Exploring the FTIR, Optical and Nuclear Radiation Shielding Properties of Samarium-Borate Glass: A Characterization through Experimental and Simulation Methods
Abstract
:1. Introduction
2. Methods and Materials
3. Results and Discussion
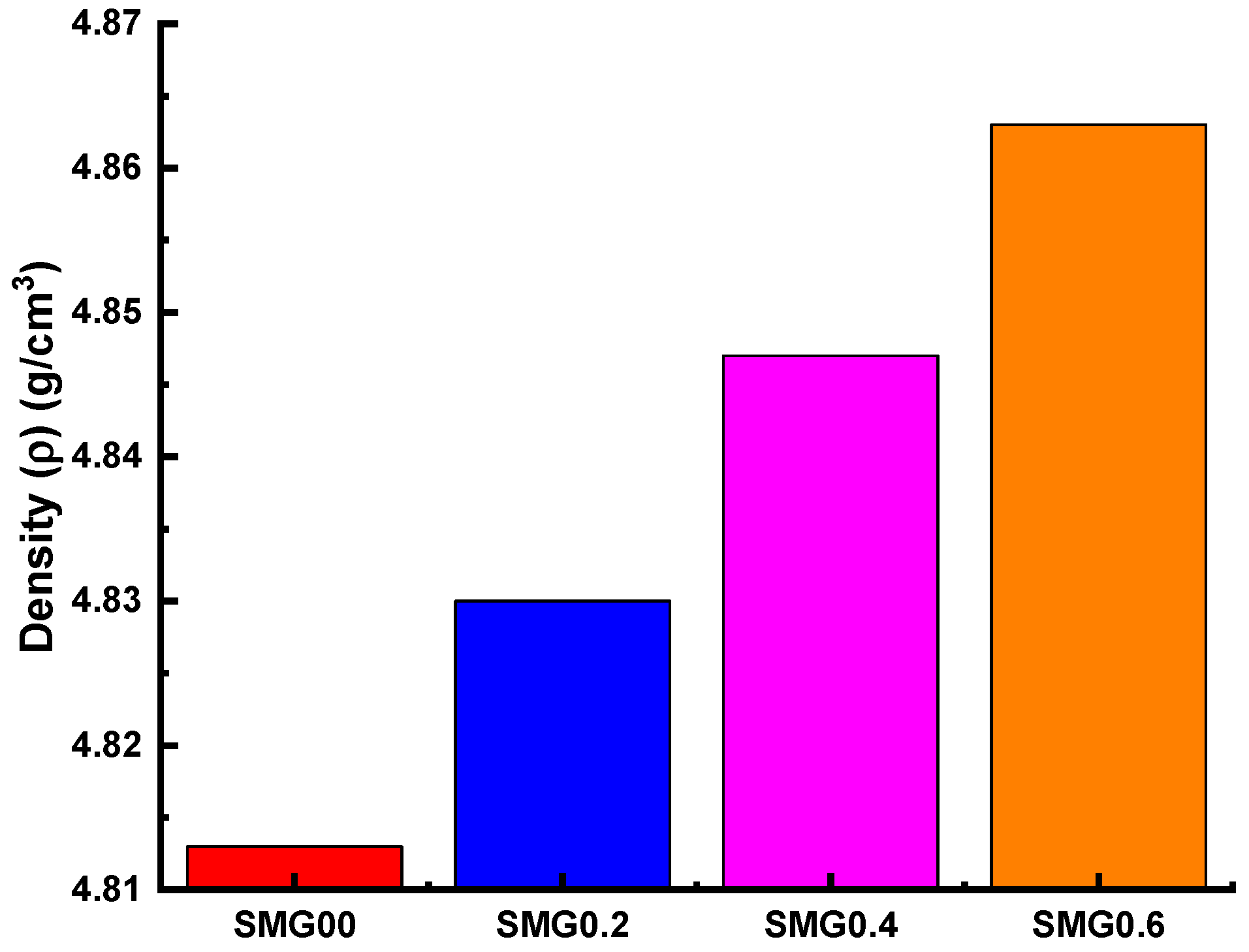
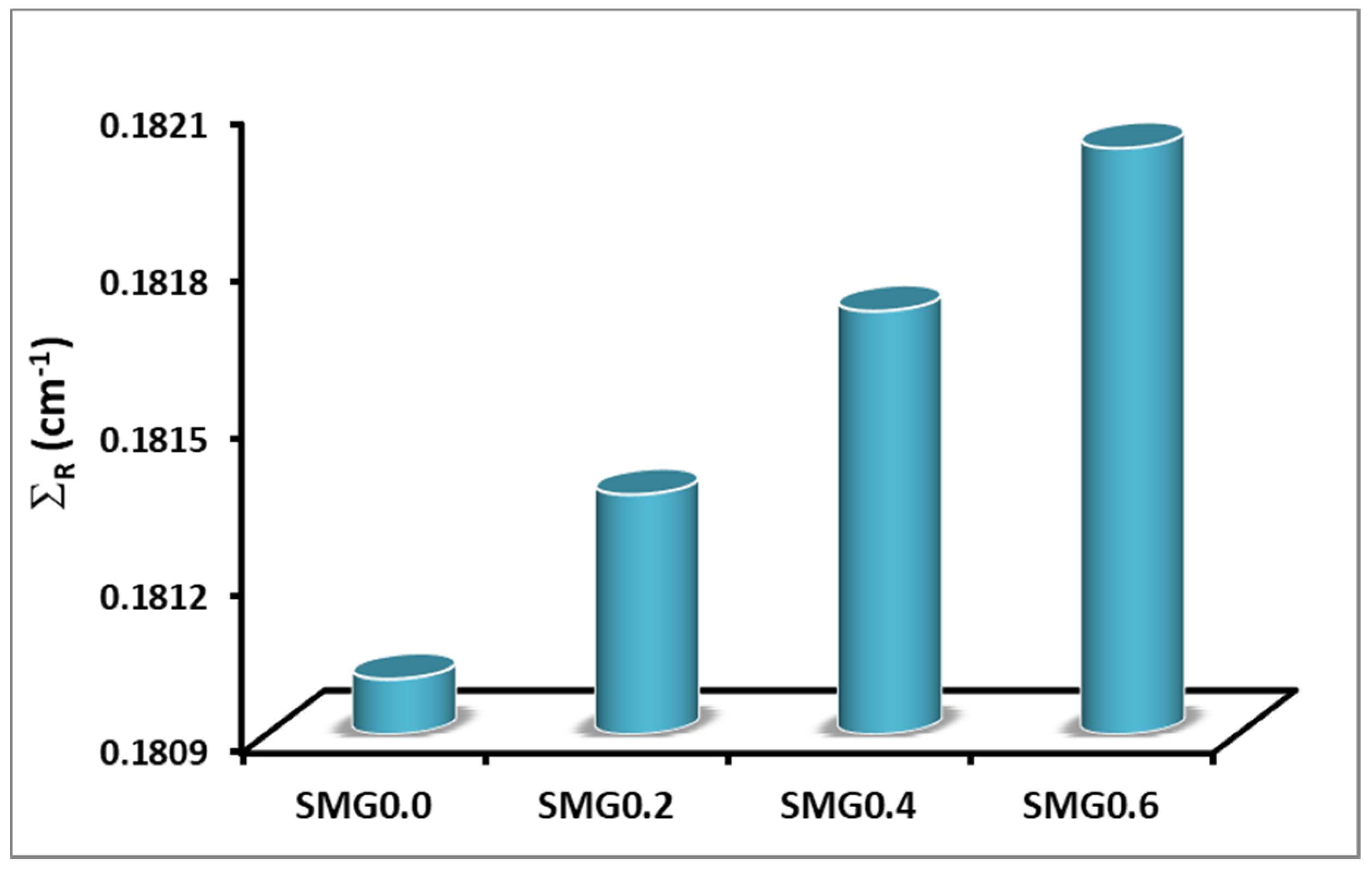
3.1. Investigations on Structural Properties
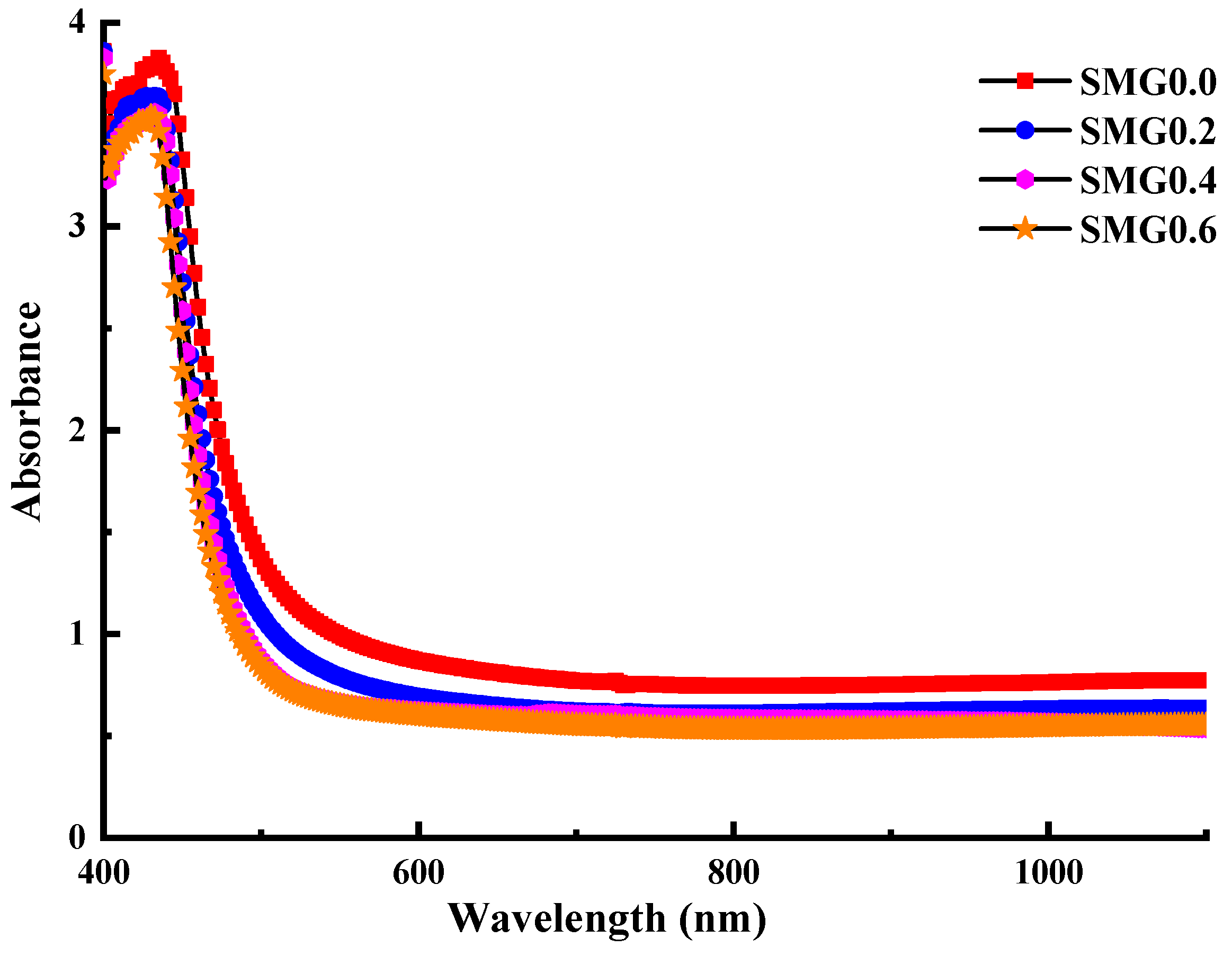
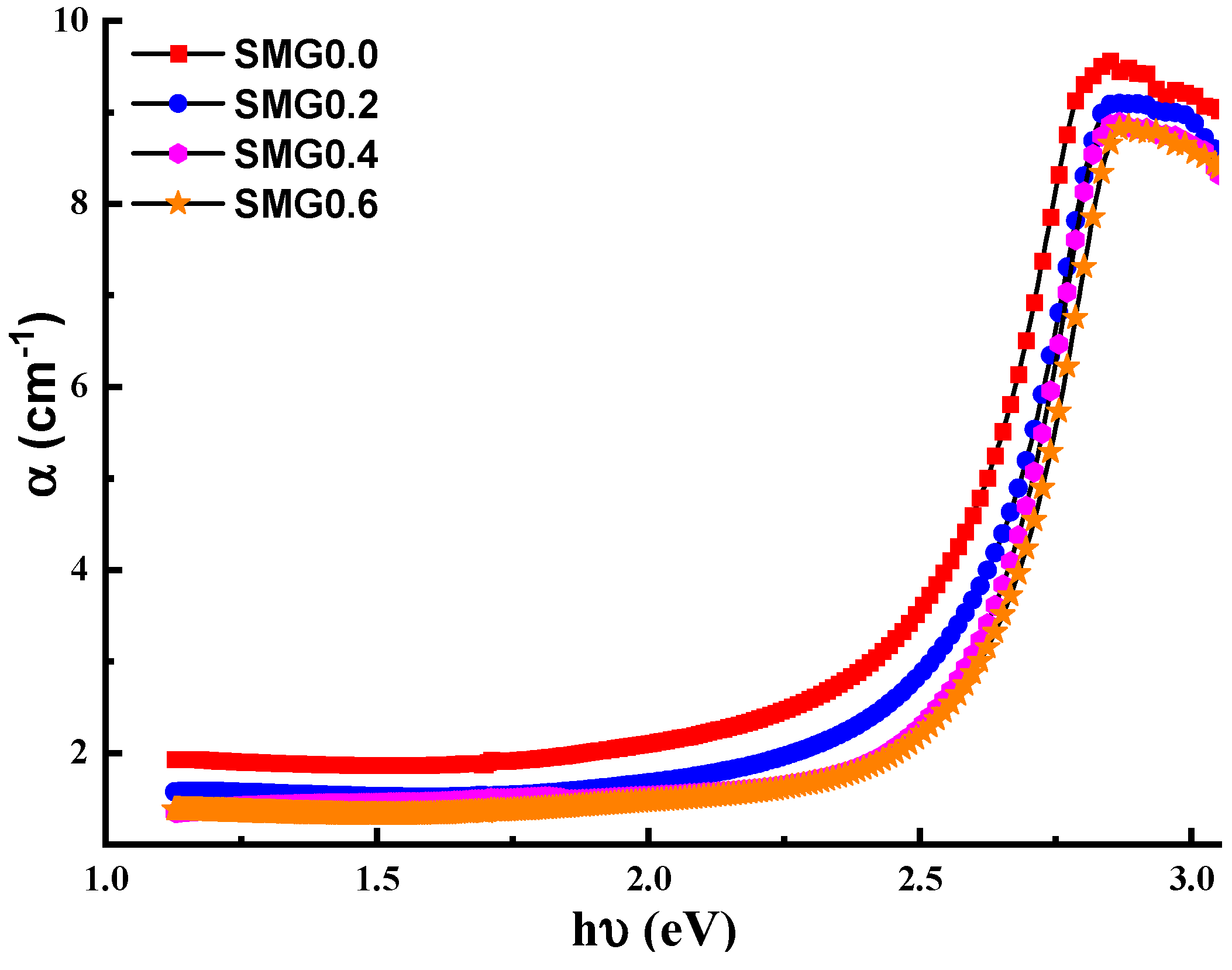
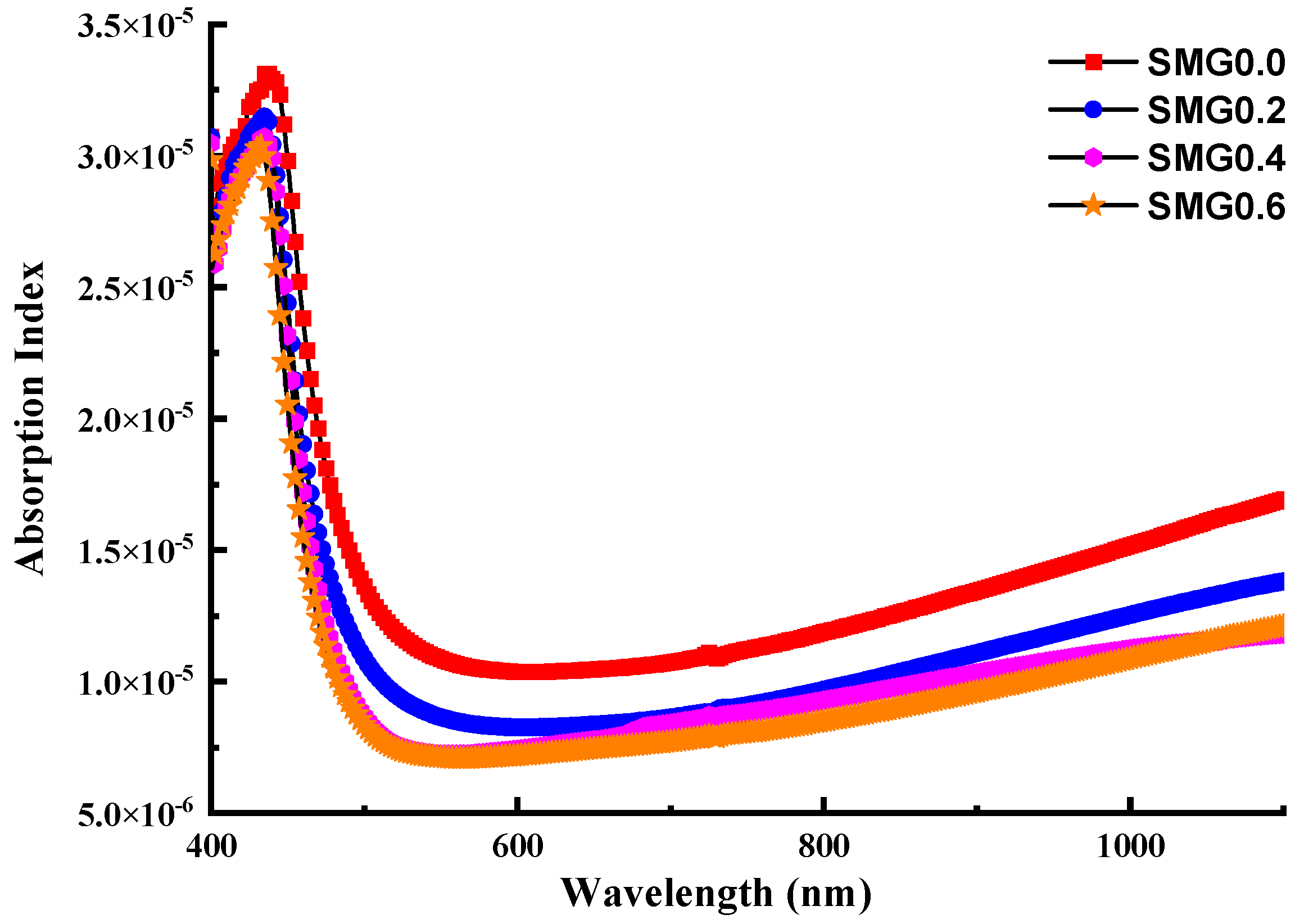
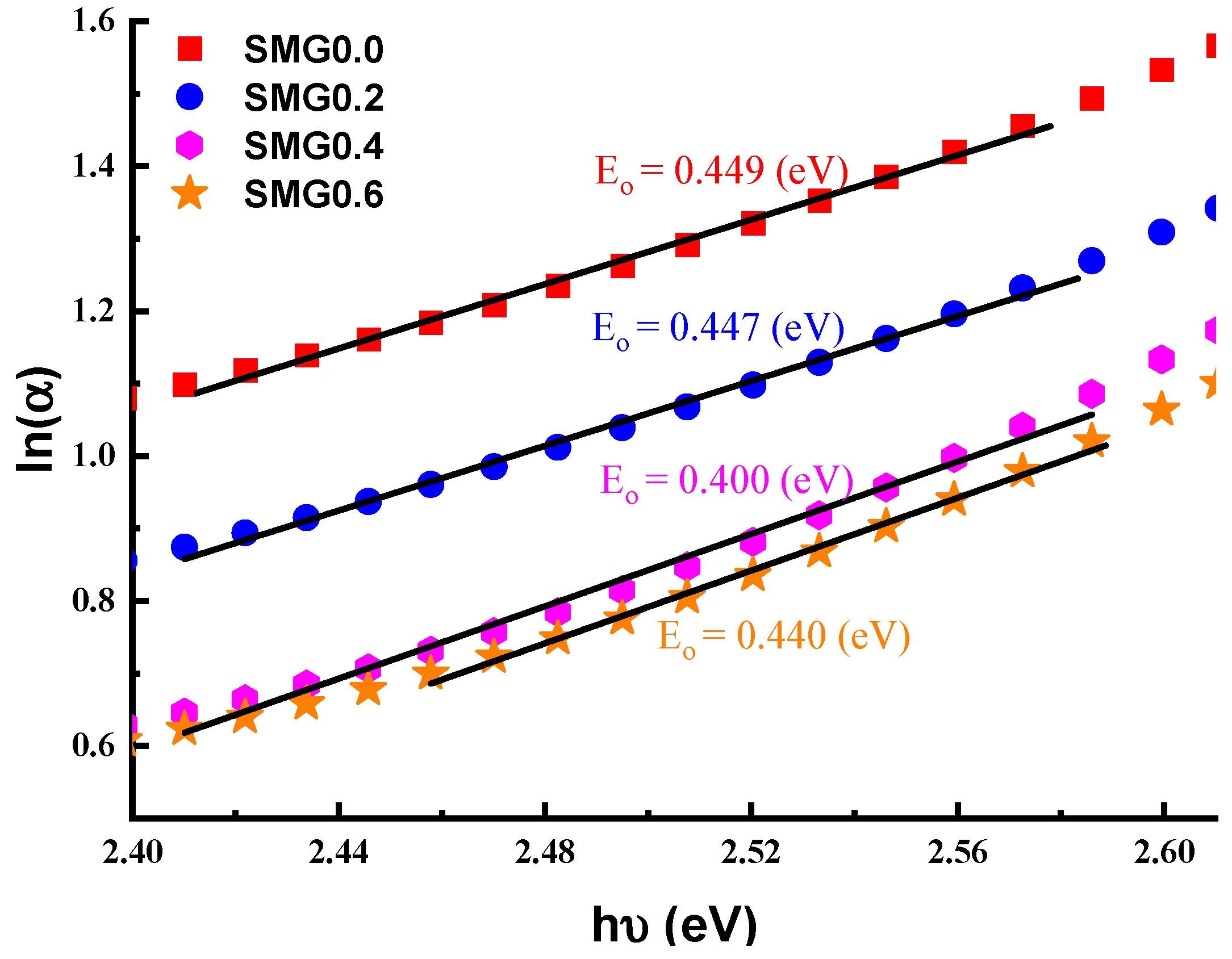
3.2. Investigations on Optical Properties
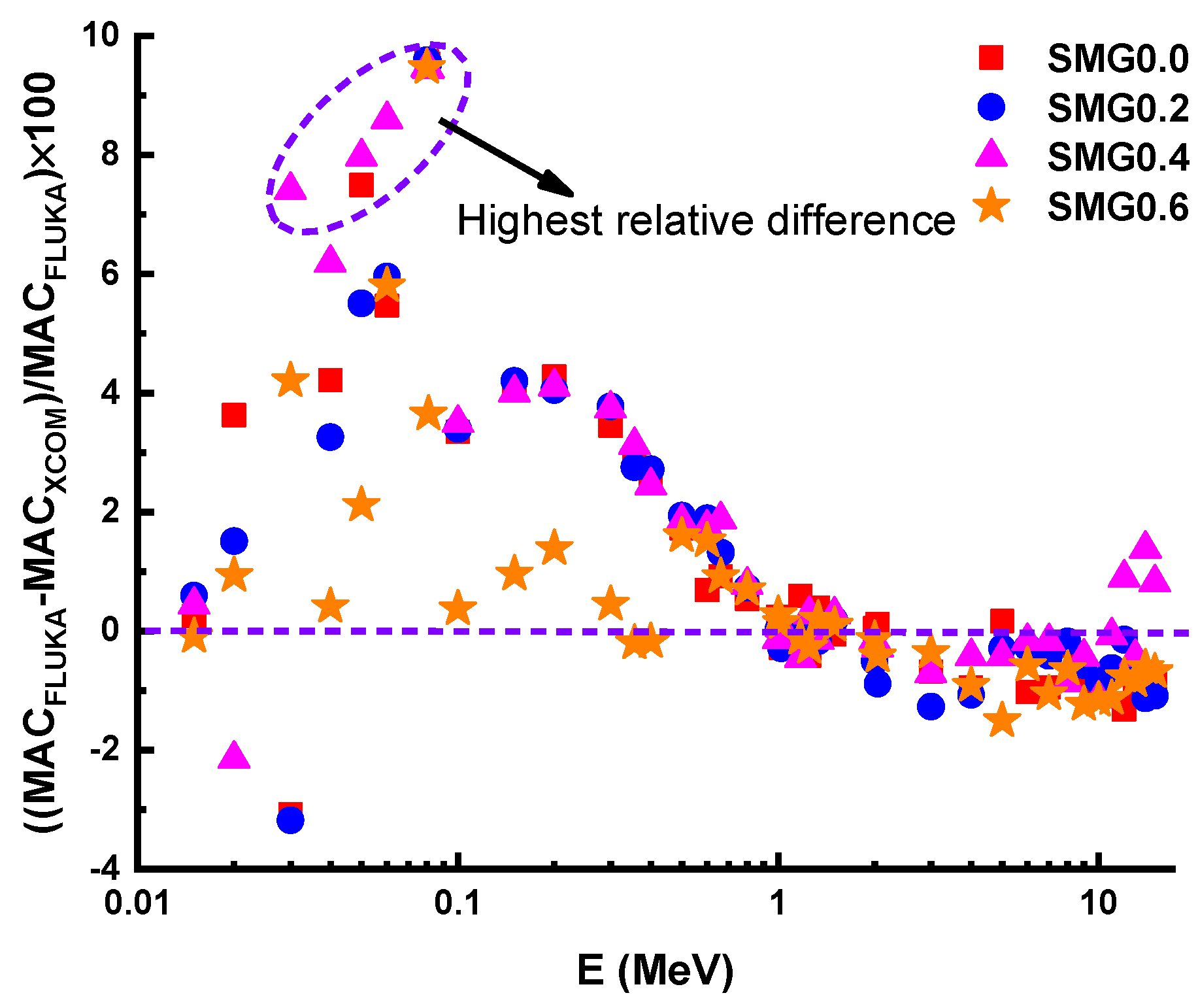
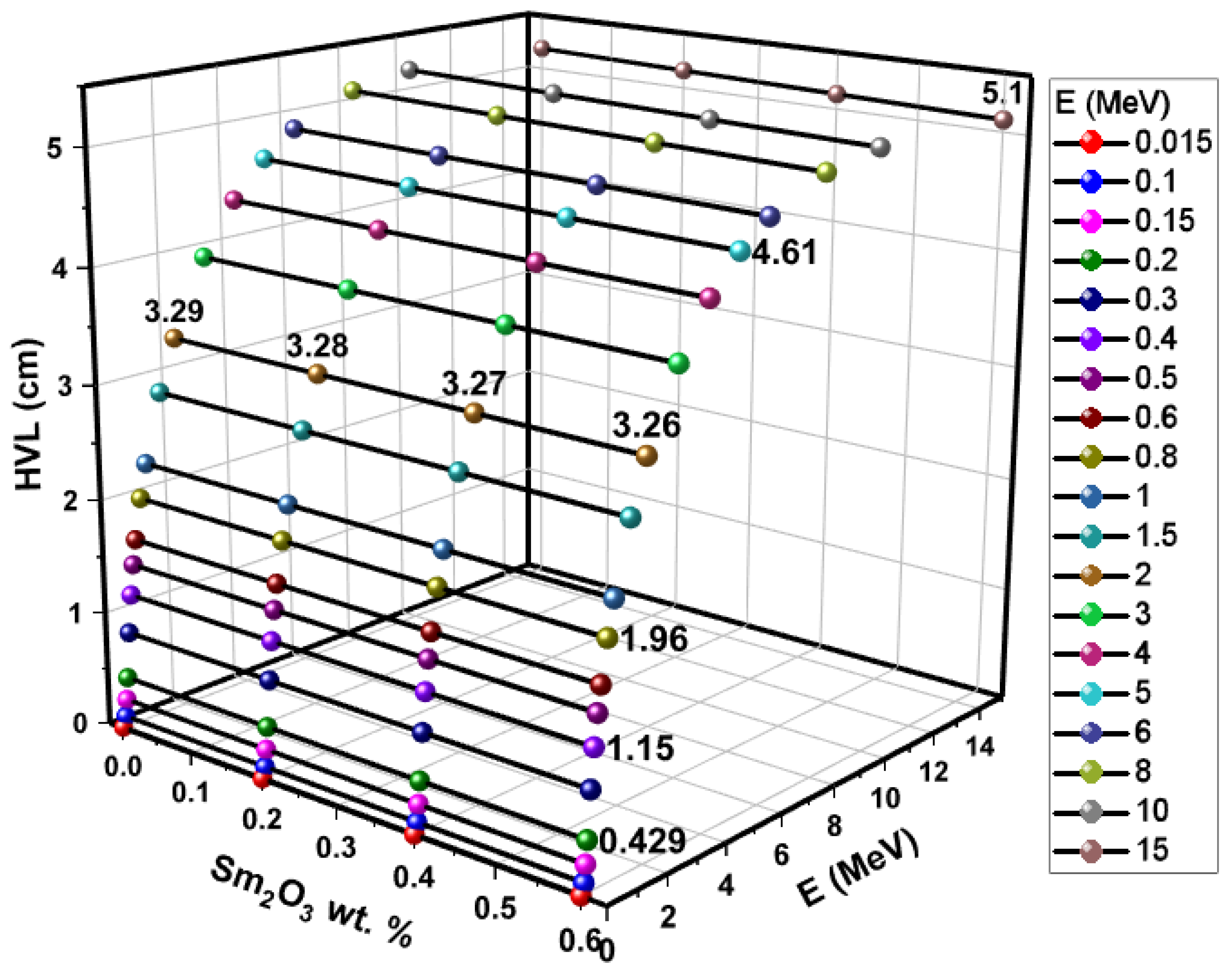
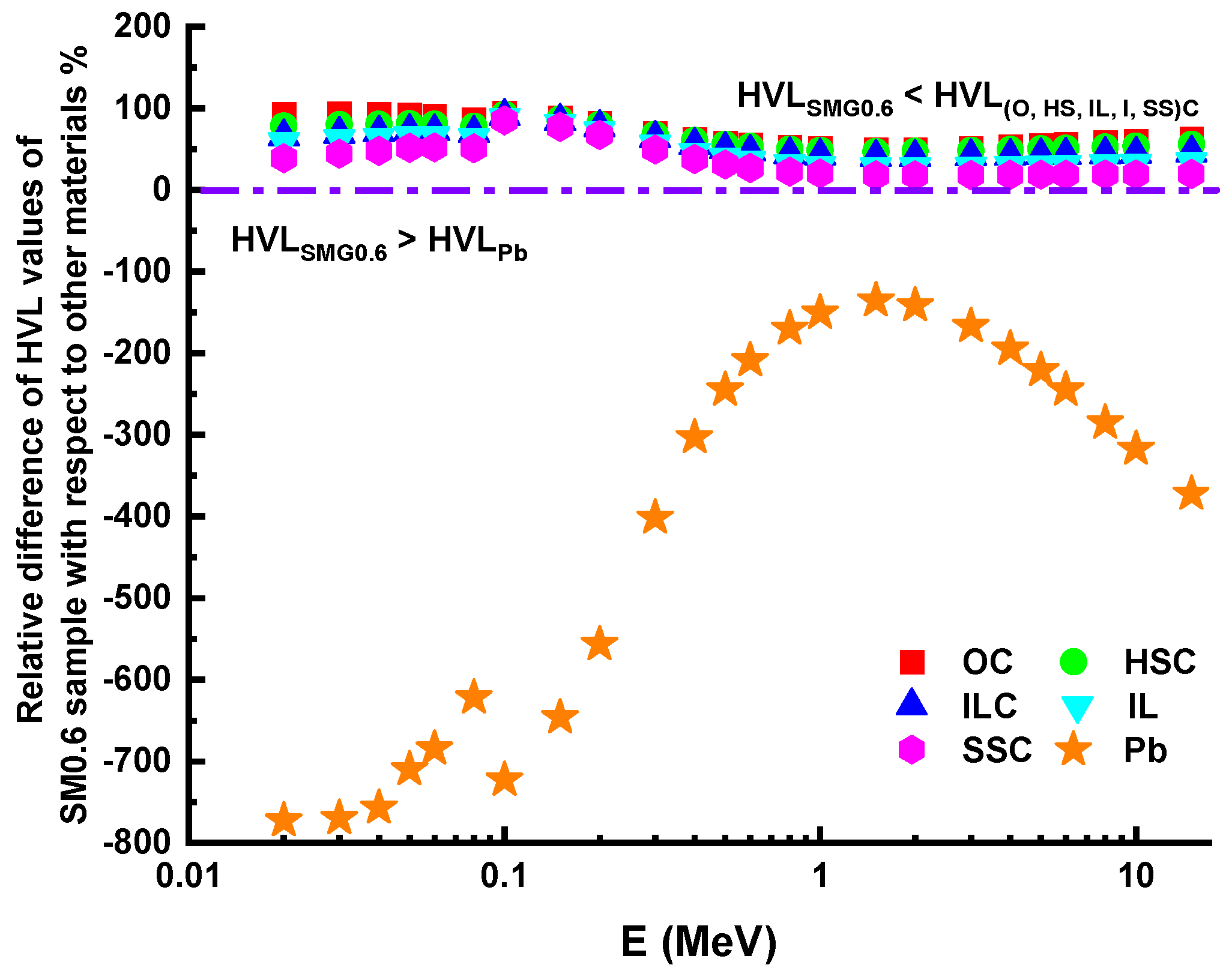
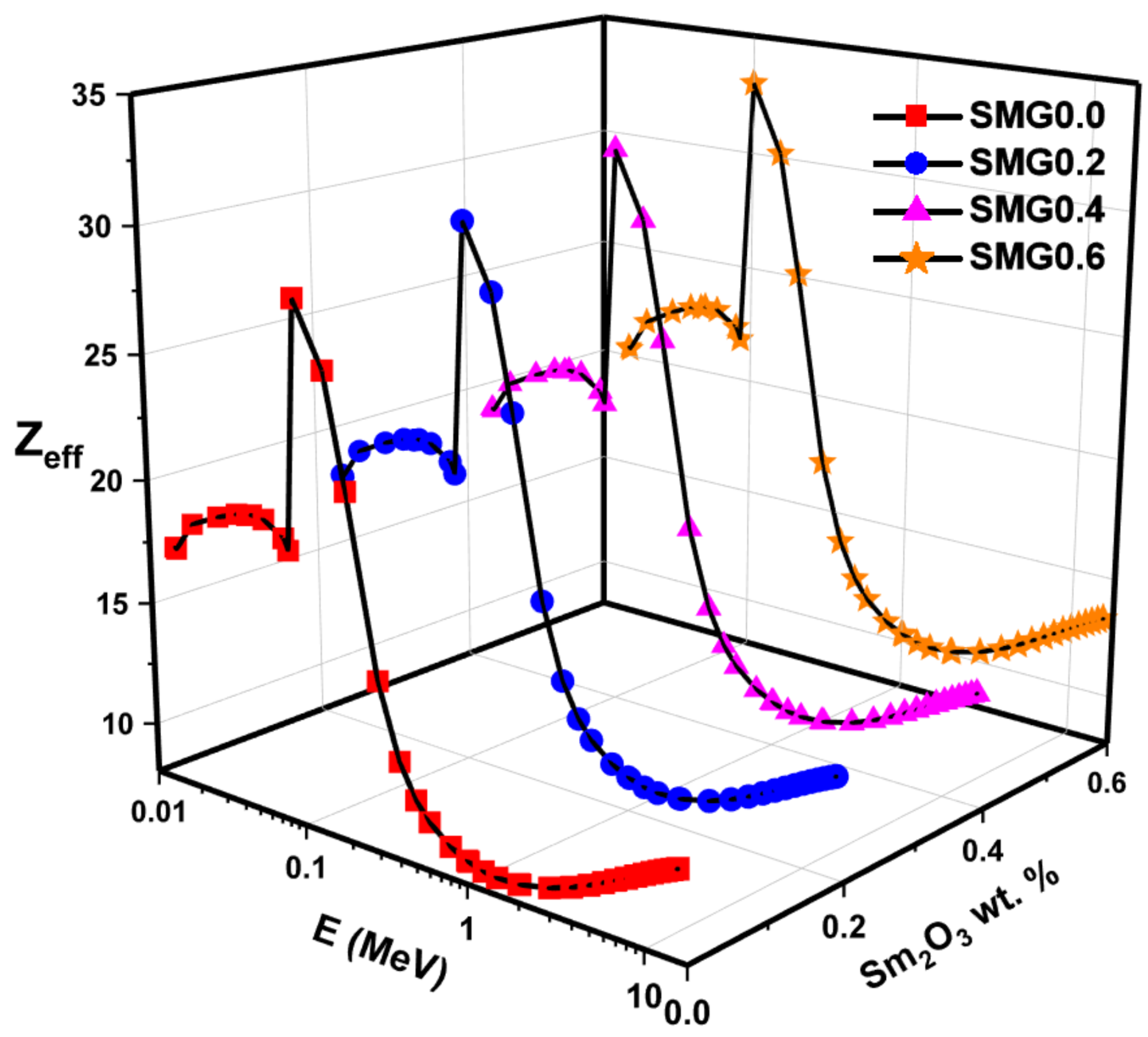
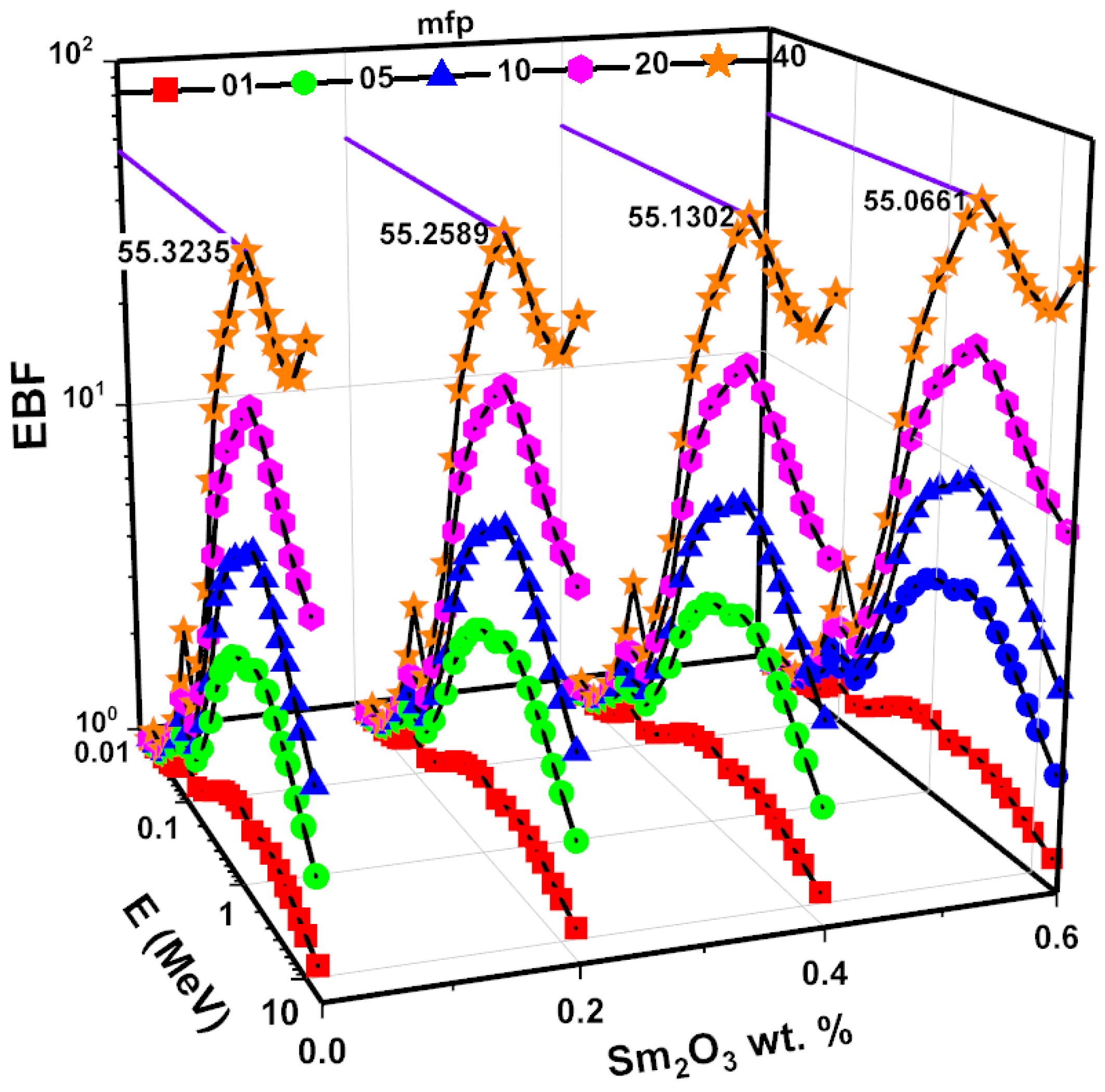
3.3. Investigations on Nuclear Radiation Shielding Competencies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yim, M.-S.; Ocken, H. Radiation dose management in nuclear power plants. Prog. Nucl. Energy 2001, 39, 31–51. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; El-Agawany, F.I.; Sriwunkum, C.; Akyıldırım, H.; Arslan, H.; Tonguc, B.T.; El-Mallawany, R.; Rammah, Y.S. Influence of Bi2O3/PbO on nuclear shielding characteristics of lead-zinc-tellurite glasses. Phys. B Condens. Matter 2020, 581, 411946. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Saudi, H.A.; Issa, S.A.M.; Rashad, M.; Elazaka, A.I.; Tekin, H.O.; Saddeek, Y.B. Alteration of optical, structural, mechanical durability and nuclear radiation attenuation properties of barium borosilicate glasses through BaO reinforcement: Experimental and numerical analyses. Ceram. Int. 2021, 47, 5587–5596. [Google Scholar] [CrossRef]
- Zakaly, H.M.H.; Rashad, M.; Tekin, H.O.; Saudi, H.A.; Issa, S.A.M.; Henaish, A.M.A. Synthesis, optical, structural and physical properties of newly developed dolomite reinforced borate glasses for nuclear radiation shielding utilizations: An experimental and simulation study. Opt. Mater. 2021, 114, 110942. [Google Scholar] [CrossRef]
- Alalawi, A. Experimental and Monte Carlo investigations on the optical properties and nuclear shielding capability of Bi2O3–Na2O-B2O3–Cu2O glasses. J. Non. Cryst. Solids 2020, 548, 120321. [Google Scholar] [CrossRef]
- El-Mallawany, R.; Rammah, Y.S.; El-Agawany, F.I.; Lima, S.M.; Mutuwong, C.; Al-Buriahi, M.S. Evaluation of optical features and ionizing radiation shielding competences of TeO2–Li2O (TL) glasses via Geant4 simulation code and Phy-X/PSD program. Opt. Mater. 2020, 108, 110394. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Mhareb, M.H.A.; Alalawi, A.; Al-Buriahi, M.S. Physical, structural, optical, and radiation shielding properties of B2O3- 20Bi2O3- 20Na2O2- Sb2O3 glasses: Role of Sb2O3. J. Non. Cryst. Solids 2020, 543, 120130. [Google Scholar] [CrossRef]
- Alalawi, A.; Al-Buriahi, M.S.; Rammah, Y.S. Radiation shielding properties of PNCKM bioactive glasses at nuclear medicine energies. Ceram. Int. 2020, 46, 15027–15033. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Kavaz, E. A novel B2O3-Na2O-BaO-HgO glass system: Synthesis, physical, optical and nuclear shielding features. Ceram. Int. 2020, 46, 16166–16177. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, A.; Khanna, A.; Gonzàlez, F.; Pesquera, C.; Iordanova, R.; Chen, B. Structure-property correlations in TiO 2 -Bi2 O3 -B2 O3 -TeO2 glasses. J. Non. Cryst. Solids 2017, 470, 168–177. [Google Scholar] [CrossRef]
- Sadeq, M.S.; Morshidy, H.Y. Effect of samarium oxide on structural, optical and electrical properties of some alumino-borate glasses with constant copper chloride. J. Rare Earths 2020, 38, 770–775. [Google Scholar] [CrossRef]
- Mostafa, A.M.A.; Zakaly, H.M.H.; Pyshkina, M.; Issa, S.A.M.; Tekin, H.O.; Sidek, H.A.A.; Matori, K.A.; Zaid, M.H.M. Multi-objective optimization strategies for radiation shielding performance of BZBB glasses using Bi2O3: A FLUKA Monte Carlo code calculations. J. Mater. Res. Technol. 2020, 9, 12335–12345. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Zakaly, H.M.H.; Pyshkina, M.; Mostafa, M.Y.A.; Rashad, M.; Soliman, T.S. Structure, optical, and radiation shielding properties of PVA–BaTiO3 nanocomposite films: An experimental investigation. Radiat. Phys. Chem. 2021, 180, 109281. [Google Scholar] [CrossRef]
- Ballarini, F.; Battistoni, G.; Brugger, M.; Campanella, M.; Carboni, M.; Cerutti, F.; Empl, A.; Fassò, A.; Ferrari, A.; Gadioli, E.; et al. The physics of the FLUKA code: Recent developments. Adv. Space Res. 2007, 40, 1339–1349. [Google Scholar] [CrossRef]
- Ferrari, A.; Sala, P.R.; Fasso, A.; Ranft, J. FLUKA: A Multi-Particle Transport Code; Stanford Linear Accelerator Center: Menlo Park, CA, USA, 2005. [Google Scholar]
- Henaish, A.M.A.; Mostafa, M.; Salem, B.I.; Zakaly, H.M.H.; Issa, S.A.M.; Weinstein, I.A.; Hemeda, O.M. Spectral, electrical, magnetic and radiation shielding studies of Mg-doped Ni–Cu–Zn nanoferrites. J. Mater. Sci. Mater. Electron. 2020, 31, 20210–20222. [Google Scholar] [CrossRef]
- Tekin, H.O.; Issa, S.A.M.; Kavaz, E.; Altunsoy Guclu, E.E. The direct effect of Er2 O3 on bismuth barium telluro borate glasses for nuclear security applications. Mater. Res. Express 2019, 6, 115212. [Google Scholar] [CrossRef]
- Gong, J.-T.; Zhang, Z.; Yang, X.-Z. Theoretical investigation of the local structure of Cu2+ doped Bi2O3-ZnO-B2O3-Li2O glasses by their EPR and optical spectra. Optik 2014, 125, 1698–1700. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Stefanovich, S.Y. Structural and spectroscopic properties of new noncentrosymmetric self-activated borate Rb3EuB6O12 with B5O10 units. Mater. Des. 2018, 140, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Atuchin, V.V.; Subanakov, A.K.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Dorzhieva, S.G.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; et al. Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Ichoja, A.; Hashim, S.; Ghoshal, S.K. Unique optical traits of Sm3+ -doped magnesium borate glass. Chinese J. Phys. 2020, 66, 36–49. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V.; Sidorenko, M.Y.; Dmitrushkov, M.S. Crystal structure and properties of the precursor [Ni(H2O) 6](HTBA)2·2H2O and the complexes M(HTBA)2(H2O)2 (M = Ni, Co, Fe). Polyhedron 2014, 70, 71–76. [Google Scholar] [CrossRef]
- Golovnev, N.N.; Molokeev, M.S.; Vereshchagin, S.N.; Atuchin, V.V. Calcium and strontium thiobarbiturates with discrete and polymeric structures. J. Coord. Chem. 2013, 66, 4119–4130. [Google Scholar] [CrossRef]
- Kaçal, M.R.; Akman, F.; Sayyed, M.I.; Akman, F. Evaluation of gamma-ray and neutron attenuation properties of some polymers. Nucl. Eng. Technol. 2019, 51, 818–824. [Google Scholar] [CrossRef]
- Poltabtim, W.; Wimolmala, E.; Saenboonruang, K. Properties of lead-free gamma-ray shielding materials from metal oxide/EPDM rubber composites. Radiat. Phys. Chem. 2018, 153, 1–9. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Tekin, H.O.; Altunsoy, E.E.; Obaid, S.S.; Almatari, M. Radiation shielding study of tellurite tungsten glasses with different antimony oxide as transparent shielding materials using MCNPX code. J. Non. Cryst. Solids 2018, 498, 167–172. [Google Scholar] [CrossRef]
- Ali, A.; Singh, B.N.; Yadav, S.; Ershad, M.; Singh, S.K.; Mallick, S.P.; Pyare, R. CuO assisted borate 1393B3 glass scaffold with enhanced mechanical performance and cytocompatibility: An In vitro study. J. Mech. Behav. Biomed. Mater. 2020, 104231. [Google Scholar] [CrossRef]
- Othman, H.; Elkholy, H.; Cicconi, M.R.; Palles, D.; de Ligny, D.; Kamitsos, E.I.; Möncke, D. Spectroscopic study of the role of alkaline earth oxides in mixed borate glasses—site basicity, polarizability and glass structure. J. Non. Cryst. Solids 2020, 533, 119892. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Darwish, A.A.A.; El-Nahass, M.M. The evolution of gamma-rays sensing properties of pure and doped phthalocyanine. Prog. Nucl. Energy 2017, 100, 276–282. [Google Scholar] [CrossRef]
- Bashter, I.I. Calculation of radiation attenuation coefficients for shielding concretes. Ann. Nucl. Energy 1997, 24, 1389–1401. [Google Scholar] [CrossRef]
- El-Taher, A.; Zakaly, H.M.H.; Pyshkina, M.; Allam, E.A.; El-Sharkawy, R.M.; Mahmoud, M.E.; Abdel-Rahman, M.A.E. A comparative Study between Fluka and Microshield Modeling Calculations to study the Radiation-Shielding of Nanoparticles and Plastic Waste composites. Z. Anorg. Allg. Chem. 2021, 647, 1083–1090. [Google Scholar] [CrossRef]
- Issa, S.A.M.; Tekin, H.O. The multiple characterization of gamma, neutron and proton shielding performances of xPbO-(99-x)B2O3–Sm2O3 glass system. Ceram. Int. 2019, 45, 23561–23571. [Google Scholar] [CrossRef]

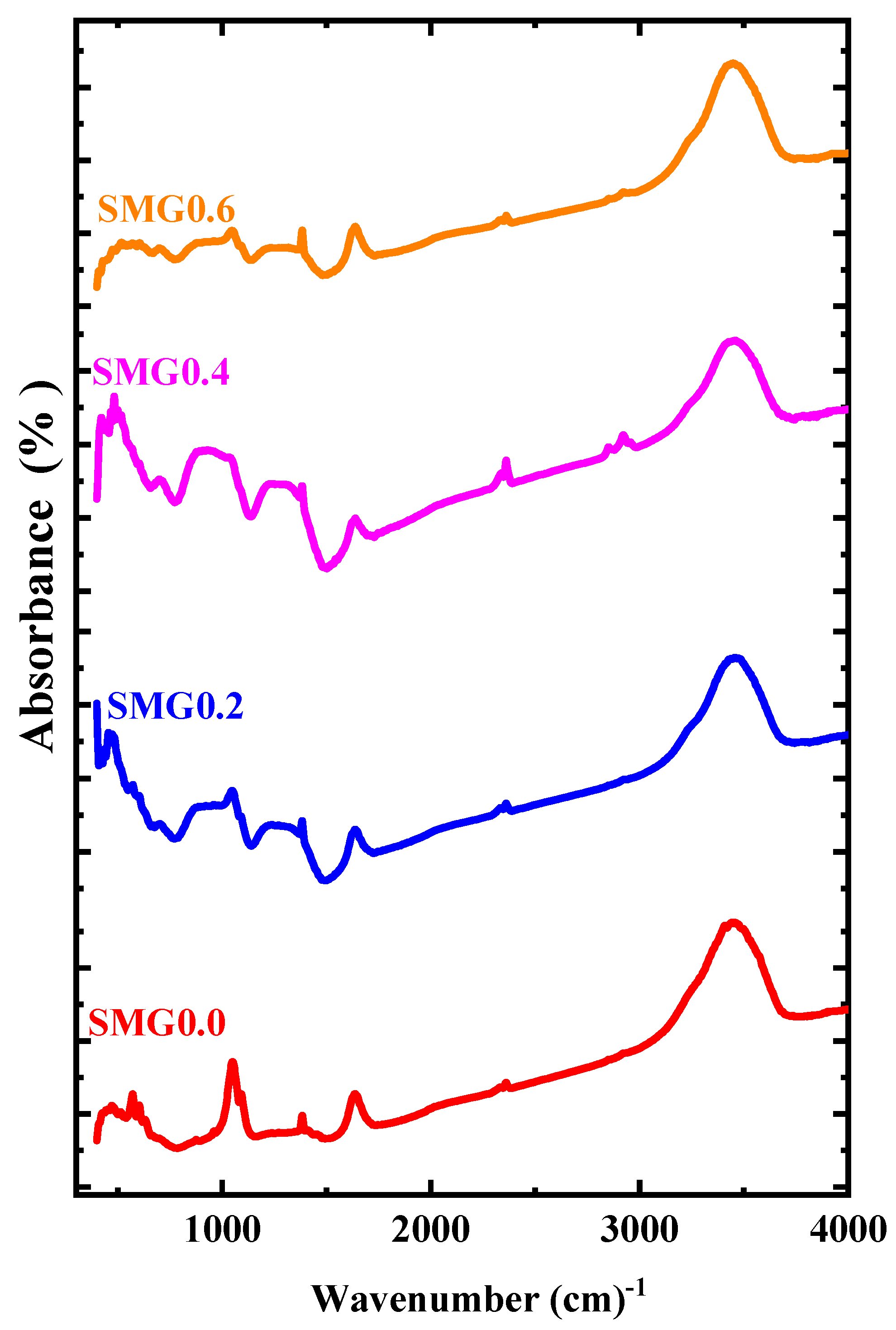



| Code | SMG0.0 | SMG0.2 | SMG0.4 | SMG0.6 | IR Band Assignments | Reference |
|---|---|---|---|---|---|---|
| IR band positions | 1035 | 1049 | 929 | 1049 | (B–O stretching of tetrahedral units) | [24] |
| 1643 | 1643 | 1643 | 1643 | Crystal water with H−O−H bending mode | [25] | |
| 3434 | 3434 | 3434 | 3434 | B–OH | [25] | |
| - | 2355 | 2342 | 2355 | Sm–O | [26] |
| E (MeV) | TVL | MFP | ||||||
|---|---|---|---|---|---|---|---|---|
| SMG0.0 | SMG0.2 | SMG0.4 | SMG0.6 | SMG0.0 | SMG0.2 | SMG0.4 | SMG0.6 | |
| 0.015 | 0.01189 | 0.01181 | 0.01172 | 0.01165 | 0.00516 | 0.00513 | 0.00509 | 0.00506 |
| 0.02 | 0.02148 | 0.02135 | 0.02121 | 0.02108 | 0.00933 | 0.00927 | 0.00921 | 0.00916 |
| 0.03 | 0.06211 | 0.06172 | 0.06133 | 0.06096 | 0.02697 | 0.02680 | 0.02663 | 0.02648 |
| 0.04 | 0.13194 | 0.13111 | 0.13033 | 0.12955 | 0.05730 | 0.05694 | 0.05660 | 0.05626 |
| 0.05 | 0.23451 | 0.23041 | 0.22632 | 0.22251 | 0.10185 | 0.10007 | 0.09829 | 0.09663 |
| 0.06 | 0.37029 | 0.36391 | 0.35745 | 0.35151 | 0.16081 | 0.15805 | 0.15524 | 0.15266 |
| 0.08 | 0.72806 | 0.71634 | 0.70504 | 0.69406 | 0.31619 | 0.31110 | 0.30619 | 0.30143 |
| 0.1 | 0.31915 | 0.31718 | 0.31502 | 0.31316 | 0.13861 | 0.13775 | 0.13681 | 0.13600 |
| 0.15 | 0.80731 | 0.80257 | 0.79774 | 0.79325 | 0.35061 | 0.34855 | 0.34645 | 0.34450 |
| 0.2 | 1.44666 | 1.43895 | 1.43132 | 1.42403 | 0.62828 | 0.62493 | 0.62161 | 0.61845 |
| 0.3 | 2.76378 | 2.75246 | 2.73964 | 2.72748 | 1.20029 | 1.19538 | 1.18981 | 1.18453 |
| 0.4 | 3.85814 | 3.84146 | 3.82491 | 3.80926 | 1.67557 | 1.66833 | 1.66114 | 1.65434 |
| 0.5 | 4.72270 | 4.70144 | 4.68495 | 4.66953 | 2.05104 | 2.04181 | 2.03465 | 2.02795 |
| 0.6 | 5.43153 | 5.41119 | 5.39160 | 5.37264 | 2.35889 | 2.35005 | 2.34154 | 2.33331 |
| 0.8 | 6.58785 | 6.56466 | 6.54164 | 6.52011 | 2.86107 | 2.85100 | 2.84100 | 2.83165 |
| 1 | 7.55066 | 7.52408 | 7.49769 | 7.47420 | 3.27921 | 3.26767 | 3.25621 | 3.24600 |
| 1.5 | 9.48661 | 9.45509 | 9.42380 | 9.39279 | 4.11998 | 4.10629 | 4.09270 | 4.07924 |
| 2 | 10.93258 | 10.89410 | 10.85589 | 10.82017 | 4.74796 | 4.73125 | 4.71465 | 4.69914 |
| 3 | 13.04279 | 12.99334 | 12.94777 | 12.90165 | 5.66441 | 5.64294 | 5.62314 | 5.60312 |
| 4 | 14.48409 | 14.42874 | 14.37379 | 14.31783 | 6.29036 | 6.26632 | 6.24246 | 6.21815 |
| 5 | 15.48752 | 15.42302 | 15.35899 | 15.29857 | 6.72615 | 6.69813 | 6.67033 | 6.64408 |
| 6 | 16.18436 | 16.11104 | 16.04369 | 15.97472 | 7.02878 | 6.99694 | 6.96769 | 6.93773 |
| 8 | 16.98294 | 16.89917 | 16.82201 | 16.74295 | 7.37560 | 7.33922 | 7.30571 | 7.27137 |
| 10 | 17.29608 | 17.21031 | 17.12522 | 17.04430 | 7.51159 | 7.47434 | 7.43739 | 7.40225 |
| 15 | 17.23377 | 17.13608 | 17.04534 | 16.95276 | 7.48453 | 7.44210 | 7.40270 | 7.36249 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, S.A.M.; Zakaly, H.M.H.; Tekin, H.O.; Saudi, H.A.; Badawi, A.; Pyshkina, M.; Susoy, G.; Elazaka, A.I.; Ene, A. Exploring the FTIR, Optical and Nuclear Radiation Shielding Properties of Samarium-Borate Glass: A Characterization through Experimental and Simulation Methods. Nanomaterials 2021, 11, 1713. https://doi.org/10.3390/nano11071713
Issa SAM, Zakaly HMH, Tekin HO, Saudi HA, Badawi A, Pyshkina M, Susoy G, Elazaka AI, Ene A. Exploring the FTIR, Optical and Nuclear Radiation Shielding Properties of Samarium-Borate Glass: A Characterization through Experimental and Simulation Methods. Nanomaterials. 2021; 11(7):1713. https://doi.org/10.3390/nano11071713
Chicago/Turabian StyleIssa, Shams A. M., Hesham M. H. Zakaly, Huseyin O. Tekin, Heba A. Saudi, Ali Badawi, Mariia Pyshkina, Gulfem Susoy, Ahmed I. Elazaka, and Antoaneta Ene. 2021. "Exploring the FTIR, Optical and Nuclear Radiation Shielding Properties of Samarium-Borate Glass: A Characterization through Experimental and Simulation Methods" Nanomaterials 11, no. 7: 1713. https://doi.org/10.3390/nano11071713
APA StyleIssa, S. A. M., Zakaly, H. M. H., Tekin, H. O., Saudi, H. A., Badawi, A., Pyshkina, M., Susoy, G., Elazaka, A. I., & Ene, A. (2021). Exploring the FTIR, Optical and Nuclear Radiation Shielding Properties of Samarium-Borate Glass: A Characterization through Experimental and Simulation Methods. Nanomaterials, 11(7), 1713. https://doi.org/10.3390/nano11071713











