Inclusion of 2D Transition Metal Dichalcogenides in Perovskite Inks and Their Influence on Solar Cell Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of 2D MoS2 Nanosheets
2.3. Preparation of the Perovskite Precursors Solutions
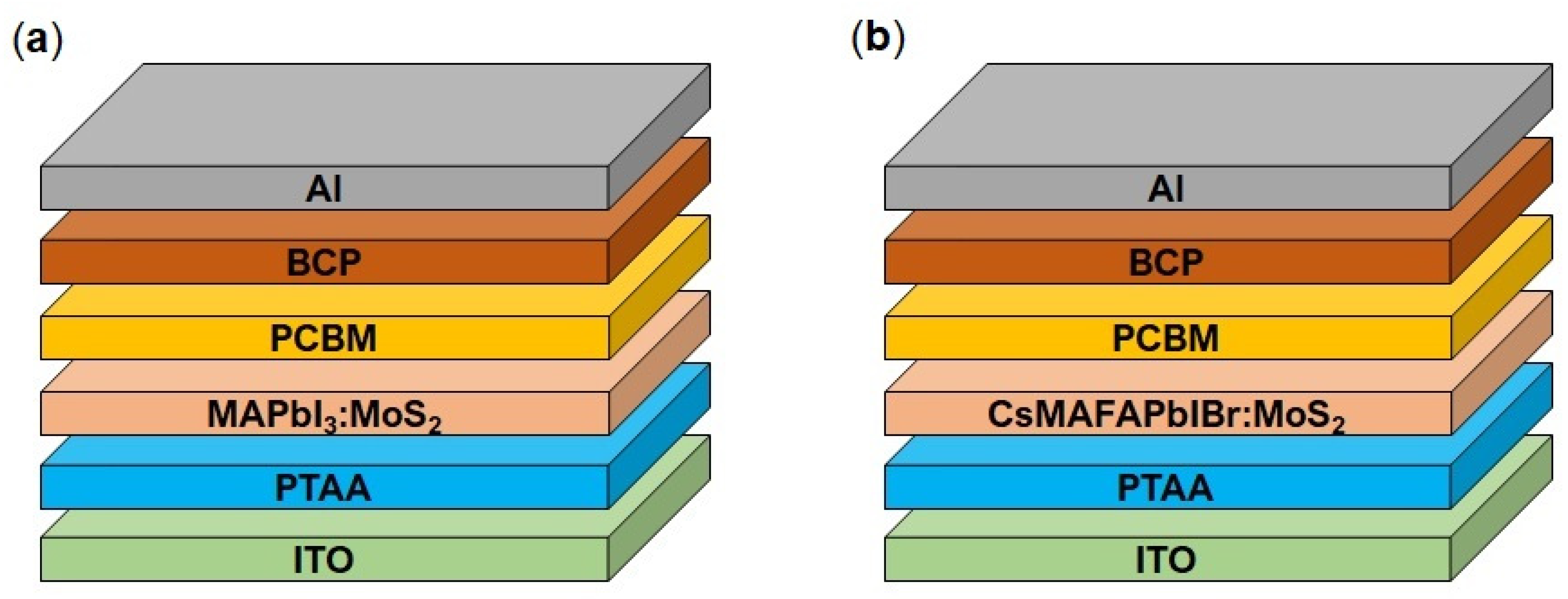
2.4. Photovoltaic Device Fabrication
2.5. Materials Characterization
2.6. Photovoltaic Device Characterization
3. Results and Discussion
3.1. Properties and Characterizations of Materials
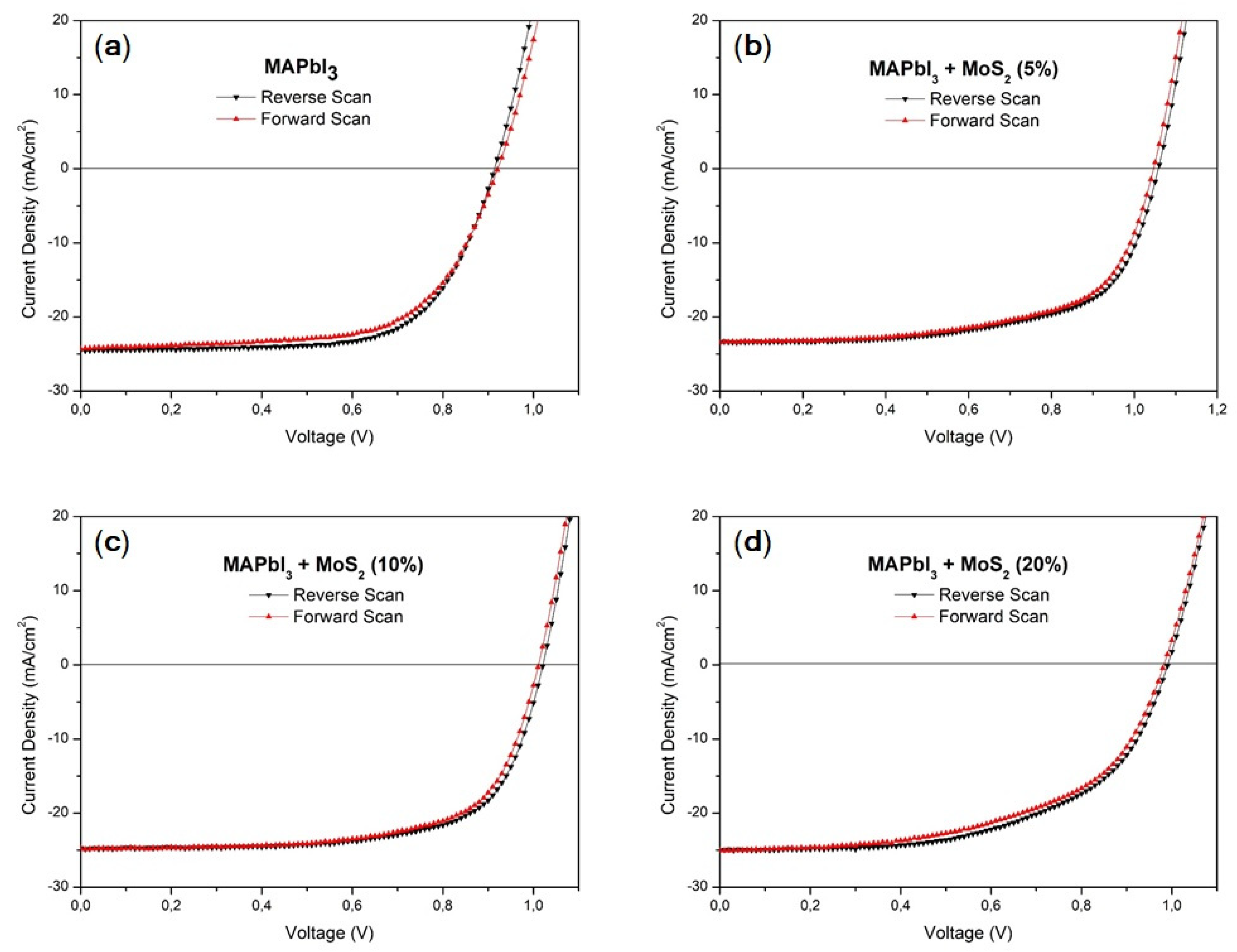
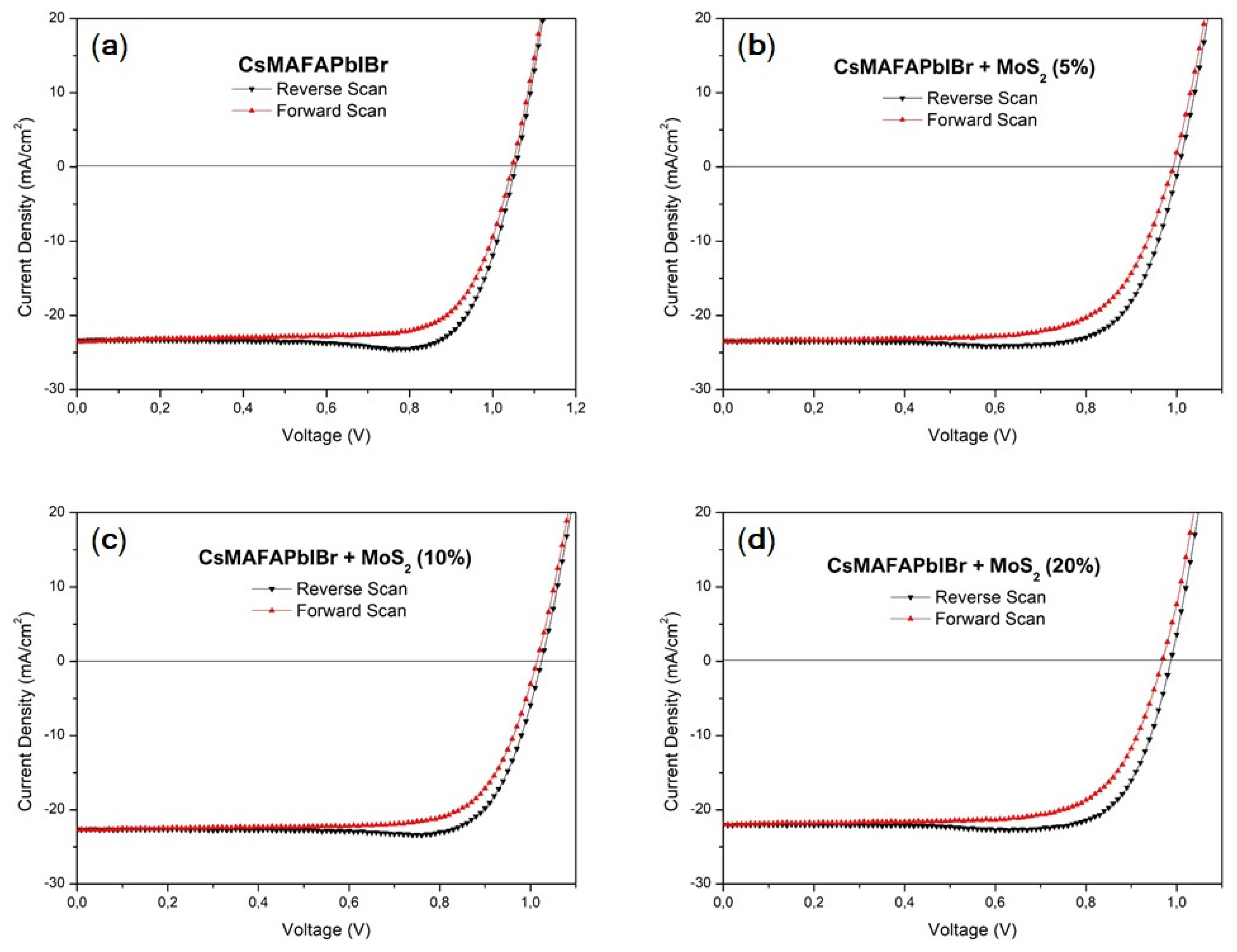
3.2. Photovoltaic Performances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chi, W.; Banerjee, S.K. Progress in materials development for the rapid efficiency advancement of perovskite solar cells. Small 2020, 16, 1907531. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-efficiency perovskite solar cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Ann, M.H.; Kim, J.; Kim, M.; Alosaimi, G.; Kim, D.; Ha, N.Y.; Seidel, J.; Park, N.; Yun, J.S.; Kim, J.H. Device design rules and operation principles of high-power perovskite solar cells for indoor applications. Nano Energy 2020, 68, 104321. [Google Scholar] [CrossRef]
- Tailor, N.K.; Kim, J.; Kim, M.; Alosaimi, G.; Kim, D.; Ha, N.Y.; Seidel, J.; Park, N.; Yun, J.S.; Kim, J.H. Recent progress in morphology optimization in perovskite solar cell. J. Mater. Chem. A 2020, 8, 21356–21386. [Google Scholar] [CrossRef]
- Kim, H.S.; Hagfeldt, A.; Park, N.G. Morphological and compositional progress in halide perovskite solar cells. Chem. Commun. 2019, 55, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Arain, Z.; Liu, C.; Yang, Y.; Mateen, M.; Ren, Y.; Ding, Y.; Liu, X.; Ali, Z.; Kumar, M.; Dai, S. Elucidating the dynamics of solvent engineering for perovskite solar cells. Sci. China Mater. 2019, 62, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Park, N.G. Research direction toward scalable, stable, and high efficiency perovskite solar cells. Adv. Energy Mater. 2020, 10, 1903106. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Qaid, S.M.H.; Hezam, M.; Bedja, I.; Ghaithan, H.M.; Aldwayyan, A.S. Effect of deposition method on the structural and optical properties of CH3NH3PbI3 perovskite thin films. Opt. Mater. 2020, 103, 109836. [Google Scholar] [CrossRef]
- Mahapatra, A.; Mahapatra, A.; Prochowicz, D.; Tavakolic, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Masi, S.; Rizzo, A.; Munir, R.; Listorti, A.; Giuri, A.; Corcione, C.E.; Treat, N.D.; Gigli, G.; Amassian, A.; Stingelin, N.; et al. Organic Gelators as Growth Control Agents for Stable and Reproducible Hybrid Perovskite-Based Solar Cells. Adv. Energy Mater. 2017, 7, 1602600. [Google Scholar] [CrossRef] [Green Version]
- Masi, S.; Sestu, N.; Valenzano, V.; Higashino, T.; Imahori, H.; Saba, M.; Bongiovanni, G.; Armenise, V.; Milella, A.; Gigli, G.; et al. Simple processing additive-driven 20% efficiency for inverted planar heterojunction perovskite solar cells. ACS Appl. Mater. Interfaces 2020, 12, 18431–18436. [Google Scholar] [CrossRef]
- Giuri, A.; Masi, S.; Listorti, A.; Gigli, G.; Colella, S.; Corcione, C.E.; Rizzo, A. Polymeric rheology modifier allows single-step coating of perovskite ink for highly efficient and stable solar cells. Nano Energy 2018, 54, 400–408. [Google Scholar] [CrossRef]
- Masi, S.; Masi, S.; Rizzo, A.; Aiello, F.; Balzano, F.; Uccello-Barretta, G.; Listorti, A.; Gigliac, G.; Colella, S. Multiscale morphology design of hybrid halide perovskites through a polymeric template. Nanoscale 2015, 7, 18956–18963. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- You, P.; Tang, G.; Cao, J.; Shen, D.; Ng, T.; Hawash, Z.; Wang, N.; Liu, C.; Lu, W.; Tai, Q. 2D materials for conducting holes from grain boundaries in perovskite solar cells. Light Sci. Appl. 2021, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Aiello, F.; Listorti, A.; Balzano, F.; Altamura, D.; Giannini, C.; Caliandro, R.; Uccello-Barretta, G.; Rizzo, A.; Colella, S. Connecting the solution chemistry of PbI2 and MAI: A cyclodextrin-based supramolecular approach to the formation of hybrid halide perovskites. Chem. Sci. 2018, 9, 3200–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastria, R.; Colella, S.; Qualtieri, A.; Listorti, A.; Gigliab, G.; Rizzo, A. Elucidating the effect of the lead iodide complexation degree behind the morphology and performance of perovskite solar cells. Nanoscale 2017, 9, 3889–3897. [Google Scholar] [CrossRef]
- Chen, H.-C.; Lan, J.M.; Hsu, H.L.; Li, C.W.; Shieh, T.S.; Wong, K.T.; Chen, C.P. Synergistic improvements in performance and stability of inverted planar MAPbI3 based perovskite solar cells incorporating benzylammonium halide salt additives. Mater. Chem. Front. 2021, 5, 3378–3387. [Google Scholar] [CrossRef]
- Liu, Z.; Ono, L.K.; Qi, Y. Additives in metal halide perovskite films and their applications in solar cells. J. Energy Chem. 2020, 46, 215–228. [Google Scholar] [CrossRef]
- Haque, F.; Wright, M.; Mahmud, M.A.; Yi, H.; Wang, D.; Duan, L.; Xu, C.; Upama, M.B.; Uddin, A. Effects of hydroiodic acid concentration on the properties of CsPbI3 perovskite solar cells. ACS Omega 2018, 3, 11937–11944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, V.; Silva, S.R.P.; Zhang, W. Carbon materials in perovskite solar cells: Prospects and future challenges. Energy Environ. Mater. 2019, 2, 107–118. [Google Scholar] [CrossRef] [Green Version]
- You, P.; Tang, G.; Yan, F. Two-dimensional materials in perovskite solar cells. Mater. Today Energy 2019, 11, 128–158. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Batmunkh, M.; Shapter, J.G. Emerging 2D layered materials for perovskite solar cells. Adv. Energy Mater. 2020, 10, 1902253. [Google Scholar] [CrossRef]
- Carlo, A.; Di Agresti, A.; Brunetti, F.; Pescetelli, S. Two-dimensional materials in perovskite solar cells. J. Phys. Energy 2020, 2, 128–158. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Gouda, L.; Tischler, Y.; Kaliakatsos, I.; Petridis, K. 2D Transition Metal. Dichalcogenides for Solution-Processed Organic and Perovskite Solar Cells; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Capasso, A.; Matteocci, F.; Najafi, L.; Prato, M.; Buha, J.; Cinà, L.; Pellegrini, V.; di Carlo, A.; Bonaccorso, F. Few-Layer MoS2 Flakes as Active Buffer Layer for Stable Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1600920. [Google Scholar] [CrossRef]
- Huang, P.; Chen, Q.; Zhang, K.; Yuan, L.; Zhou, Y.; Song, B.; Li, Y. 21.7% efficiency achieved in planar n-i-p perovskite solar cells via interface engineering with water-soluble 2D TiS2. J. Mater. Chem. A 2019, 7, 6213–6219. [Google Scholar] [CrossRef]
- Liang, M.; Ali, A.; Belaidi, A.; Hossain, M.I.; Ronan, O.; Downing, C.; Tabet, N.; Sanvito, S.; I-Mellouhi, F.E.; Nicolosi, V. Improving stability of organometallic-halide perovskite solar cells using exfoliation two-dimensional molybdenum chalcogenides. NPJ 2D Mater. Appl. 2020, 4, 40. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, K.; Zhang, F.; Jain, S.M.; He, T.; Jiang, Y.; Yuan, M. CH3NH3PbI3:MoS2 heterostructure for stable and efficient inverted perovskite solar cell. Sol. Energy 2020, 195, 436–445. [Google Scholar] [CrossRef]
- Vasilopoulou, M.; Fakharuddin, A.; Coutsolelos, A.G.; Falaras, P.; Argitis, P.; Yusoffd, A.R.B.M.; Nazeeruddin, M.K. Molecular materials as interfacial layers and additives in perovskite solar cells. Chem. Soc. Rev. 2020, 49, 4496–4526. [Google Scholar] [CrossRef]
- Bermudez, V.M. Theoretical study of the adsorption of Lewis acids on MoS2 in relation to atomic layer deposition of Al2O3. J. Vac. Sci. Technol. A 2020, 38, 062412. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.; Fu, Q.; Chen, L.; Ji, T.; Hu, X.; Chen, Y. Enhancing the grain size of organic halide perovskites by sulfonate-carbon nanotube incorporation in high performance perovskite solar cells. Chem. Commun. 2016, 52, 5674–5677. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.B.; Ge, Z. Understanding of perovskite crystal growth and film formation in scalable deposition processes. Chem. Soc. Rev. 2020, 49, 1653–1687. [Google Scholar] [CrossRef]
- Ke, L.; Luo, S.; Ren, X.; Yuan, Y. Factors influencing the nucleation and crystal growth of solution-processed organic lead halide perovskites: A review. J. Phys. D Appl. Phys. 2021, 54, 163001. [Google Scholar] [CrossRef]
- Li, H.; Wu, G.; Li, W.; Zhang, Y.; Liu, Z.; Wang, D.; Liu, S. Additive Engineering to Grow Micron-Sized Grains for Stable High Efficiency Perovskite Solar Cells. Adv. Sci. 2019, 6, 1901241. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.; Shang, Q.; Zhao, L.; Wang, R.; Zhang, Z.; Yang, P.; Sui, X.; Qiu, X.; Liu, X.; Zhang, Q.; et al. Highly Efficient Charge Transfer between Perovskite Nanocrystals and g-C3N4 Nanosheets. Phys. Status Solidi Basic Res. 2020, 257, 1655–1662. [Google Scholar]
- Fang, Q.; Shang, Q.; Zhao, L.; Wang, R.; Zhang, Z.; Yang, P.; Sui, X.; Qiu, X.; Liu, X.; Zhang, Q.; et al. Ultrafast charge transfer in perovskite nanowire/2D transition metal dichalcogenide heterostructures. J. Phys. Chem. Lett. 2018, 9, 1655–1662. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Meng, L.; You, J.; Guo, T.F.; Yang, Y. Recent advances in the inverted planar structure of perovskite solar cells. Acc. Chem. Res. 2016, 49, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, R.; Zhang, Z.; Xiong, J.; He, Z.; Fan, B.; Zhang, J. Achieving efficient inverted planar perovskite solar cells with nondoped PTAA as a hole transport layer. Org. Electron. 2019, 71, 106–112. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Chen, X.; Li, Z.; Wang, J.; Lia, T.; Deng, X. Largely enhanced: VOC and stability in perovskite solar cells with modified energy match by coupled 2D interlayers. J. Mater. Chem. A 2018, 6, 4860–4867. [Google Scholar] [CrossRef]
- Wu, W.Q.; Yang, Z.; Rudd, P.N.; Shao, Y.; Dai, X.; Wei, H.; Huang, J. Bilateral alkylamine for suppressing charge recombination and improving stability in blade-coated perovskite solar cells. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [Green Version]






| Average Thickness (nm) | |
|---|---|
| MAPbI3 | 422 ± 7 |
| MAPbI3 + MoS2 (5%) | 439 ± 15 |
| MAPbI3 + MoS2 (10%) | 436 ± 9 |
| MAPbI3 + MoS2 (20%) | 442 ± 20 |
| Mean (nm) | St. Dev. (nm) | |
|---|---|---|
| MAPbI3 | 116.32 | 33.27 |
| MAPbI3 + MoS2 (5%) | 177.17 | 48.83 |
| MAPbI3 + MoS2 (10%) | 187.54 | 40.97 |
| MAPbI3 + MoS2 (20%) | 125.63 | 28.17 |
| Mean (nm) | St. Dev. (nm) | |
|---|---|---|
| CsMAFAPbIBr | 237.17 | 71.32 |
| CsMAFAPbIBr + MoS2 (5%) | 248.74 | 70.56 |
| CsMAFAPbIBr + MoS2 (10%) | 240.10 | 72.42 |
| CsMAFAPbIBr + MoS2 (20%) | 214.23 | 69.25 |
| FF (%) | VOC (V) | JSC (mA cm−2) | PCE (%) | |
|---|---|---|---|---|
| MAPbI3 | 67.4 (67.5 ± 1.8) 64.2 (66.9 ± 1.9) | 0.91 (0.96 ± 0.04) 0.92 (0.96 ± 0.03) | 24.49 (22.16 ± 1.65) 24.31 (22.20 ± 1.50) | 15.09 (14.24 ± 0.60) 14.38 (14.20 ± 0.46) |
| MAPbI3 + MoS2 (5%) | 64.5 (60.1 ± 8.6) 63.3 (58.7 ± 7.8) | 1.06 (1.05 ± 0.01) 1.05 (1.03 ± 0.02) | 23.45 (22.37 ± 0.78) 23.48 (22.25 ± 0.86) | 16.01 (14.13 ± 2.31) 15.54 (13.51 ± 2.36) |
| MAPbI3 + MoS2 (10%) | 68.9 (68.9 ± 1.1) 67.6 (67.5 ± 1.4) | 1.02 (1.01 ± 0.01) 1.01 (1.00 ± 0.01) | 24.76 (21.88 ± 2.05) 24.77 (21.91 ± 2.04) | 17.42 (15.25 ± 1.55) 16.92 (14.85 ± 1.47) |
| MAPbI3 + MoS2 (20%) | 67.4 (71.1 ± 3.2) 63.7 (67.8 ± 3.9) | 0.92 (0.92 ± 0.01) 0.91 (0.92 ± 0.01) | 21.46 (19.75 ± 2.00) 21.44 (19.71 ± 2.01) | 13.30 (12.88 ± 1.59) 12.45 (12.30 ± 1.60) |
| FF (%) | VOC (V) | JSC (mA cm−2) | PCE (%) | |
|---|---|---|---|---|
| CsMAFAPbIBr | 78.6 (78.4 ± 4.9) 68.8 (70.3 ± 4.0) | 1.03 (1.03 ± 0.01) 1.00 (1.03 ± 0.01) | 23.37 (21.38 ± 1.34) 23.56 (20.58 ± 0.69) | 18.89 (17.29 ± 1.13) 16.97 (14.89 ± 0.90) |
| CsMAFAPbIBr + MoS2 (5%) | 78.2 (77.6 ± 4.8) 69.8 (70.6 ± 3.6) | 1.01 (1.01 ± 0.03) 0.99 (1.01 ± 0.03) | 23.47 (21.91 ± 1.66) 23.54 (21.86 ± 1.72) | 18.45 (17.20 ± 1.19) 16.31 (15.45 ± 0.64) |
| CsMAFAPbIBr + MoS2 (10%) | 81.3 (78.5 ± 4.3) 73.5 (71.8 ± 3.2) | 1.03 (1.01 ± 0.02) 1.01 (1.00 ± 0.02) | 22.62 (22.41 ± 1.41) 22.76 (22.46 ± 1.32) | 18.85 (17.78 ± 0.81) 16.98 (16.19 ± 0.58) |
| CsMAFAPbIBr + MoS2 (20%) | 78.8 (77.6 ± 3.2) 71.7 (70.7 ± 1.8) | 1.01 (1.00 ± 0.02) 1.00 (0.99 ± 0.03) | 22.08 (21.78 ± 0.76) 22.10 (21.81 ± 0.78) | 17.59 (16.85 ± 0.63) 15.80 (15.21 ± 0.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taurisano, N.; Bravetti, G.; Carallo, S.; Liang, M.; Ronan, O.; Spurling, D.; Coelho, J.; Nicolosi, V.; Colella, S.; Gigli, G.; et al. Inclusion of 2D Transition Metal Dichalcogenides in Perovskite Inks and Their Influence on Solar Cell Performance. Nanomaterials 2021, 11, 1706. https://doi.org/10.3390/nano11071706
Taurisano N, Bravetti G, Carallo S, Liang M, Ronan O, Spurling D, Coelho J, Nicolosi V, Colella S, Gigli G, et al. Inclusion of 2D Transition Metal Dichalcogenides in Perovskite Inks and Their Influence on Solar Cell Performance. Nanomaterials. 2021; 11(7):1706. https://doi.org/10.3390/nano11071706
Chicago/Turabian StyleTaurisano, Nicola, Gianluca Bravetti, Sonia Carallo, Meiying Liang, Oskar Ronan, Dahnan Spurling, João Coelho, Valeria Nicolosi, Silvia Colella, Giuseppe Gigli, and et al. 2021. "Inclusion of 2D Transition Metal Dichalcogenides in Perovskite Inks and Their Influence on Solar Cell Performance" Nanomaterials 11, no. 7: 1706. https://doi.org/10.3390/nano11071706
APA StyleTaurisano, N., Bravetti, G., Carallo, S., Liang, M., Ronan, O., Spurling, D., Coelho, J., Nicolosi, V., Colella, S., Gigli, G., Listorti, A., & Rizzo, A. (2021). Inclusion of 2D Transition Metal Dichalcogenides in Perovskite Inks and Their Influence on Solar Cell Performance. Nanomaterials, 11(7), 1706. https://doi.org/10.3390/nano11071706









