Doxorubicin Embedded into Nanofibrillated Bacterial Cellulose (NFBC) Produces a Promising Therapeutic Outcome for Peritoneally Metastatic Gastric Cancer in Mice Models via Intraperitoneal Direct Injection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of CM-NFBC
2.4. Preparation of DXR/CM-NFBC
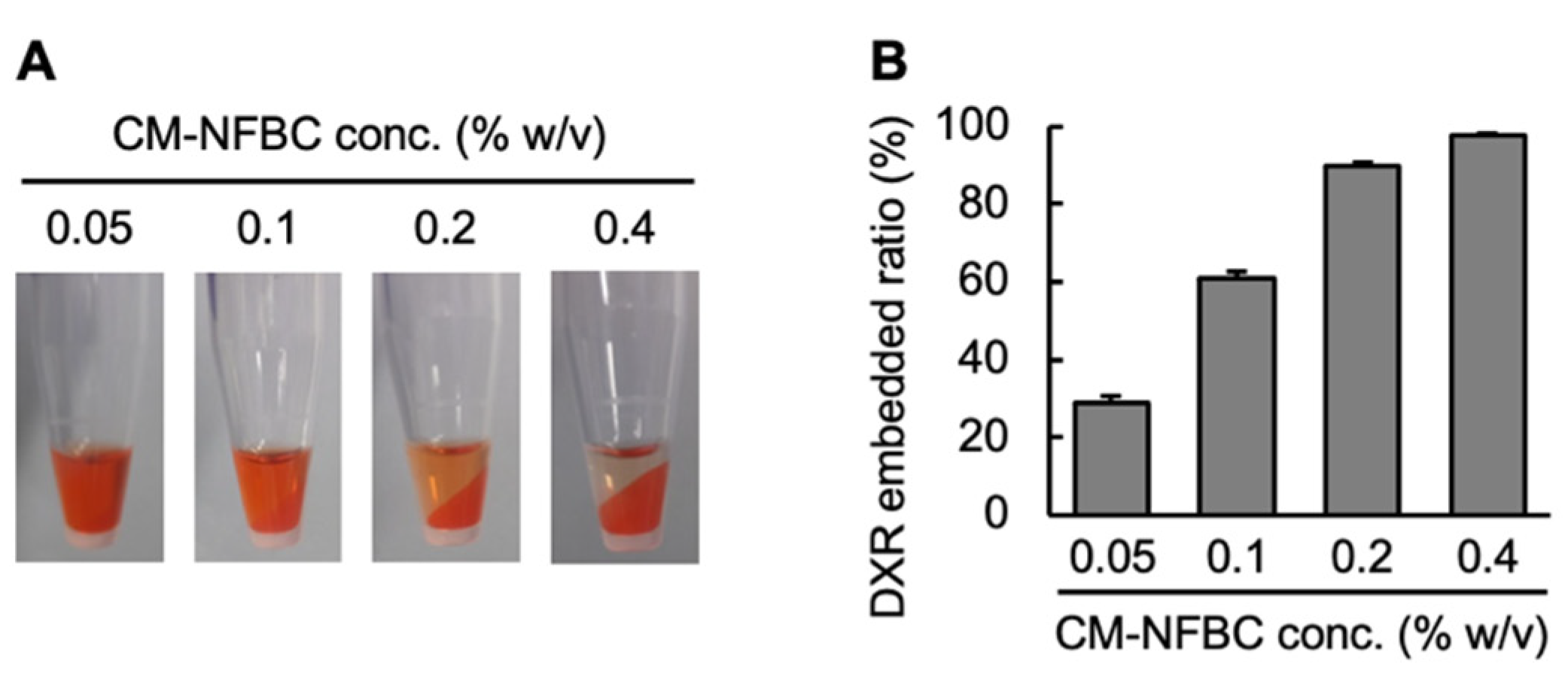
2.5. Determining the Drug-Embedded Ratio in CM-NFBC
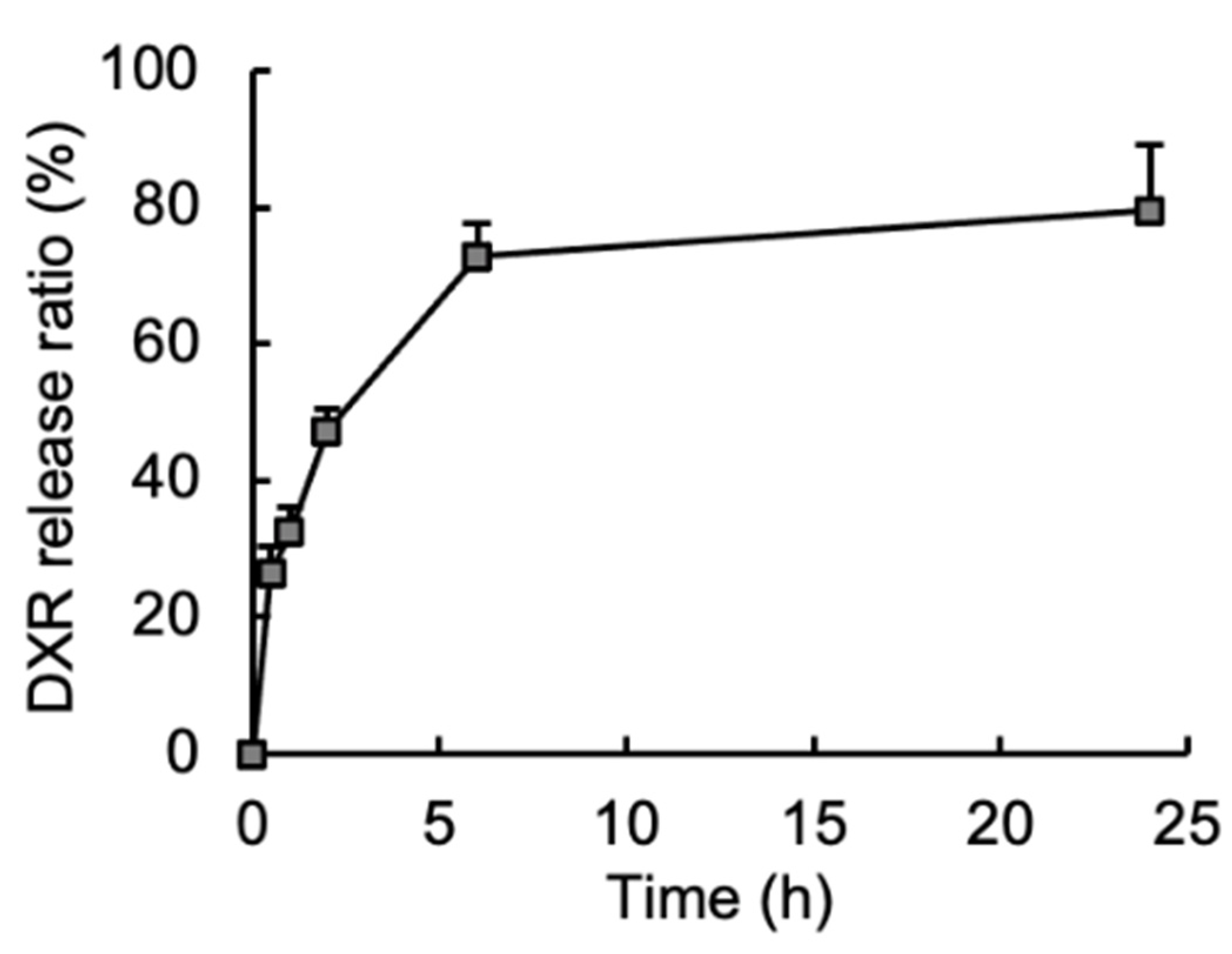
2.6. Release of DXR from DXR/CM-NFBC In Vitro
2.7. Preparation of Peritoneally Disseminated Gastric Cancer Xenograft Mouse Model
2.8. Evaluating the Tumor-Growth Inhibitory Effect of DXR/CM-NFBC on a Peritoneally Disseminated NCI-N87-Luc Xenograft Mouse Model
2.9. Statistical Analysis
3. Results
3.1. Preparation of DXR/CM-NFBC Formulation
3.2. Release of Embedded DXR from the DXR/CM-NFBC Formulation in Peritoneal Fluid
3.3. Growth Inhibitory Effect of an Intraperitoneally Injected DXR/CM-NFBC Formulation on Peritoneally Inoculated NCI-N87-Luc Tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Somerville, C. Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–78. [Google Scholar] [CrossRef]
- Doblin, M.S.; Kurek, I.; Jacob-Wilk, D.; Delmer, D.P. Cellulose biosynthesis in plants: From genes to rosettes. Plant Cell Physiol. 2002, 43, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [CrossRef] [PubMed]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshk, S.M. Vitamin C enhances bacterial cellulose production in Gluconacetobacter xylinus. Carbohydr. Polym. 2014, 99, 98–100. [Google Scholar] [CrossRef]
- Saxena, I.M.; Brown, R.M., Jr. Cellulose biosynthesis: Current views and evolving concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Haigler, C.H.; White, A.R.; Brown, R.M., Jr.; Cooper, K.M. Alteration of in vivo cellulose ribbon assembly by carboxymethylcellulose and other cellulose derivatives. J. Cell Biol. 1982, 94, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Lopez-Sanchez, P.; Li, R.; Li, Z. Production of bacterial cellulose by Gluconacetobacter hansenii CGMCC 3917 using only waste beer yeast as nutrient source. Bioresour. Technol. 2014, 151, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Lobmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef]
- Akagi, S.; Ando, H.; Fujita, K.; Shimizu, T.; Ishima, Y.; Tajima, K.; Matsushima, T.; Kusano, T.; Ishida, T. Therapeutic efficacy of a paclitaxel-loaded nanofibrillated bacterial cellulose (PTX/NFBC) formulation in a peritoneally disseminated gastric cancer xenograft model. Int. J. Biol. Macromol. 2021, 174, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Kose, R.; Sunagawa, N.; Yoshida, M.; Tajima, K. One-step production of nanofibrillated bacterial cellulose (NFBC) from waste glycerol using Gluconacetobacter intermedius NEDO-01. Cellulose 2013, 20, 2971–2979. [Google Scholar] [CrossRef]
- Tajima, K.; Kusumoto, R.; Kose, R.; Kono, H.; Matsushima, T.; Isono, T.; Yamamoto, T.; Satoh, T. One-Step Production of Amphiphilic Nanofibrillated Cellulose Using a Cellulose-Producing Bacterium. Biomacromolecules 2017, 18, 3432–3438. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef] [PubMed]
- Valero, V.; Perez, E.; Dieras, V. Doxorubicin and taxane combination regimens for metastatic breast cancer: Focus on cardiac effects. Semin. Oncol. 2001, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.J.; Bajorin, D.F. Advanced bladder cancer: The need to identify new agents in the post-M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) world. J. Urol. 1995, 153, 894–900. [Google Scholar] [CrossRef]
- Aisner, J.; Whitacre, M.; Abrams, J.; Propert, K. Doxorubicin, cyclophosphamide, etoposide and platinum, doxorubicin, cyclophosphamide and etoposide for small-cell carcinoma of the lung. Semin. Oncol. 1986, 13, 54–62. [Google Scholar]
- Zhu, Q.; Qi, H.; Long, Z.; Liu, S.; Huang, Z.; Zhang, J.; Wang, C.; Dong, L. Extracellular control of intracellular drug release for enhanced safety of anti-cancer chemotherapy. Sci. Rep. 2016, 6, 28596. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Li, H.; Yin, C.; Tang, F. Research progress in the application of in situ hydrogel system in tumor treatment. Drug Deliv. 2020, 27, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, B.; Devi, K.S.; Banerjee, R.; Maiti, T.K.; Pramanik, P.; Dhara, D. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl. Mater. Interfaces 2013, 5, 3884–3893. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Layard, M.W.; Basa, P.; Davis, H.L., Jr.; Von Hoff, A.L.; Rozencweig, M.; Muggia, F.M. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979, 91, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Doroshow, J.H. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J. Biol. Chem. 1986, 261, 3060–3067. [Google Scholar] [CrossRef]
- Kono, K.; Yong, W.P.; Okayama, H.; Shabbir, A.; Momma, T.; Ohki, S.; Takenoshita, S.; So, J. Intraperitoneal chemotherapy for gastric cancer with peritoneal disease: Experience from Singapore and Japan. Gastric Cancer 2017, 20, 122–127. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kodera, Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer 2017, 20, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, S.K.; Chow, H.H.; Janicek, M.F.; Cragun, J.M.; Hatch, K.D.; Cui, H.; Laughren, C.; Clouser, M.C.; Cohen, J.L.; Wright, H.M.; et al. Phase I trial of intraperitoneal pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer. Clin. Cancer Res. 2012, 18, 2668–2678. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatment | Day 14 | Day 21 | Day 28 | Day 35 | ||||
|---|---|---|---|---|---|---|---|---|
| BLI (×107) | TGI (%) | BLI (×107) | TGI (%) | BLI (×107) | TGI (%) | BLI (×107) | TGI (%) | |
| Control | 43.0 ± 16.1 | ----- | 35.6 ± 16.8 | ----- | 44.7 ± 14.2 | ----- | 49.3 ± 14.3 | ----- |
| Free DXR | 24.9 ± 10.3 * | 42.1 | 20.3 ± 9.8 | 42.9 | 23.2 ± 11.6 ** | 48.0 | 18.6 ± 11.1 *** | 62.4 |
| DXR/CM-NFBC | 18.0 ± 12.1 ** | 58.2 | 11.4 ± 11.0 ** | 67.9 | 10.0 ± 11.3 *** | 77.7 | 7.0 ± 8.0 *** | 85.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, H.; Mochizuki, T.; Lila, A.S.A.; Akagi, S.; Tajima, K.; Fujita, K.; Shimizu, T.; Ishima, Y.; Matsushima, T.; Kusano, T.; et al. Doxorubicin Embedded into Nanofibrillated Bacterial Cellulose (NFBC) Produces a Promising Therapeutic Outcome for Peritoneally Metastatic Gastric Cancer in Mice Models via Intraperitoneal Direct Injection. Nanomaterials 2021, 11, 1697. https://doi.org/10.3390/nano11071697
Ando H, Mochizuki T, Lila ASA, Akagi S, Tajima K, Fujita K, Shimizu T, Ishima Y, Matsushima T, Kusano T, et al. Doxorubicin Embedded into Nanofibrillated Bacterial Cellulose (NFBC) Produces a Promising Therapeutic Outcome for Peritoneally Metastatic Gastric Cancer in Mice Models via Intraperitoneal Direct Injection. Nanomaterials. 2021; 11(7):1697. https://doi.org/10.3390/nano11071697
Chicago/Turabian StyleAndo, Hidenori, Takashi Mochizuki, Amr S. Abu Lila, Shunsuke Akagi, Kenji Tajima, Kenji Fujita, Taro Shimizu, Yu Ishima, Tokuo Matsushima, Takatomo Kusano, and et al. 2021. "Doxorubicin Embedded into Nanofibrillated Bacterial Cellulose (NFBC) Produces a Promising Therapeutic Outcome for Peritoneally Metastatic Gastric Cancer in Mice Models via Intraperitoneal Direct Injection" Nanomaterials 11, no. 7: 1697. https://doi.org/10.3390/nano11071697







