 2015, 127, 274–280. [Google Scholar] [CrossRef]
- Kundu, S.; Nithiyanantham, U. In Situ Formation of Curcumin Stabilized Shape-Selective Ag Nanostructures in Aqueous Solution and Their Pronounced SERS Activity. RSC Adv. 2013, 3, 25278–25290. [Google Scholar] [CrossRef]
- Jaiswal, S.; Mishra, P. Antimicrobial and Antibiofilm Activity of Curcumin-Silver Nanoparticles with Improved Stability and Selective Toxicity to Bacteria over Mammalian Cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef]
- Verma, A.D.; Jain, N.; Singha, S.K.; Quraishi, M.A.; Sinha, I. Green Synthesis and Catalytic Application of Curcumin Stabilized Silver Nanoparticles. J. Chem. Sci. 2016, 128, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Mohana Raju, K. Synthesis and Characterization of Hydrogel-Silver Nanoparticle-Curcumin Composites for Wound Dressing and Antibacterial Application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic Antibacterial Effects of Curcumin Modified Silver Nanoparticles through ROS-Mediated Pathways. Mater. Sci. Eng. C 2019, 99, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Lambert, J.D. Antioxidant Effects of Green Tea. Mol. Nutr. Food Res. 2011, 55, 844–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valcic, S.; Timmermann, B.N.; Alberts, D.S.; Wächter, G.A.; Krutzsch, M.; Wymer, J.; Guillén, J.M. Inhibitory Effect of Six Green Tea Catechins and Caffeine on the Growth of Four Selected Human Tumor Cell Lines. Anticancer. Drugs 1996, 7, 461–468. [Google Scholar] [CrossRef]
- Moulton, M.C.; Braydich-Stolle, L.K.; Nadagouda, M.N.; Kunzelman, S.; Hussain, S.M.; Varma, R.S. Synthesis, Characterization and Biocompatibility of “Green” Synthesized Silver Nanoparticles Using Tea Polyphenols. Nanoscale 2010, 2, 763–770. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Green Synthesis of Silver and Palladium Nanoparticles at Room Temperature Using Coffee and Tea Extract. Green Chem. 2008, 10, 859–862. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, X.; Li, J.; Zheng, M.; Chen, Z.; Yu, C.-P. Green Synthesis of Silver Nanoparticles Using Tea Leaf Extract and Evaluation of Their Stability and Antibacterial Activity. Colloids Surf. Physicochem. Eng. Asp. 2014, 444, 226–231. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef] [Green Version]
- Rolim, W.R.; Pelegrino, M.T.; de Araújo Lima, B.; Ferraz, L.S.; Costa, F.N.; Bernardes, J.S.; Rodigues, T.; Brocchi, M.; Seabra, A.B. Green Tea Extract Mediated Biogenic Synthesis of Silver Nanoparticles: Characterization, Cytotoxicity Evaluation and Antibacterial Activity. Appl. Surf. Sci. 2019, 463, 66–74. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Lee, J.Y.; Aguilar, L.E.; Park, C.H.; Kim, C.S. UV Light Assisted Coating Method of Polyphenol Caffeic Acid and Mediated Immobilization of Metallic Silver Particles for Antibacterial Implant Surface Modification. Polymers 2019, 11, 1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, L.E.; Lee, J.Y.; Park, C.H.; Kim, C.S. Biomedical Grade Stainless Steel Coating of Polycaffeic Acid via Combined Oxidative and Ultraviolet Light-Assisted Polymerization Process for Bioactive Implant Application. Polymers 2019, 11, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matei, P.M.; Iacomi, B.M.; Martín-Gil, J.; Pérez-Lebeña, E.; Ramos-Sánchez, M.C.; Barrio-Arredondo, M.T.; Martín-Ramos, P. In Vitro Antifungal Activity of Composites of AgNPs and Polyphenol Inclusion Compounds against Fusarium culmorum in Different Dispersion Media. Agronomy 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. Tannic Acid Modified Silver Nanoparticles Show Antiviral Activity in Herpes Simplex Virus Type 2 Infection. PLoS ONE 2014, 9, e0104113. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Sun, B.; Zhou, Z.; Wu, Y.; Zhu, M. Size-Controlled and Large-Scale Synthesis of Organic-Soluble Ag Nanocrystals in Water and Their Formation Mechanism. Prog. Nat. Sci. Mater. Int. 2011, 21, 447–454. [Google Scholar] [CrossRef] [Green Version]
- Le, A.-T.; Huy, P.T.; Tam, P.D.; Huy, T.Q.; Cam, P.D.; Kudrinskiy, A.A.; Krutyakov, Y.A. Green Synthesis of Finely-Dispersed Highly Bactericidal Silver Nanoparticles via Modified Tollens Technique. Curr. Appl. Phys. 2010, 10, 910–916. [Google Scholar] [CrossRef]
- Anwar, A.; Abdalla, S.A.O.; Aslam, Z.; Shah, M.R.; Siddiqui, R.; Khan, N.A. Oleic Acid–Conjugated Silver Nanoparticles as Efficient Antiamoebic Agent against Acanthamoeba castellanii. Parasitol. Res. 2019, 118, 2295–2304. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [Green Version]
- Speert, D.P.; Wannamaker, L.W.; Gray, E.D.; Clawson, C.C. Bactericidal Effect of Oleic Acid on Group A Streptococci: Mechanism of Action. Infect. Immun. 1979, 26, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, M.W. Pathogenesis of Group A Streptococcal Infections. Clin. Microbiol. Rev. 2000, 13, 470–511. [Google Scholar] [CrossRef] [PubMed]
- Le, A.-T.; Tam, L.T.; Tam, P.D.; Huy, P.T.; Huy, T.Q.; Van Hieu, N.; Kudrinskiy, A.A.; Krutyakov, Y.A. Synthesis of Oleic Acid-Stabilized Silver Nanoparticles and Analysis of Their Antibacterial Activity. Mater. Sci. Eng. C 2010, 30, 910–916. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. The Citric Acid Cycle. In Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
- Al-Rousan, W.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Ajo, R.Y.; Holley, R.A. Use of Acetic and Citric Acids to Inhibit Escherichia Coli O157:H7, Salmonella typhimurium and Staphylococcus aureus in Tabbouleh Salad. Food Microbiol. 2018, 73, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Xie, G.; Edmondson, A.S. The Fate of Salmonella Enteritidis PT4 in Home-Made Mayonnaise Prepared with Citric Acid. Lett. Appl. Microbiol. 1999, 28, 36–40. [Google Scholar] [CrossRef]
- Pundir, R.; Jain, P. Evaluation of Five Chemical Food Preservatives for Their Antibacterial Activity against Bacterial Isolates from Bakery Products and Mango Pickles. J. Chem. Pharm. Res. 2011, 3, 24–31. [Google Scholar]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of Organic Acids on Biofilm Formation and Quorum Signaling of Pathogens from Fresh Fruits and Vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-Coated Silver Nanoparticles in Biological and Environmental Conditions: Fate, Stability and Toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Kim, I.; Lee, B.-T.; Kim, H.-A.; Kim, K.-W.; Kim, S.D.; Hwang, Y.-S. Citrate Coated Silver Nanoparticles Change Heavy Metal Toxicities and Bioaccumulation of Daphnia Magna. Chemosphere 2016, 143, 99–105. [Google Scholar] [CrossRef]
- Gutierrez, L.; Aubry, C.; Cornejo, M.; Croue, J.-P. Citrate-Coated Silver Nanoparticles Interactions with Effluent Organic Matter: Influence of Capping Agent and Solution Conditions. Langmuir 2015, 31, 8865–8872. [Google Scholar] [CrossRef] [Green Version]
- Mitra, C.; Gummadidala, P.M.; Afshinnia, K.; Merrifield, R.C.; Baalousha, M.; Lead, J.R.; Chanda, A. Citrate-Coated Silver Nanoparticles Growth-Independently Inhibit Aflatoxin Synthesis in Aspergillus Parasiticus. Environ. Sci. Technol. 2017, 51, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
- Gopisetty, M.K.; Kovács, D.; Igaz, N.; Kiricsi, M.; Miklós, B.I. Size-Dependent Inhibition of P-Glycoprotein Function by Citrate Coated Silver Nanoparticles in Multidrug Resistant Breast Cancer Cells. Role of Autophagy and ER Stress. Eur. J. Cancer 2018, 92, S139. [Google Scholar] [CrossRef]
- Kubo, A.-L.; Capjak, I.; Vrček, I.V.; Bondarenko, O.M.; Kurvet, I.; Vija, H.; Ivask, A.; Kasemets, K.; Kahru, A. Antimicrobial Potency of Differently Coated 10 and 50 nm Silver Nanoparticles against Clinically Relevant Bacteria Escherichia Coli and Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 170, 401–410. [Google Scholar] [CrossRef]
- Scatolino, M.V.; Dias, M.C.; Silva, D.W.; Bufalino, L.; Martins, M.A.; Piccoli, R.H.; Tonoli, G.H.D.; Londero, A.A.; Neto, V.O.; Mendes, L.M. Tannin-Stabilized Silver Nanoparticles and Citric Acid Added Associated to Cellulose Nanofibrils: Effect on Film Antimicrobial Properties. SN Appl. Sci. 2019, 1, 1243. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.; Ferreira, C.; Saavedra, M.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids against Pathogenic Bacteria. Microb. Drug Resist. Larchmt. N 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of Gallic Acid against Food-Related Pseudomonas Strains and Its Use as Biocontrol Tool to Improve the Shelf Life of Fresh Black Truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Naz, S.; Khaskheli, A.R.; Aljabour, A.; Kara, H.; Talpur, F.N.; Sherazi, S.T.H.; Khaskheli, A.A.; Jawaid, S. Synthesis of Highly Stable Cobalt Nanomaterial Using Gallic Acid and Its Application in Catalysis. Available online: https://www.hindawi.com/journals/ac/2014/686925/ (accessed on 29 April 2020).
- Farrokhnia, M.; Karimi, S.; Askarian, S. Strong Hydrogen Bonding of Gallic Acid during Synthesis of an Efficient AgNPs Colorimetric Sensor for Melamine Detection via Dis-Synthesis Strategy. ACS Sustain. Chem. Eng. 2019, 7, 6672–6684. [Google Scholar] [CrossRef]
- Sunil Gowda, S.N.; Rajasowmiya, S.; Vadivel, V.; Banu Devi, S.; Celestin Jerald, A.; Marimuthu, S.; Devipriya, N. Gallic Acid-Coated Sliver Nanoparticle Alters the Expression of Radiation-Induced Epithelial-Mesenchymal Transition in Non-Small Lung Cancer Cells. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2018, 52, 170–177. [Google Scholar] [CrossRef]
- Matei, P.M.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.T.; Martín-Ramos, P. Silver Nanoparticles and Polyphenol Inclusion Compounds Composites for Phytophthora cinnamomi Mycelial Growth Inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi. Mol. Plant Pathol. 2017, 19, 260–285. [Google Scholar] [CrossRef] [Green Version]
- Jana, S.; Maiti, S.; Jana, S. Biopolymer-Based Composites: Drug Delivery and Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2017; ISBN 9780081019153. [Google Scholar]
- Nuti, R.; Goud, N.S.; Saraswati, A.P.; Alvala, R.; Alvala, M. Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Curr. Med. Chem. 2017, 24, 4303–4314. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The Antimicrobial Peptides and Their Potential Clinical Applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Bechinger, B.; Gorr, S.-U. Antimicrobial Peptides: Mechanisms of Action and Resistance. J. Dent. Res. 2017, 96, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuri, R.; Shprung, T.; Shai, Y. Defensive Remodeling: How Bacterial Surface Properties and Biofilm Formation Promote Resistance to Antimicrobial Peptides. Biochim. Biophys. Acta 2015, 1848, 3089–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial Peptides and Their Interaction with Biofilms of Medically Relevant Bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Núñez, C.; Cardoso, M.H.; de Souza Cândido, E.; Franco, O.L.; Hancock, R.E.W. Synthetic Antibiofilm Peptides. Biochim. Biophys. Acta 2016, 1858, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.; Mirkalantari, S.; Amirmozafari, N. Bacterial Resistance to Antimicrobial Peptides. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2019, 25, e3210. [Google Scholar] [CrossRef] [PubMed]
- Bhopale, G.M. Antimicrobial Peptides: A Promising Avenue for Human Healthcare. Curr. Pharm. Biotechnol. 2020, 21, 90–96. [Google Scholar] [CrossRef]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Bardanca, M.G.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652. [Google Scholar] [CrossRef] [Green Version]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The Value of Antimicrobial Peptides in the Age of Resistance. Lancet Infect. Dis. 2020, 20, e216–e230. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial Peptides: Application Informed by Evolution. Science 2020, 368. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Strempel, N.; Strehmel, J.; Overhage, J. Potential Application of Antimicrobial Peptides in the Treatment of Bacterial Biofilm Infections. Curr. Pharm. Des. 2015, 21, 67–84. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Nielsen, C.G.; Aarestrup, F.M.; Hansen, E.B. Comparative Evaluation of the Antimicrobial Activity of Different Antimicrobial Peptides against a Range of Pathogenic Bacteria. PLoS ONE 2015, 10, e0144611. [Google Scholar] [CrossRef] [Green Version]
- Agrillo, B.; Balestrieri, M.; Gogliettino, M.; Palmieri, G.; Moretta, R.; Proroga, Y.T.R.; Rea, I.; Cornacchia, A.; Capuano, F.; Smaldone, G.; et al. Functionalized Polymeric Materials with Bio-Derived Antimicrobial Peptides for “Active” Packaging. Int. J. Mol. Sci. 2019, 20, 601. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xia, L.; Haapasalo, M.; Wei, W.; Zhang, D.; Ma, J.; Shen, Y. A Novel Hydroxyapatite-Binding Antimicrobial Peptide against Oral Biofilms. Clin. Oral Investig. 2019, 23, 2705–2712. [Google Scholar] [CrossRef]
- Lim, K.; Chua, R.R.Y.; Ho, B.; Tambyah, P.A.; Hadinoto, K.; Leong, S.S.J. Development of a Catheter Functionalized by a Polydopamine Peptide Coating with Antimicrobial and Antibiofilm Properties. Acta Biomater. 2015, 15, 127–138. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Sun, P.; Zhang, N.; Zhao, Y.; Qin, S.; Zhao, Y. Antimicrobial Peptide-Modified Silver Nanoparticles for Enhancing the Antibacterial Efficacy. RSC Adv. 2020, 10, 38746–38754. [Google Scholar] [CrossRef]
- Chen, L.; Ai, J.; Cai, H.; Chen, X.; Liu, Z.; Li, Z.; Dai, F. Antibacterial Gauze Based on the Synergistic Antibacterial Mechanism of Antimicrobial Peptides and Silver Nanoparticles. J. Polym. Res. 2021, 28, 32. [Google Scholar] [CrossRef]
- Ye, Z.; Zhu, H.; Zhang, S.; Li, J.; Wang, J.; Wang, E. Highly Efficient Nanomedicine from Cationic Antimicrobial Peptide-Protected Ag Nanoclusters. J. Mater. Chem. B 2021, 9, 307–313. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic Interaction between Silver Nanoparticles and Membrane-Permeabilizing Antimicrobial Peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taglietti, A.; Diaz Fernandez, Y.A.; Amato, E.; Cucca, L.; Dacarro, G.; Grisoli, P.; Necchi, V.; Pallavicini, P.; Pasotti, L.; Patrini, M. Antibacterial Activity of Glutathione-Coated Silver Nanoparticles against Gram Positive and Gram Negative Bacteria. Langmuir 2012, 28, 8140–8148. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Xie, J.; Luo, Z.; Jiang, J.; Yang, Y.Y.; Liu, S. The Potent Antimicrobial Properties of Cell Penetrating Peptide-Conjugated Silver Nanoparticles with Excellent Selectivity for Gram-Positive Bacteria over Erythrocytes. Nanoscale 2013, 5, 3834–3840. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Na, H.; Zhong, R.; Yuan, M.; Guo, J.; Zhao, L.; Wang, Y.; Wang, L.; Zhang, F. One Step Synthesis of Antimicrobial Peptide Protected Silver Nanoparticles: The Core-Shell Mutual Enhancement of Antibacterial Activity. Colloids Surf. B Biointerfaces 2020, 186, 110704. [Google Scholar] [CrossRef]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic Antimicrobial Peptides and Biogenic Silver Nanoparticles Kill Mycobacteria without Eliciting DNA Damage and Cytotoxicity in Mouse Macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef] [Green Version]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced Antimicrobial Activity of the Food-Protecting Nisin Peptide by Bioconjugation with Silver Nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, L.; Guan, S.; Shi, G.; Luo, Q.; Miao, L.; Thistlethwaite, I.; Huang, Z.; Xu, J.; Liu, J. Silver Mineralization on Self-Assembled Peptide Nanofibers for Long Term Antimicrobial Effect. J. Mater. Chem. 2012, 22, 2575–2581. [Google Scholar] [CrossRef]
- Pardhi, D.M.; Şen Karaman, D.; Timonen, J.; Wu, W.; Zhang, Q.; Satija, S.; Mehta, M.; Charbe, N.; McCarron, P.A.; Tambuwala, M.M.; et al. Anti-Bacterial Activity of Inorganic Nanomaterials and Their Antimicrobial Peptide Conjugates against Resistant and Non-Resistant Pathogens. Int. J. Pharm. 2020, 586, 119531. [Google Scholar] [CrossRef] [PubMed]
- Gakiya-Teruya, M.; Palomino-Marcelo, L.; Pierce, S.; Angeles-Boza, A.M.; Krishna, V.; Rodriguez-Reyes, J.C.F. Enhanced Antimicrobial Activity of Silver Nanoparticles Conjugated with Synthetic Peptide by Click Chemistry. J. Nanopart. Res. 2020, 22, 90. [Google Scholar] [CrossRef]









| Class of Organic Compounds | Organic Antimicrobial Compound | Advantages on Combination with AgNPs | Particular Features of the Compound |
|---|---|---|---|
| Polysaccharides and derivatives | Chitosan | Can be conjugated with AgNPs in many ways, both complex and one-pot synthesis (can be used as both stabilizing and reducing agent); system thoroughly studied, including toxicity and stability | Easily available, cheap, does not need additional modifications |
| Chitosan derivatives | Provides flexibility in terms of complexation/conjugation with AgNPs and structural organization of the composite material; antimicrobial activity could be reached in a controlled manner | Tunable and enhanced antimicrobial activity; can be designed against specific pathogens | |
| Cellulose derivatives | Allows functionalization with a wide range of antibacterial groups; provides better mechanical stability and broadens the potential application | ||
| Hyaluronic acid and derivatives | Provide strong antiadhesive properties; one of the main compounds used in tissue engineering | ||
| Phenolic compounds | Curcumin | Can be conjugated with AgNPs in many ways, both complex and one-pot synthesis (can be used as both stabilizing and c agent); | Pronounced activity against common human pathogens; abundant and biosafe |
| Tea extracts | Provides reduced or no toxicity to human cells | Have antibacterial and antifungal activity; tannic acid possess antiviral activity | |
| Acids (polycaffeic, tannic, ferulic) | Increased membrane permeability to allow silver ions and ROS attack pathogens more efficiently | ||
| Organic acids | Oleic acid | By providing strong negative surface charge, they show increased activity toward Gm+ bacteria | Provide several mechanisms of antibacterial activity; highly applicable in food safety |
| Gallic acid | |||
| Citric acid | |||
| Peptides | Host defense peptides (HDP) | Provide possible therapeutic window | Can be designed against specific pathogens |
| Independent-designed peptides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukushkina, E.A.; Hossain, S.I.; Sportelli, M.C.; Ditaranto, N.; Picca, R.A.; Cioffi, N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials 2021, 11, 1687. https://doi.org/10.3390/nano11071687
Kukushkina EA, Hossain SI, Sportelli MC, Ditaranto N, Picca RA, Cioffi N. Ag-Based Synergistic Antimicrobial Composites. A Critical Review. Nanomaterials. 2021; 11(7):1687. https://doi.org/10.3390/nano11071687
Chicago/Turabian StyleKukushkina, Ekaterina A., Syed Imdadul Hossain, Maria Chiara Sportelli, Nicoletta Ditaranto, Rosaria Anna Picca, and Nicola Cioffi. 2021. "Ag-Based Synergistic Antimicrobial Composites. A Critical Review" Nanomaterials 11, no. 7: 1687. https://doi.org/10.3390/nano11071687









