Anticancer Potential of Temozolomide-Loaded Eudragit-Chitosan Coated Selenium Nanoparticles: In Vitro Evaluation of Cytotoxicity, Apoptosis and Gene Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
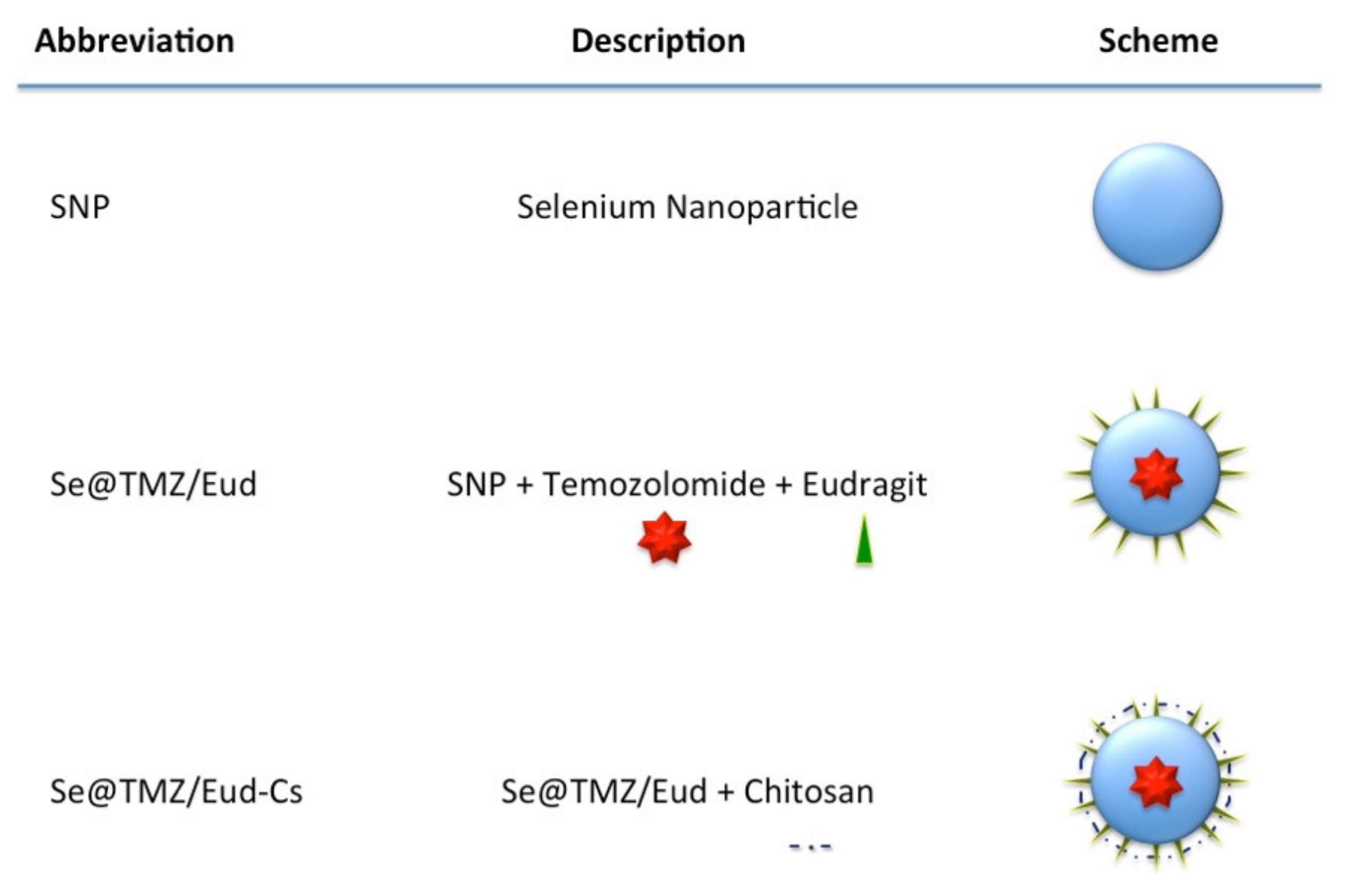
2.2. Nanoparticle Preparation
2.3. Characterization of the Nanoparticles
2.4. Evaluation of Drug Content
2.5. Evaluation of Drug Release
2.6. Evaluation of Cellular Uptake of Nanoparticles by Flow Cytometry
2.7. Cell Culture and Cell Viability Assay
2.8. Apoptosis Assay
2.9. RNA Extraction and cDNA Synthesis
2.10. Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. Characterization of Nanoparticles
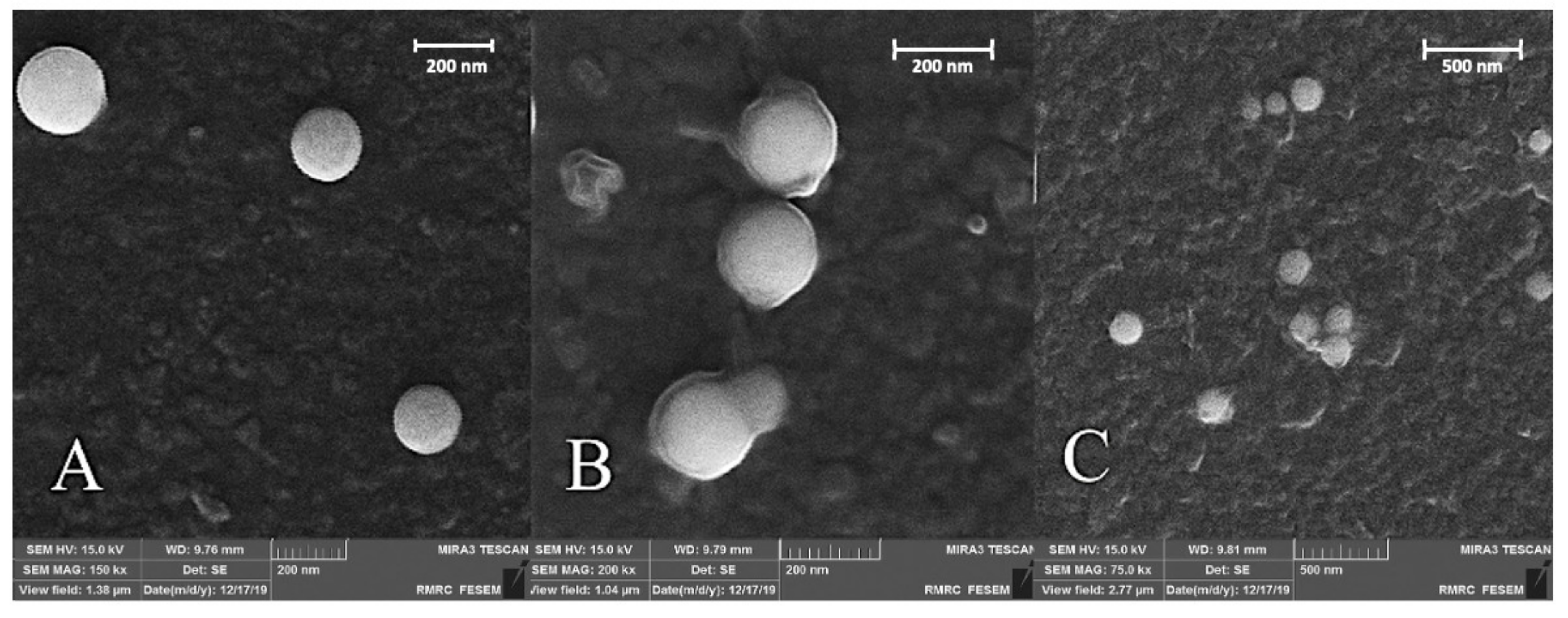
3.1.1. Morphological and Surface Properties
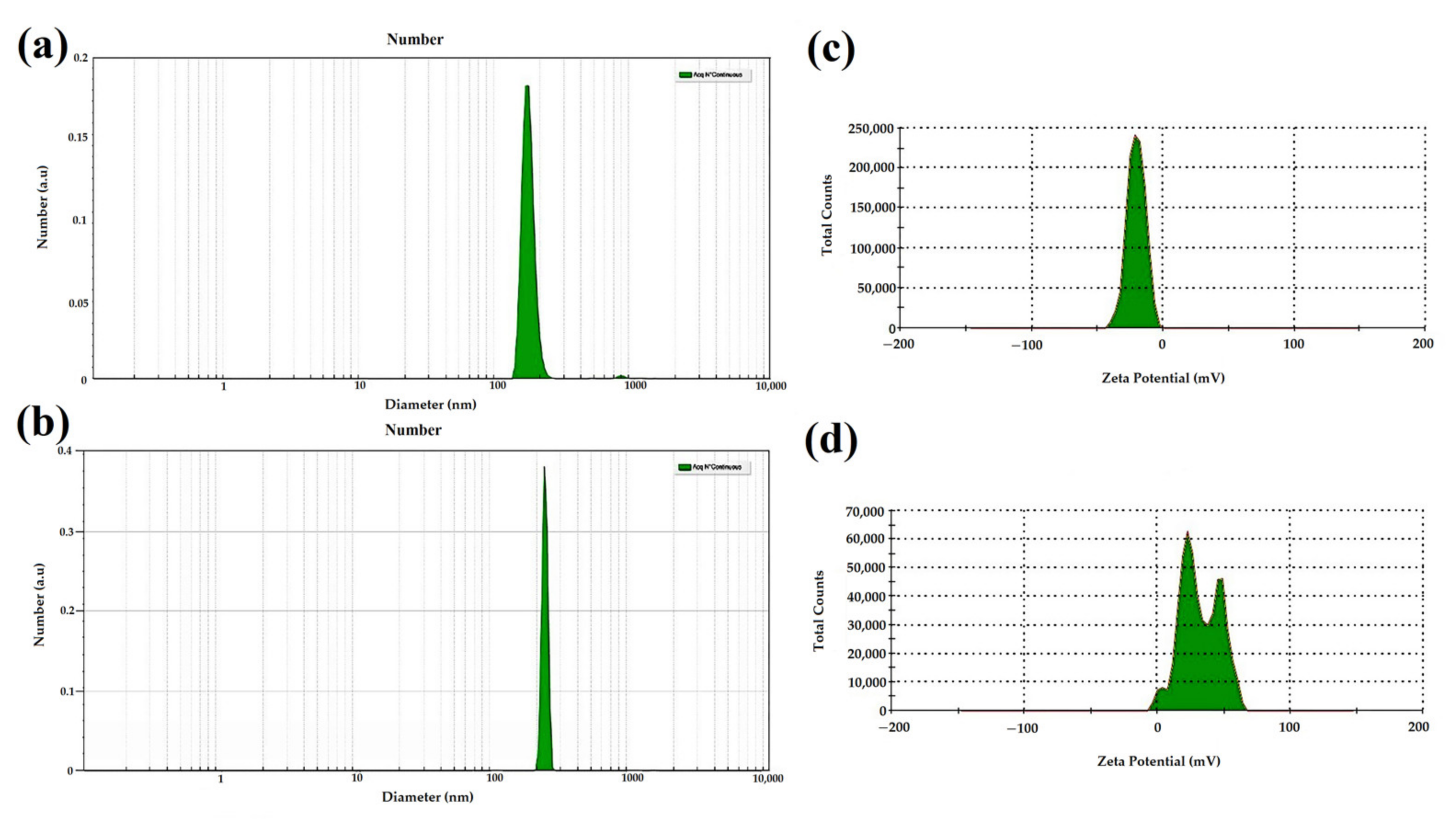
3.1.2. Evaluation by DLS and SBL
3.1.3. Validity Measurement by EDAX Assay
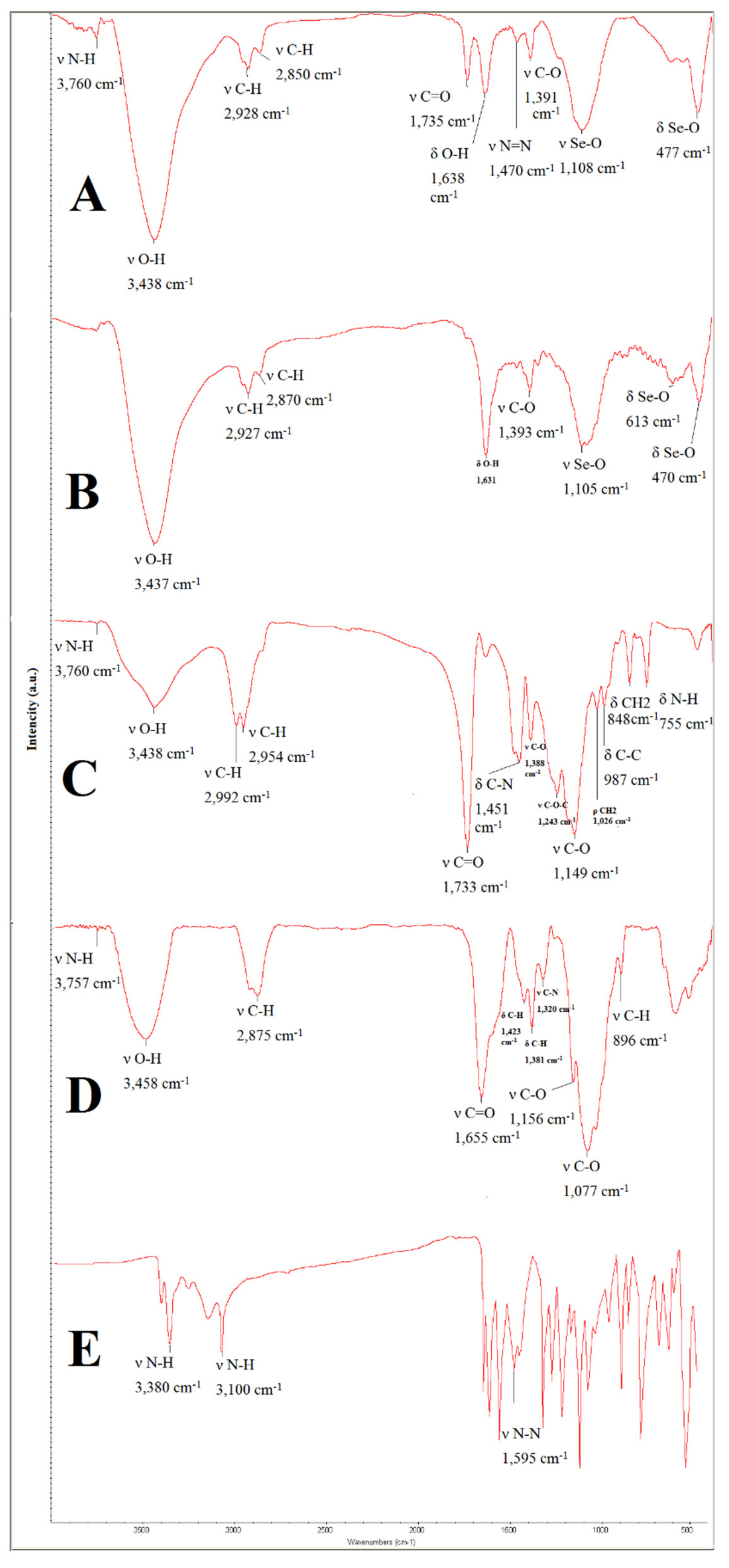
3.1.4. Fourier Transform Infrared Spectroscopy (FTIR) Assay
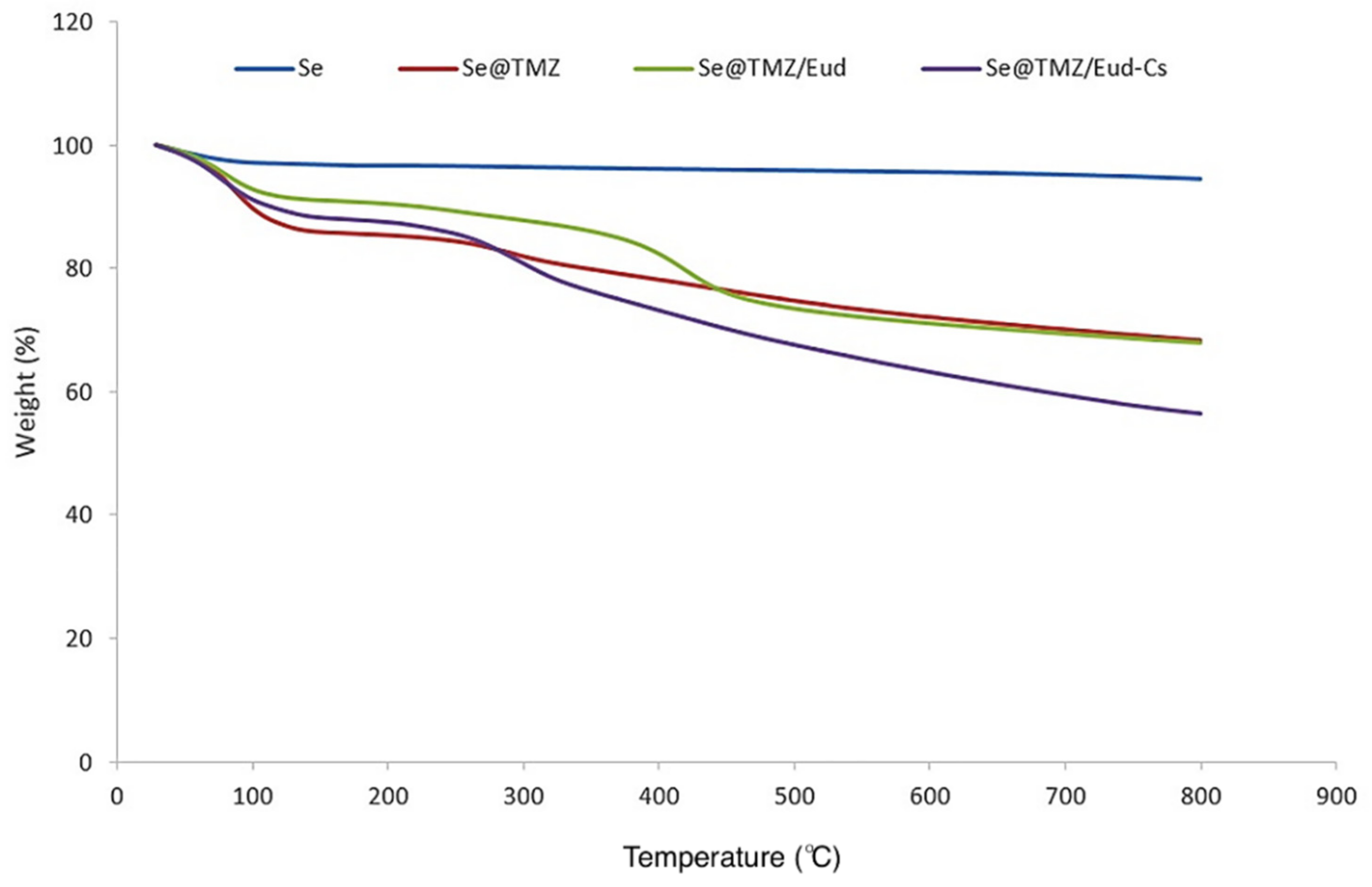
3.1.5. TGA Analysis
3.1.6. Drug Loading and In Vitro Release of Nanoparticles
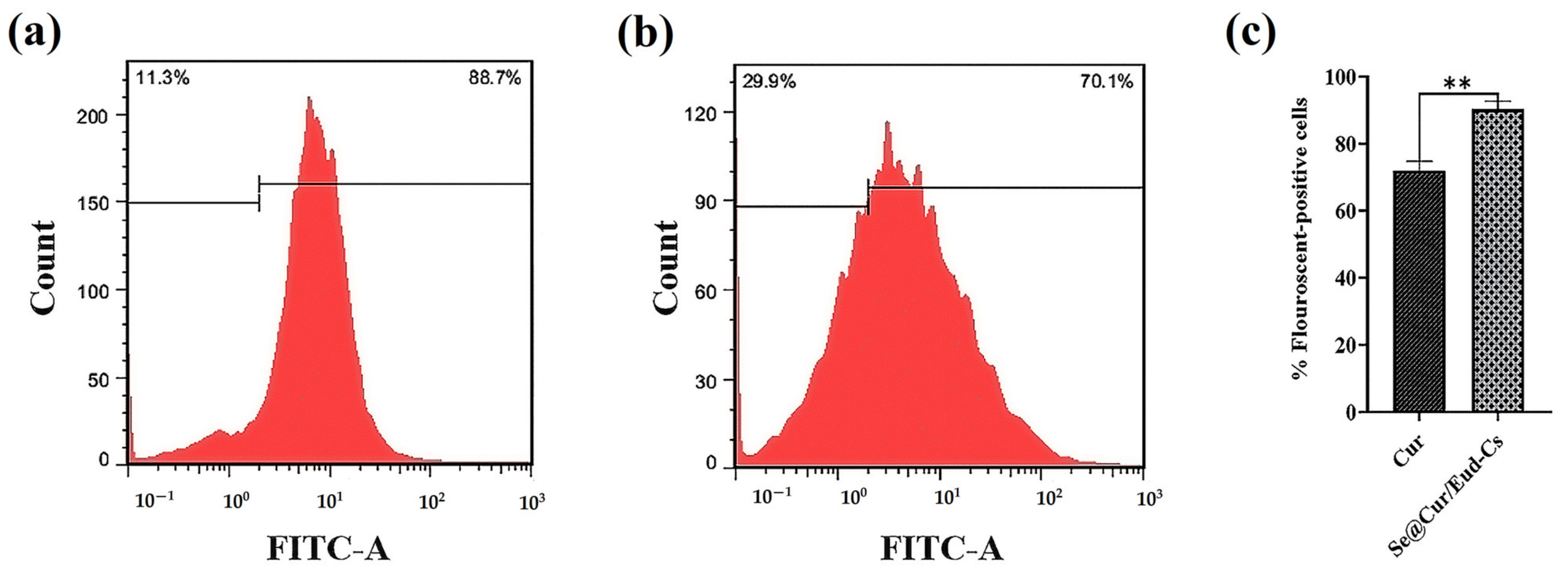
3.1.7. Cellular Uptake
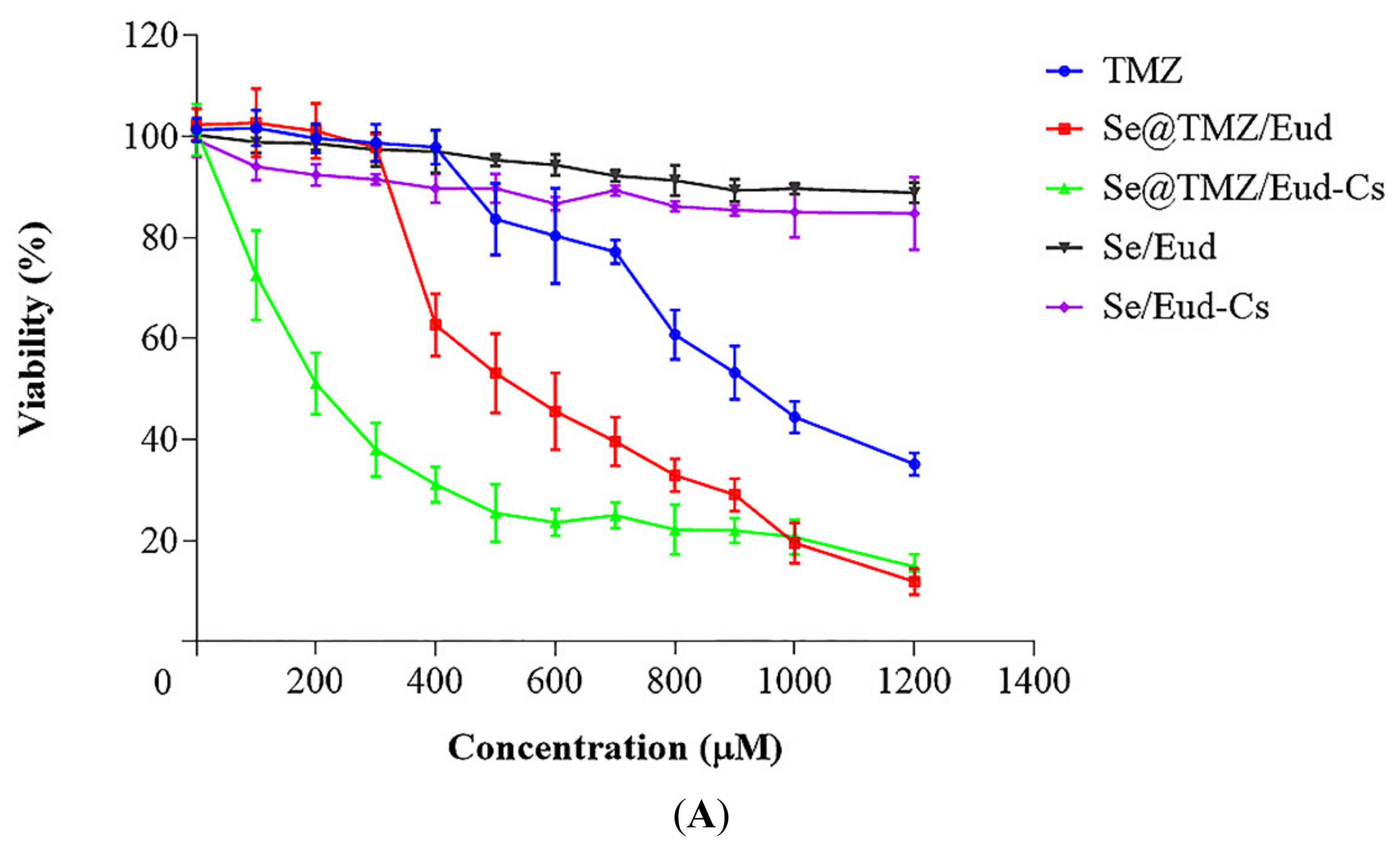
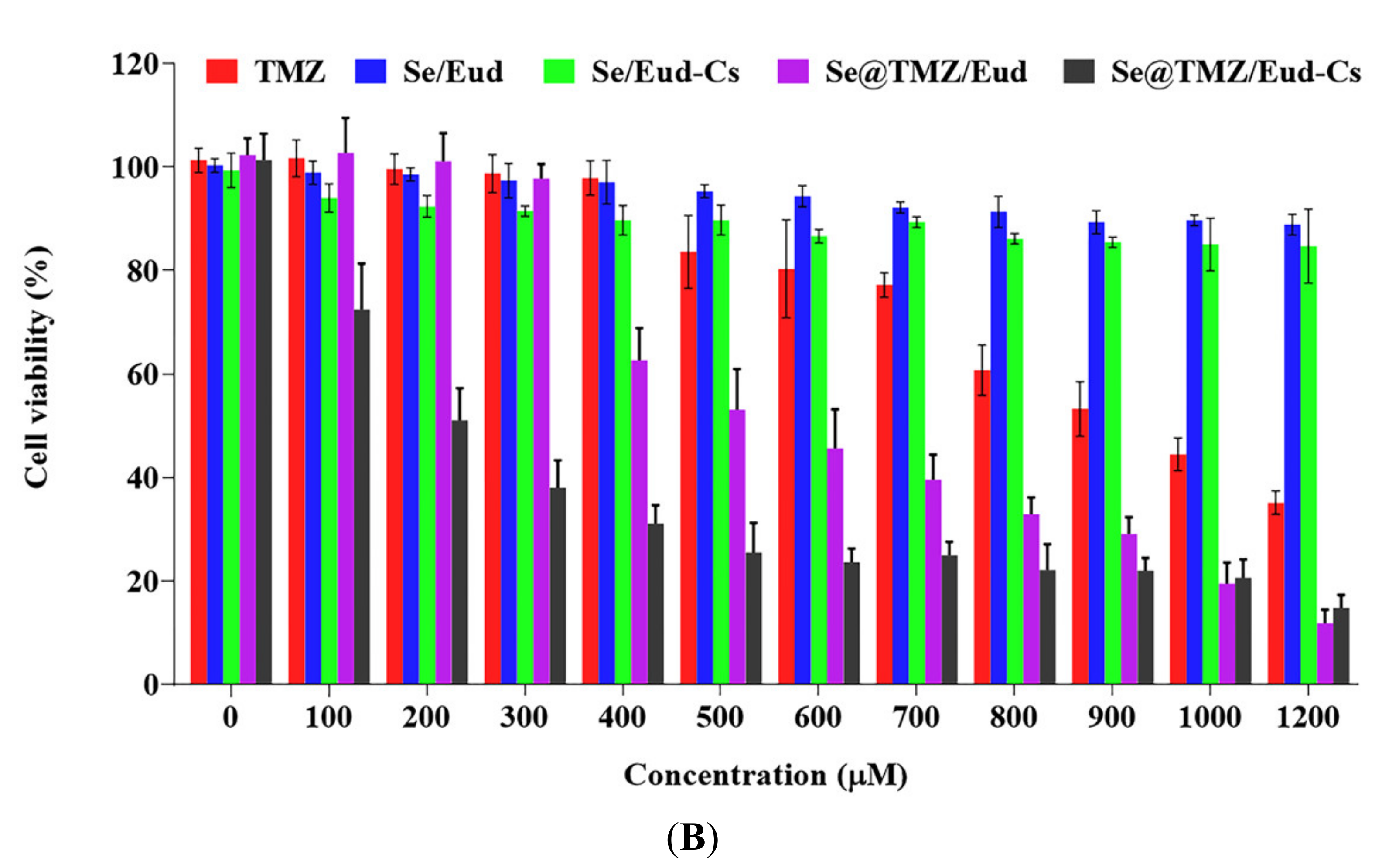
3.2. Cytotoxicity Studies
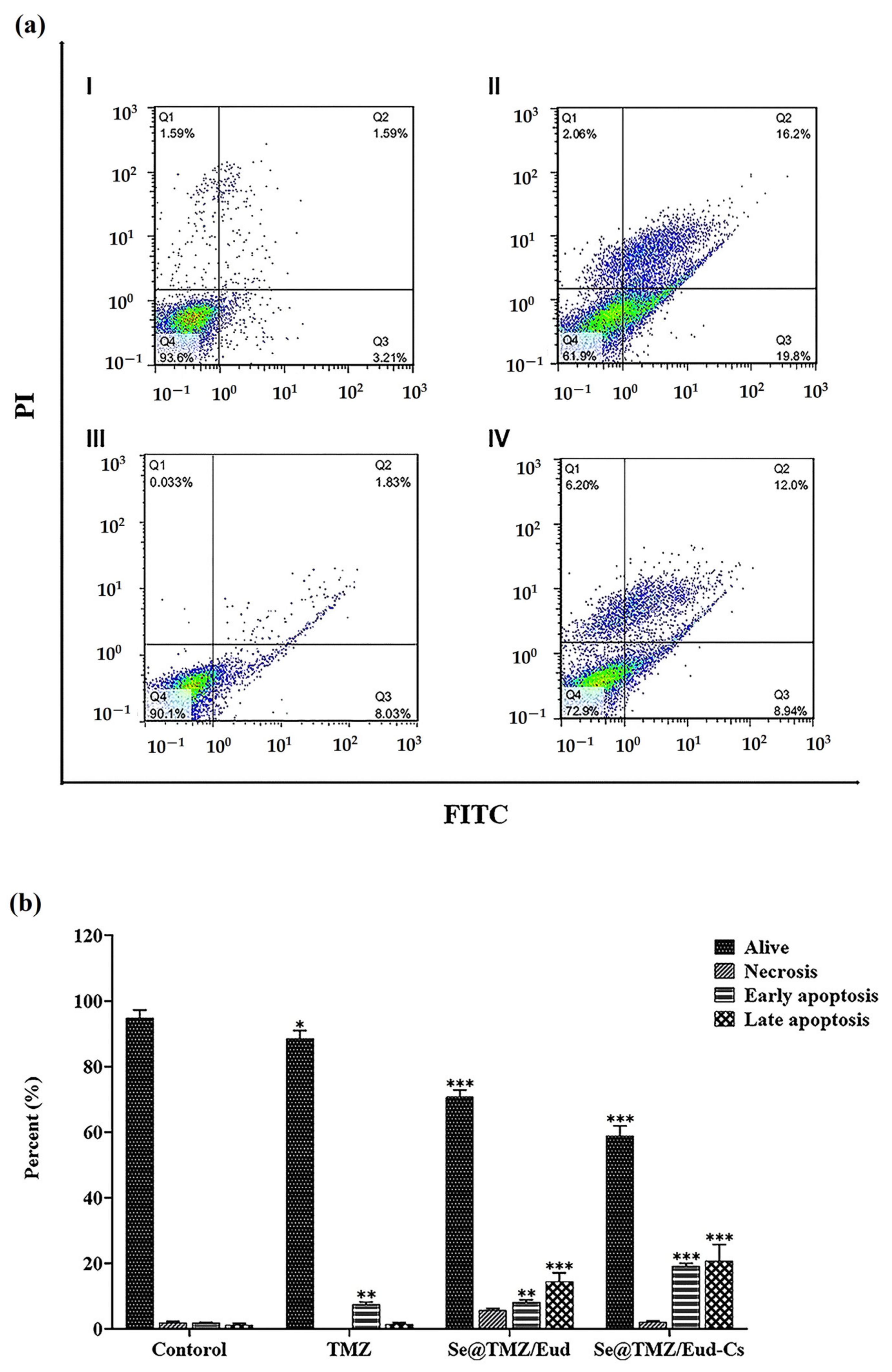
3.3. Apoptosis Assay
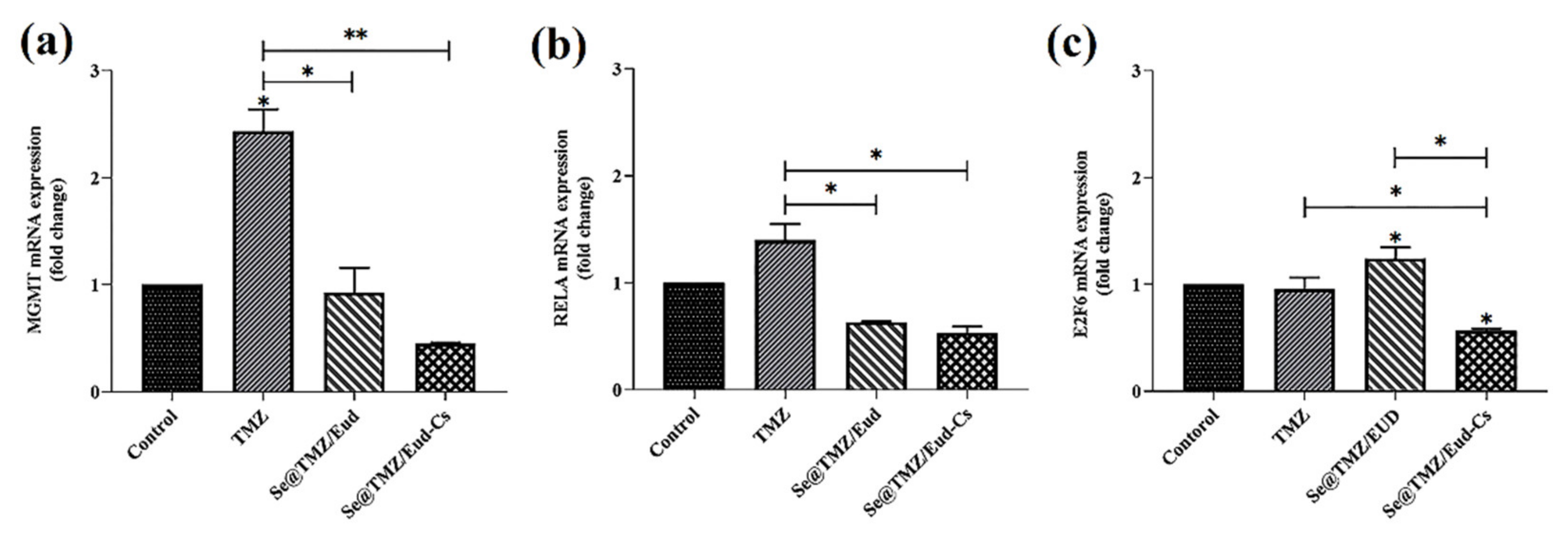
3.4. Gene Expression Level Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Cowperthwaite, M.C.; Burnett, M.G.; Shpak, M. Molecular predictors of long-term survival in glioblastoma multiforme patients. PLoS ONE 2016, 11, e0154313. [Google Scholar] [CrossRef] [Green Version]
- Yi, G.-Z.; Xiang, W.; Feng, W.-Y.; Chen, Z.-Y.; Li, Y.-M.; Deng, S.-Z.; Guo, M.-L.; Zhao, L.; Sun, X.-G.; He, M.-Y. Identification of key candidate proteins and pathways associated with temozolomide resistance in glioblastoma based on subcellular proteomics and bioinformatical analysis. BioMed Res. Int. 2018, 2018, 5238760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol. Med. Chir. 2018, 58, 405–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Liu, X.; Li, Y.; Wang, Q.; Zhou, J.; Wang, Y.; Dong, F.; Yang, C.; Sun, Z.; Fang, C. Genome-Wide CRISPR-Cas9 Screening Identifies NF-κB/E2F6 Responsible for EGFRvIII-Associated Temozolomide Resistance in Glioblastoma. Adv. Sci. 2019, 6, 1900782. [Google Scholar] [CrossRef] [Green Version]
- Kaina, B. Temozolomide in Glioblastoma Therapy: Role of Apoptosis, Senescence and Autophagy. Comment on Strobel et al., Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma—A comprehensive review. Cancer Drug Resist. 2021, 4, 17–43. [Google Scholar]
- André-Grégoire, G.; Bidère, N.; Gavard, J. Temozolomide affects extracellular vesicles released by glioblastoma cells. Biochimie 2018, 155, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, K.; Clavreul, A.; Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today 2015, 20, 899–905. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Wang, W.; Liang, J. Temozolomide inhibits cellular growth and motility via targeting ERK signaling in glioma C6 cells. Mol. Med. Rep. 2016, 14, 5732–5738. [Google Scholar] [CrossRef]
- Di Martino, A.; Kucharczyk, P.; Capakova, Z.; Humpolicek, P.; Sedlarik, V. Enhancement of temozolomide stability by loading in chitosan-carboxylated polylactide-based nanoparticles. J. Nanoparticle Res. 2017, 19, 71. [Google Scholar] [CrossRef] [Green Version]
- Erasimus, H.; Gobin, M.; Niclou, S.; Van Dyck, E. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat. Res. Rev. Mutat. Res. 2016, 769, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Hirst, T.; Vesterinen, H.; Sena, E.; Egan, K.; Macleod, M.; Whittle, I. Systematic review and meta-analysis of temozolomide in animal models of glioma: Was clinical efficacy predicted? Br. J. Cancer 2013, 108, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro Reily Rocha, C.; Reily Rocha, A.; Molina Silva, M.; Rodrigues Gomes, L.; Teatin Latancia, M.; Andrade Tomaz, M.; de Souza, I.; Karolynne Seregni Monteiro, L.; Frederico Martins Menck, C. Revealing Temozolomide Resistance Mechanisms via Genome-Wide CRISPR Libraries. Cells 2020, 9, 2573. [Google Scholar] [CrossRef]
- Soukhtanloo, M.; Mohtashami, E.; Maghrouni, A.; Mollazadeh, H.; Mousavi, S.H.; Roshan, M.K.; Tabatabaeizadeh, S.-A.; Hosseini, A.; Vahedi, M.M.; Jalili-Nik, M. Natural products as promising targets in glioblastoma multiforme: A focus on NF-κB signaling pathway. Pharmacol. Rep. 2020, 72, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, Y.; Wang, S.; Li, P.; Zhou, G.; Yuan, Y. Inhibition of NF-κB results in anti-glioma activity and reduces temozolomide-induced chemoresistance by down-regulating MGMT gene expression. Cancer Lett. 2018, 428, 77–89. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB (p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Benko, A.; Medina-Cruz, D.; Vernet-Crua, A.; O’Connell, C.P.; Świętek, M.; Barabadi, H.; Saravanan, M.; Webster, T.J. Nanocarrier drug resistant tumor interactions: Novel approaches to fight drug resistance in cancer. Cancer Drug Resist. 2021, 4, 4. [Google Scholar]
- Mozafari, M.R.; Pardakhty, A.; Azarmi, S.; Jazayeri, J.; Nokhodchi, A.; Omri, A. Role of nanocarrier systems in cancer nanotherapy. J. Liposome Res. 2009, 19, 310–321. [Google Scholar] [CrossRef]
- ElMeshad, A.N.; Mortazavi, S.; Mozafari, M.R. Formulation and characterization of nanoliposomal 5-fluorouracil for cancer nanotherapy. J. Liposome Res. 2014, 24, 1–9. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for brain drug delivery. Int. Sch. Res. Not. Biochem. 2013, 2013, 238428. [Google Scholar] [CrossRef] [Green Version]
- Caffrey, P.B.; Frenkel, G.D. Selenium compounds prevent the induction of drug resistance by cisplatin in human ovarian tumor xenografts in vivo. Cancer Chemother. Pharmacol. 2000, 46, 74–78. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer activity of chitosan, chitosan derivatives, and their mechanism of action. Int. J. Biomater. 2018, 2018, 2952085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, C.; Gogoi, P.; Maji, T. Chitosan: A promising biopolymer in drug delivery applications. J. Mol. Genet. Med. S 2015, 4, 899–910. [Google Scholar] [CrossRef]
- Nikam, V.K.; Kotade, K.; Gaware, V.; Dolas, R.; Dhamak, K.; Somwanshi, S.; Khadse, A.; Kashid, V. Eudragit a versatile polymer: A review. PharmacologyOnLine 2011, 1, 152–164. [Google Scholar]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Chen, L.; Cong, D.; Li, Y.; Wang, D.; Li, Q.; Hu, S. Combination of sonodynamic with temozolomide inhibits C6 glioma migration and promotes mitochondrial pathway apoptosis via suppressing NHE-1 expression. Ultrason. Sonochem. 2017, 39, 654–661. [Google Scholar] [CrossRef]
- Guha, A.; Biswas, N.; Bhattacharjee, K.; Das, P.; Kuotsu, K. In vitro evaluation of pH responsive Doxazosin loaded mesoporous silica nanoparticles: A smart approach in drug delivery. Curr. Drug Deliv. 2016, 13, 574–581. [Google Scholar] [CrossRef]
- Daryasari, M.P.; Akhgar, M.R.; Mamashli, F.; Bigdeli, B.; Khoobi, M. Chitosan-folate coated mesoporous silica nanoparticles as a smart and pH-sensitive system for curcumin delivery. RSC Adv. 2016, 6, 105578–105588. [Google Scholar] [CrossRef]
- Khosa, A.; Sinha, S.; Reddi, S.; Saha, R.N. Simple, Rapid and Sensitive UV Spectrophotometric Method for Determination of Temozolomide in Poly-ε-caprolactone Nanoparticles. Asian J. Chem. 2018, 30, 868–872. [Google Scholar] [CrossRef]
- Altememy, D.; Jafari, M.; Naeini, K.; Alsamarrai, S.; Khosravian, P. In-vitro evaluation of metronidazole loaded mesoporous silica nanoparticles against trichomonas vaginalis. Int. J. Pharm. Res. 2020, 12, 2773–2780. [Google Scholar]
- Altememy, D.; Khoobi, M.; Akbari Javar, H.; Alsamarrai, S.; Khosravian, P. Synthesis and Characterization of Silk Fibroin-Coated Mesoporous Silica Nanoparticles for Tioguanine Targeting to Leukemia. Int. J. Pharm. Res. 2020, 12. (Special Issue 2), Article No. 8787. [Google Scholar]
- Khosravian, P.; Ardestani, M.S.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Javar, H.A.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. OncoTargets Ther. 2016, 9, 7315–7330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primer3. (v. 0.4.0) Pick Primers from DNA Sequence. Available online: http://bioinfo.ut.ee/primer3-0.4.0 (accessed on 13 August 2018).
- Rao, J.U.; Coman, D.; Walsh, J.J.; Ali, M.M.; Huang, Y.; Hyder, F. Temozolomide arrests glioma growth and normalizes intratumoral extracellular pH. Sci. Rep. 2017, 7, 7865. [Google Scholar] [CrossRef] [Green Version]
- ChemSpider. Temozolomide. Available online: http://www.chemspider.com/Chemical-Structure.5201.html (accessed on 20 September 2018).
- Johannessen, T.-C.A.; Bjerkvig, R. Molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev. Anticancer Ther. 2012, 12, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 351. [Google Scholar] [CrossRef]
- Alavian, F.; Ghasemi, S. The Effectiveness of Nanoparticles on Gene Therapy for Glioblastoma Cells Apoptosis: A Systematic Review. Curr. Gene Ther. 2021, 21, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.-E.L.; Reddy, G.R.; Bhojani, M.; Schneider, R.; Philbert, M.A.; Rehemtulla, A.; Ross, B.D.; Kopelman, R. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. 2006, 58, 1556–1577. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.H.; Park, K.; Kim, Y.-S.; Bae, S.M.; Lee, S.; Jo, H.G.; Park, R.-W.; Kim, I.-S.; Jeong, S.Y.; Kim, K. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release 2008, 127, 208–218. [Google Scholar] [CrossRef]
- Romero-Pérez, A.; García-García, E.; Zavaleta-Mancera, A.; Ramírez-Bribiesca, J.E.; Revilla-Vázquez, A.; Hernández-Calva, L.M.; López-Arellano, R.; Cruz-Monterrosa, R.G. Designing and evaluation of sodium selenite nanoparticles in vitro to improve selenium absorption in ruminants. Vet. Res. Commun. 2010, 34, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahdati, M.; Moghadam, T.T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Peng, Z.; Leblanc, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Lee, C.Y. Strategies of temozolomide in future glioblastoma treatment. OncoTargets Ther. 2017, 10, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [Green Version]
- Kitange, G.J.; Carlson, B.L.; Schroeder, M.A.; Grogan, P.T.; Lamont, J.D.; Decker, P.A.; Wu, W.; James, C.D.; Sarkaria, J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 2009, 11, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Lan, F.; Yang, Y.; Han, J.; Wu, Q.; Yu, H.; Yue, X. Sulforaphane reverses chemo-resistance to temozolomide in glioblastoma cells by NF-κB-dependent pathway downregulating MGMT expression. Int. J. Oncol. 2016, 48, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Sato, A.; Sunayama, J.; Matsuda, K.; Seino, S.; Suzuki, K.; Watanabe, E.; Tachibana, K.; Tomiyama, A.; Kayama, T.; Kitanaka, C. MEK-ERK signaling dictates DNA-repair gene MGMT expression and temozolomide resistance of stem-like glioblastoma cells via the MDM2-p53 axis. Stem Cells 2011, 29, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol. Lett. 2015, 9, 2586–2590. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Annealing Tm (°C) | PCR Product Length |
|---|---|---|---|---|
| MGMT | GACAGGTGTTATGGAAGCTG | GGCTGCTAATTGCTGGTAAGA | 58 | 71 |
| E2F6 | ATGTGTCGCTGGTCTACTTA | ATGTGGTTCTTAGATTTCTTTTCC | 56 | 178 |
| RELA | CGTGAGGCTGTTTGGTTTGAG | GTCTTATGGCTGAGGTCTGGTC | 62 | 109 |
| Actb | AGAGGGAAATCGTGCGTGAC | AGGAAGGAAGGCTGGAAGAGA | 60 | 187 |
| Nanoparticles | TMZ Loading Efficiency (%) | TMZ Loading Capacity (%) |
|---|---|---|
| Se@TMZ/Eud-Cs | 82.77 ± 5.30 | 37.5 ± 4.43 |
| Se@TMZ/Eud | 61.70 ± 3.65 | 15.71 ± 3.43 |
| Live Cells (%) | Necrotic Cells (%) | Early Apoptotic Cells (%) | Late Apoptotic Cells (%) | |
|---|---|---|---|---|
| Control | 94.91 ± 2.39 | 1.96 ± 0.35 | 1.8 ± 0.24 | 1.14 ± 0.57 |
| Free TMZ | 88.63 ± 2.43 | 0.06 ± 0.03 | 7.54 ± 0.65 | 1.41 ± 0.44 |
| Se@TMZ/Eud | 70.72 ± 2.20 | 5.73 ± 0.50 | 8.18 ± 0.68 | 14.52 ± 2.63 |
| Se@TMZ/Eud-Cs | 58.86 ± 3.16 | 2.18 ± 0.27 | 19.16 ± 0.85 | 22.88 ± 4.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazarei, M.; Mohammadi Arvejeh, P.; Mozafari, M.R.; Khosravian, P.; Ghasemi, S. Anticancer Potential of Temozolomide-Loaded Eudragit-Chitosan Coated Selenium Nanoparticles: In Vitro Evaluation of Cytotoxicity, Apoptosis and Gene Regulation. Nanomaterials 2021, 11, 1704. https://doi.org/10.3390/nano11071704
Mazarei M, Mohammadi Arvejeh P, Mozafari MR, Khosravian P, Ghasemi S. Anticancer Potential of Temozolomide-Loaded Eudragit-Chitosan Coated Selenium Nanoparticles: In Vitro Evaluation of Cytotoxicity, Apoptosis and Gene Regulation. Nanomaterials. 2021; 11(7):1704. https://doi.org/10.3390/nano11071704
Chicago/Turabian StyleMazarei, Madineh, Pooria Mohammadi Arvejeh, M. R. Mozafari, Pegah Khosravian, and Sorayya Ghasemi. 2021. "Anticancer Potential of Temozolomide-Loaded Eudragit-Chitosan Coated Selenium Nanoparticles: In Vitro Evaluation of Cytotoxicity, Apoptosis and Gene Regulation" Nanomaterials 11, no. 7: 1704. https://doi.org/10.3390/nano11071704








