An Overview of Functionalized Graphene Nanomaterials for Advanced Applications
Abstract
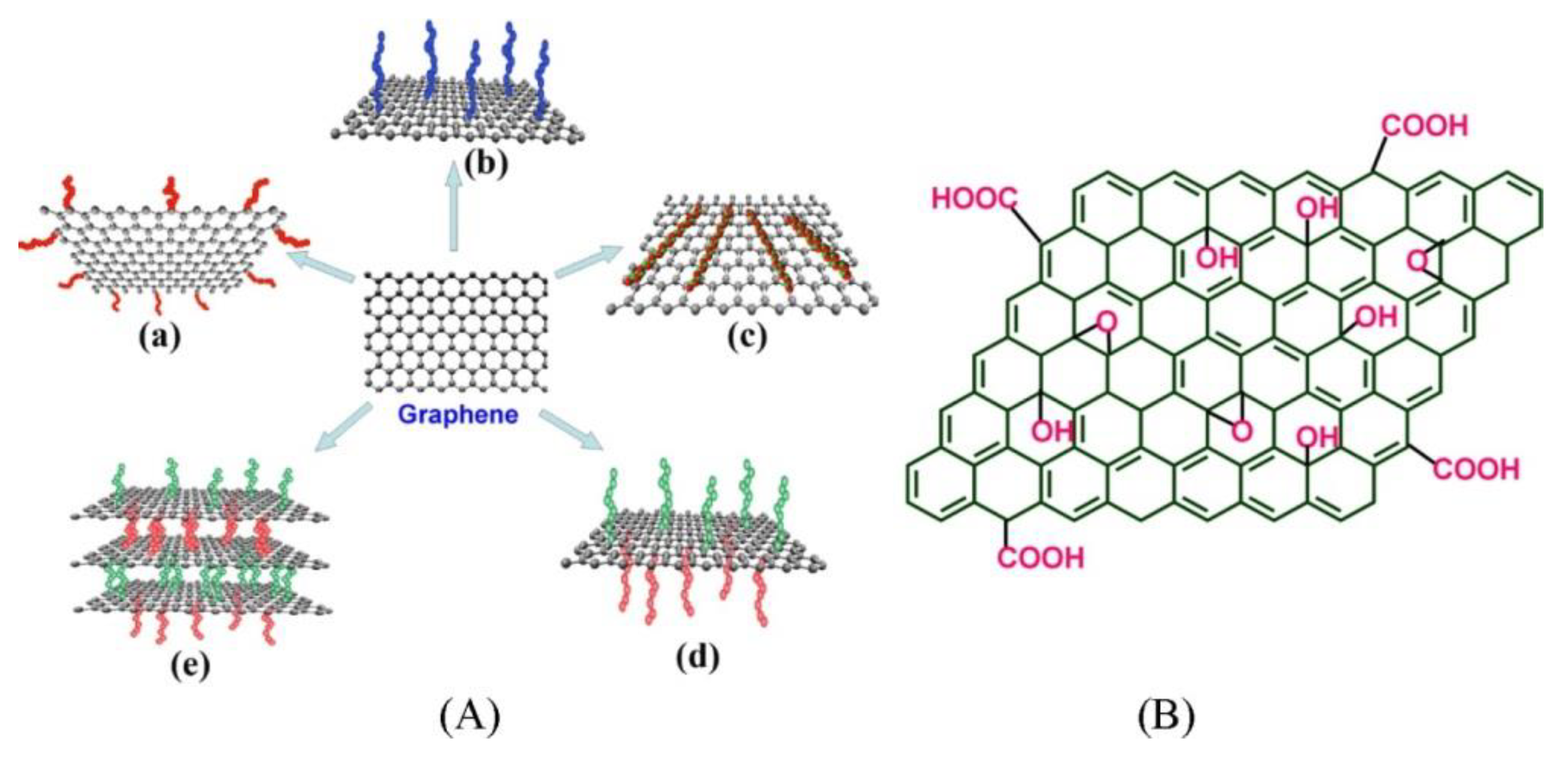
:1. Introduction
2. Materials Engineering
2.1. High Thermomechanical Performance Materials
2.2. Antibiofouling Coatings
2.3. Lubricants
2.4. Flexible Electronics
2.5. Optical Limiters
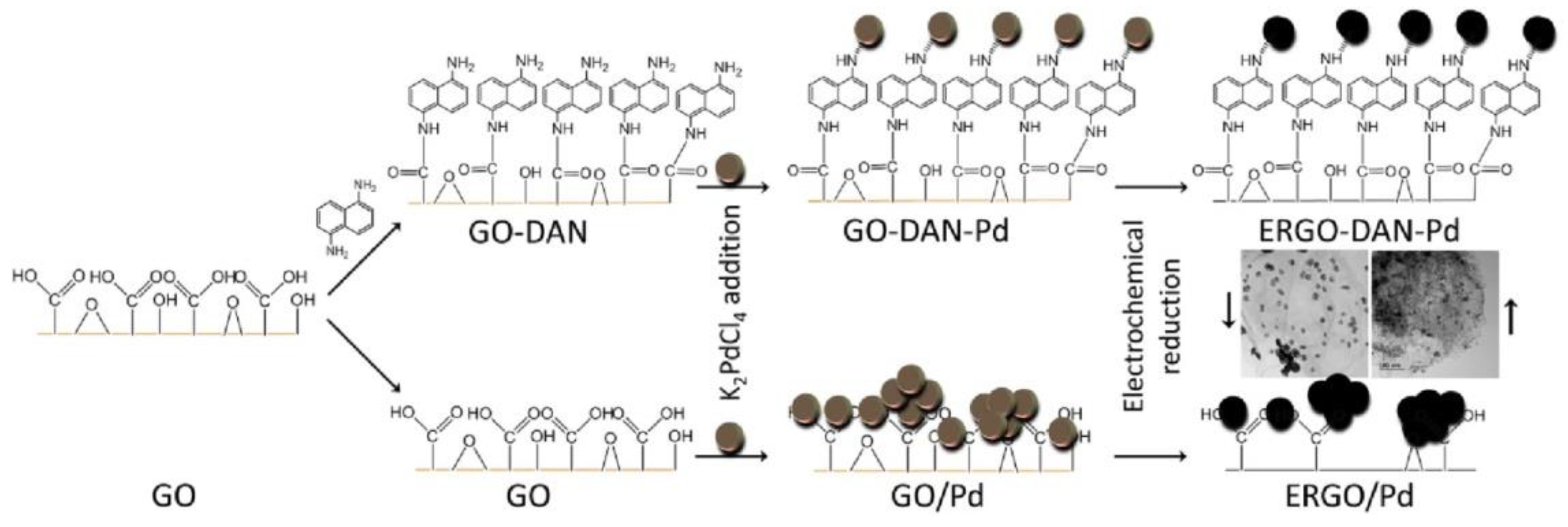
3. Energy Engineering
3.1. Electronics and Optoelectronics
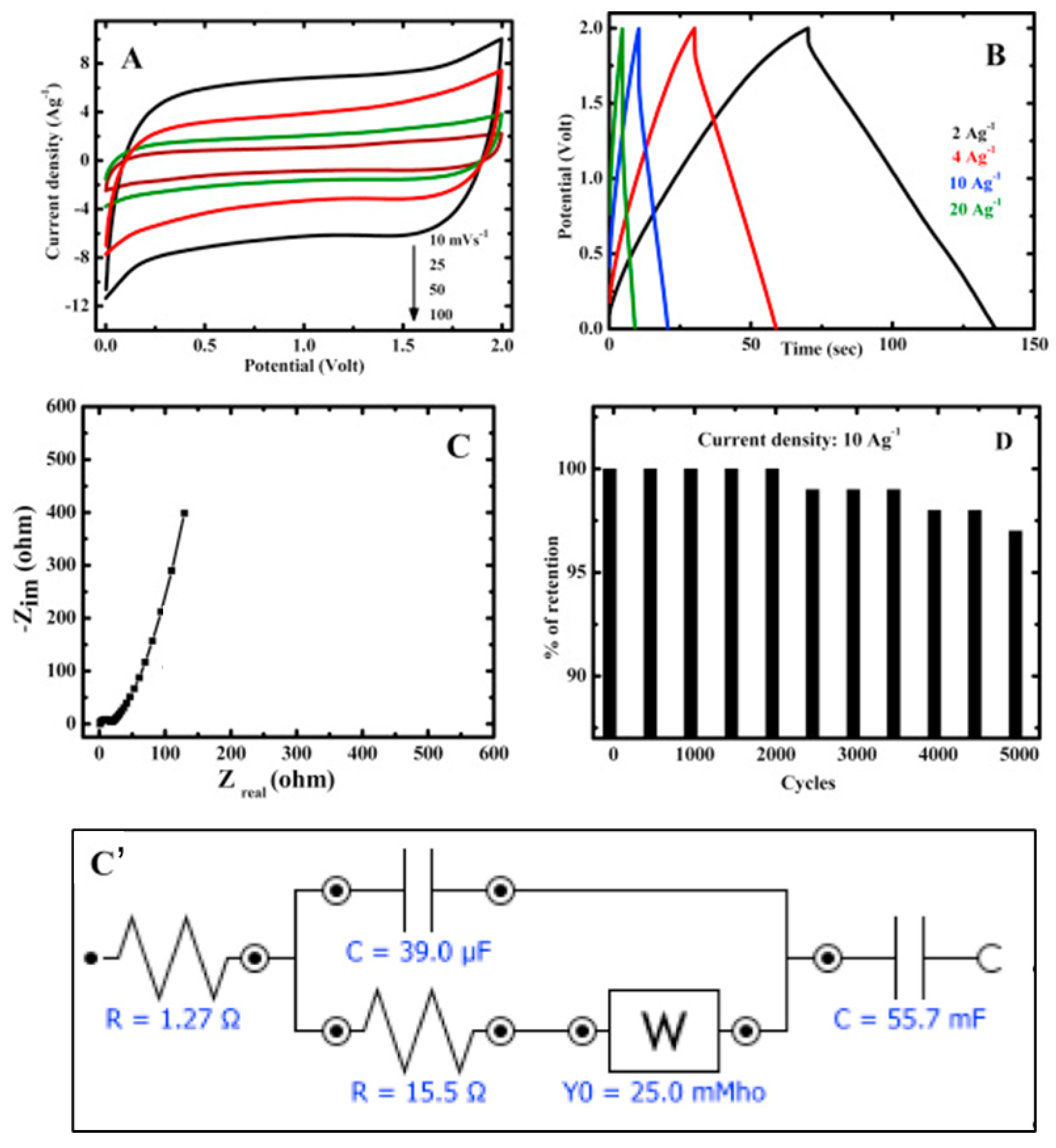
3.2. Electrochemical Supercapacitors
3.3. Fuel Cells
3.4. Solar Cells
3.5. Solar Thermal Fuels
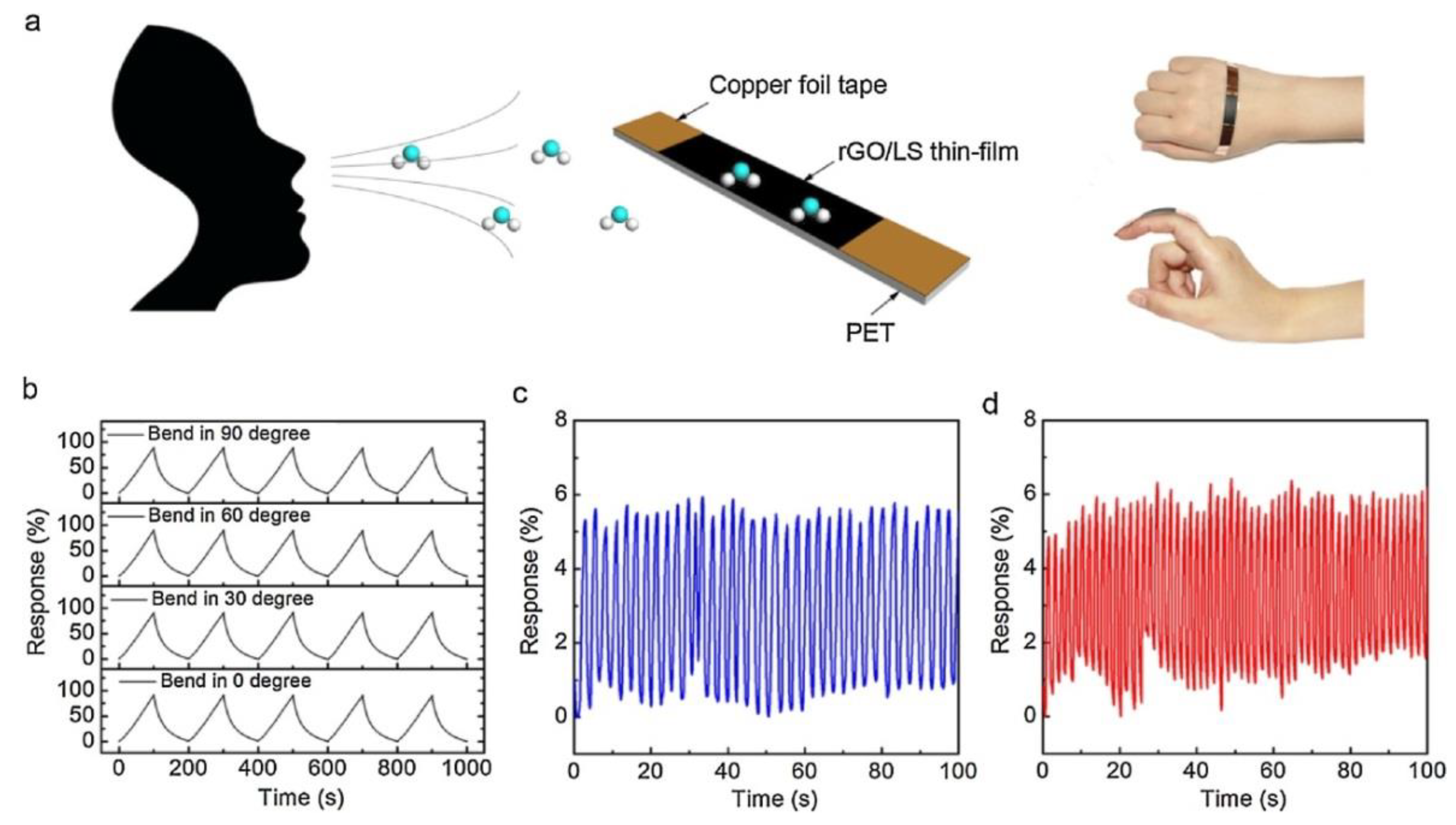
4. Sensors
5. Biomedical Engineering
5.1. Drug Delivery
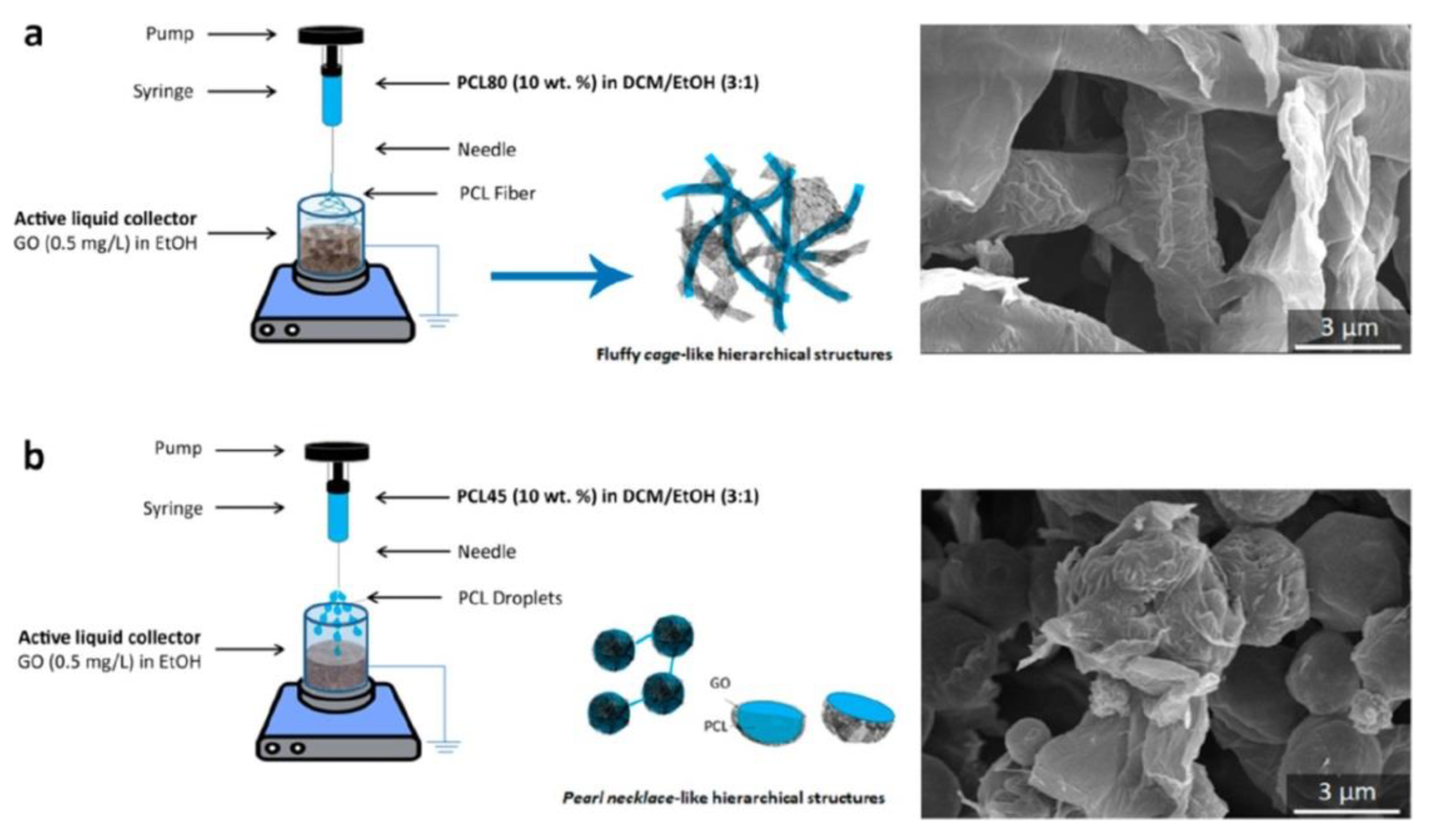
5.2. Tissue Engineering
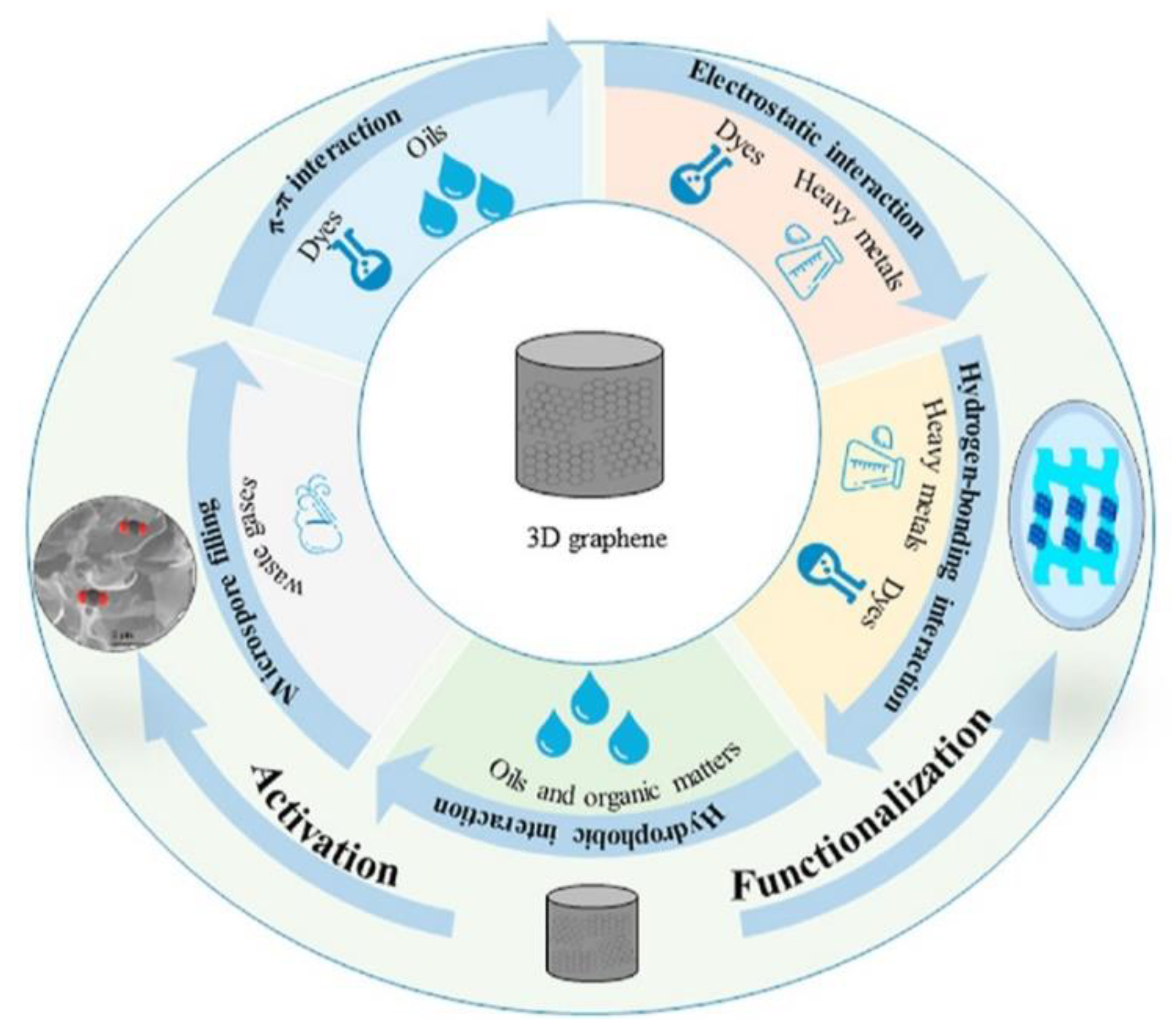
6. Water Treatment
6.1. Dyes Removal
6.2. Metal Ions Removal
6.3. Organic Molecules Removal
6.4. Desalination
7. Catalysis Engineering
7.1. Synthesis for Pharmaceutical Industry
7.2. Green Chemistry
7.3. Biocatalysis
8. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Mu, X.; Sun, M. The Thermal, Electrical and Thermoelectric Properties of Graphene Nanomaterials. Nanomaterials 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Maio, A.; Giallombardo, D.; Scaffaro, R.; Palumbo Piccionello, A.; Pibiri, I. Synthesis of a fluorinated graphene oxide–silica nanohybrid: Improving oxygen affinity. RSC Adv. 2016, 6, 46037–46047. [Google Scholar] [CrossRef]
- Maio, A.; Scaffaro, R.; Lentini, L.; Palumbo Piccionello, A.; Pibiri, I. Perfluorocarbons–graphene oxide nanoplatforms as biocompatible oxygen reservoirs. Chem. Eng. J. 2018, 334, 54–65. [Google Scholar] [CrossRef]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part. A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Botta, L. Nanocarbons in Electrospun Polymeric Nanomats for Tissue Engineering: A Review. Polymers 2017, 9, 76. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Influence of oxidation level of graphene oxide on the mechanical performance and photo-oxidation resistance of a polyamide 6. Polymers 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaffaro, R.; Maio, A.; Lo Re, G.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Y.; Sun, H.; Hagio, T. Progress in modifications of 3D graphene-based adsorbents for environmental applications. Chemosphere 2021, 270, 129420. [Google Scholar] [CrossRef]
- Dai, L. Functionalization of Graphene for Efficient Energy Conversion and Storage. Acc. Chem. Res. 2013, 46, 31–42. [Google Scholar] [CrossRef]
- Dai, L. Graphene: Tunable superdoping. Nat. Energy 2016, 1, 16041. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. A Review of Strategies for the Synthesis of N-Doped Graphene-Like Materials. Nanomaterials 2020, 10, 2286. [Google Scholar] [CrossRef]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A. Integrated ternary bionanocomposites with superior mechanical performance via the synergistic role of graphene and plasma treated carbon nanotubes. Compos. Part. B Eng. 2019, 168, 550–559. [Google Scholar] [CrossRef]
- Zhou, E.; Xi, J.; Guo, Y.; Liu, Y.; Xu, Z.; Peng, L.; Gao, W.; Ying, J.; Chen, Z.; Gao, C. Synergistic effect of graphene and carbon nanotube for high-performance electromagnetic interference shielding films. Carbon N. Y. 2018, 133, 316–322. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Kazi, S.N.; Chew, B.T.; Zubir, M.N.M. Synthesis of ethylene glycol-treated Graphene Nanoplatelets with one-pot, microwave-assisted functionalization for use as a high performance engine coolant. Energy Convers. Manag. 2015, 101, 767–777. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nafezarefi, F.; Tai, N.H.; Schlagenhauf, L.; Nüesch, F.A.; Chu, B.T.T. Size and synergy effects of nanofiller hybrids including graphene nanoplatelets and carbon nanotubes in mechanical properties of epoxy composites. Carbon N. Y. 2012, 50, 5380–5386. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. Investigation of thermal and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2010, 108, 124308. [Google Scholar] [CrossRef]
- Sonvane, Y.; Gupta, S.K.; Raval, P.; Lukačević, I.; Thakor, P.B. Length, width and roughness dependent thermal conductivity of graphene nanoribbons. Chem. Phys. Lett. 2015, 634, 16–19. [Google Scholar] [CrossRef]
- Yu, Z.G.; Zhang, Y.W. Electronic properties of mutually embedded h-BN and graphene: A first principles study. Chem. Phys. Lett. 2016, 666, 33–37. [Google Scholar] [CrossRef]
- Li, W.; Bu, Y.; Jin, H.; Wang, J.; Zhang, W.; Wang, S.; Wang, J. The preparation of hierarchical flowerlike NiO/reduced graphene oxide composites for high performance supercapacitor applications. Energy Fuels 2013, 27, 6304–6310. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.J.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymers 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
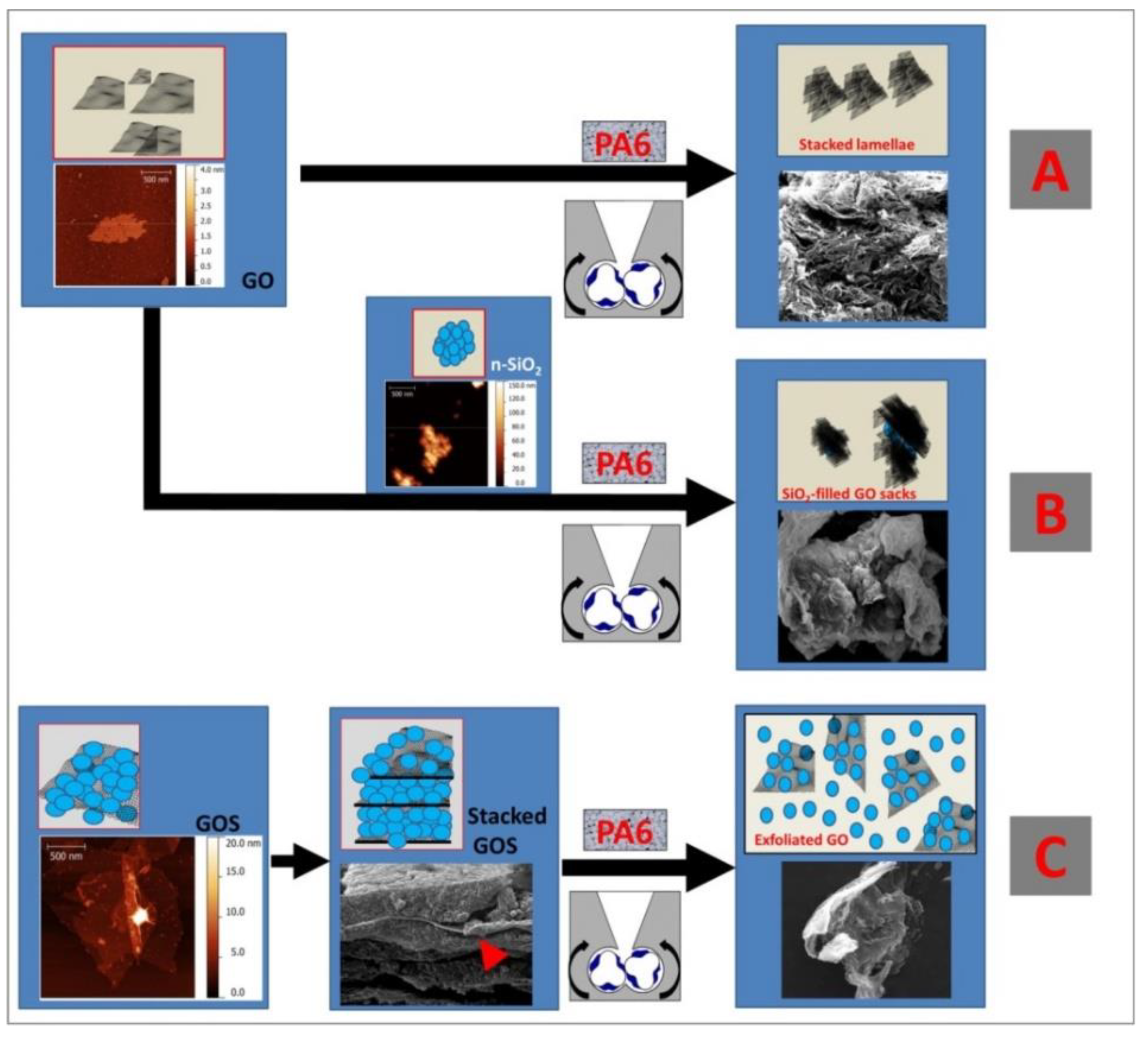
- Scaffaro, R.; Maio, A. Optimization of two-step techniques engineered for the preparation of polyamide 6 graphene oxide nanocomposites. Compos. Part. B Eng. 2019, 165, 55–64. [Google Scholar] [CrossRef]
- Gan, L.; Shang, S.; Yuen, C.W.M.; Jiang, S.-X. Covalently functionalized graphene with d-glucose and its reinforcement to poly(vinyl alcohol) and poly(methyl methacrylate). RSC Adv. 2015, 5, 15954–15961. [Google Scholar] [CrossRef]
- Chhetri, S.; Adak, N.C.; Samanta, P.; Mallisetty, P.K.; Murmu, N.C.; Kuila, T. Interface engineering for the improvement of mechanical and thermal properties of covalent functionalized graphene/epoxy composites. J. Appl. Polym. Sci. 2017, 46124, 46124. [Google Scholar] [CrossRef]
- Qian, Y.; Wu, H.; Yuan, D.; Li, X.; Yu, W.; Wang, C. In situ polymerization of polyimide-based nanocomposites via covalent incorporation of functionalized graphene nanosheets for enhancing mechanical, thermal, and electrical properties. J. Appl. Polym. Sci. 2015, 132, 42724. [Google Scholar] [CrossRef]
- Bian, J.; Wang, G.; Lin, H.L.; Zhou, X.; Wang, Z.J.; Xiao, W.Q.; Zhao, X.W. HDPE composites strengthened–toughened synergistically by l-aspartic acid functionalized graphene/carbon nanotubes hybrid nanomaterials. J. Appl. Polym. Sci. 2017, 134, 45055. [Google Scholar] [CrossRef]
- Gouvêa, R.F.; Del Aguila, E.M.; Paschoalin, V.M.F.; Andrade, C.T. Extruded hybrids based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and reduced graphene oxide composite for active food packaging. Food Packag. Shelf Life 2018, 16, 77–85. [Google Scholar] [CrossRef]
- Illyefalvi-Vitez, Z. Graphene and its potential applications in electronics packaging—A review. In Proceedings of the 36th International Spring Seminar on Electronics Technology, Alba lulia, Romania, 8–12 May 2013; pp. 323–328. [Google Scholar] [CrossRef]
- Gui, D.; Yu, S.; Xiong, W.; Cai, X.; Liu, C.; Liu, J. Liquid crystal functionalization of graphene nanoplatelets for improved thermal and mechanical properties of silicone resin composites. RSC Adv. 2016, 6, 35210–35215. [Google Scholar] [CrossRef]
- Gui, D.; Xiong, W.; Tan, G.; Li, S.; Cai, X.; Liu, J. Improved thermal and mechanical properties of silicone resin composites by liquid crystal functionalized graphene nanoplatelets. J. Mater. Sci. Mater. Electron. 2016, 27, 2120–2127. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, S.; He, H.; Lu, Y.; Shi, J.; Liu, J.; Zhu, H. Ultra-small SiO2 nanoparticles highly dispersed on non-covalent functionalized reduced graphene oxide nanoplatelets for high-performance elastomer applications. Compos. Sci. Technol. 2020, 198, 108297. [Google Scholar] [CrossRef]
- Maio, A.; Agnello, S.; Khatibi, R.; Botta, L.; Alessi, A.; Piazza, A.; Buscarino, G.; Mezzi, A.; Pantaleo, G.; Scaffaro, R. A rapid and eco-friendly route to synthesize graphene-doped silica nanohybrids. J. Alloys Compd. 2016, 664, 428–438. [Google Scholar] [CrossRef]
- Agnello, S.; Alessi, A.; Buscarino, G.; Piazza, A.; Maio, A.; Botta, L.; Scaffaro, R. Structural and thermal stability of graphene oxide-silica nanoparticles nanocomposites. J. Alloys Compd. 2017, 695, 2054–2064. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. A green method to prepare nanosilica modified graphene oxide to inhibit nanoparticles re-aggregation during melt processing. Chem. Eng. J. 2017, 308, 1034–1047. [Google Scholar] [CrossRef] [Green Version]
- Terzopoulou, Z.; Klonos, P.A.; Kyritsis, A.; Tziolas, A.; Avgeropoulos, A.; Papageorgiou, G.Z.; Bikiaris, D.N. Interfacial interactions, crystallization and molecular mobility in nanocomposites of Poly(lactic acid) filled with new hybrid inclusions based on graphene oxide and silica nanoparticles. Polymer 2019, 166, 1–12. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, Y.; He, M.; Xu, R.; Ding, B.; Zhong, X.; Tong, Y.; Fan, L.; Cai, Z.; Shen, H.; et al. Enhanced mechanical properties of silica nanoparticle-covered cross-linking graphene oxide filled thermoplastic polyurethane composite. New J. Chem. 2018, 42, 3069–3077. [Google Scholar] [CrossRef]
- Sadri, R.; Hosseini, M.; Kazi, S.N.; Bagheri, S.; Zubir, N.; Ahmadi, G.; Dahari, M.; Zaharinie, T. A novel, eco-friendly technique for covalent functionalization of graphene nanoplatelets and the potential of their nanofluids for heat transfer applications. Chem. Phys. Lett. 2017, 675, 92–97. [Google Scholar] [CrossRef]
- Maio, A.; Scaffaro, R.; Riccobono, A.; Pibiri, I. Functionalization of Graphene with Molecules and/or Nanoparticles for Advanced Applications. In Handbook of Graphene Set; John Wiley & Sons, Ltd.: Beverly, MA, USA, 2019; pp. 559–609. ISBN 9781119468455. [Google Scholar]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xu, L.; Xu, W.; Zhang, G. Degradable polyurethane for marine anti-biofouling. J. Mater. Chem. B 2013, 1, 3099. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Chen, M.; Huang, R.; Zhou, H. Preparation and application of novel biodegradable polyurethane copolymer. RSC Adv. 2016, 6, 47138–47144. [Google Scholar] [CrossRef]
- Carrión, F.J.; Sanes, J.; Bermúdez, M.D.; Arribas, A. New single-walled carbon nanotubes-ionic liquid lubricant. Application to polycarbonate-stainless steel sliding contact. Tribol. Lett. 2011, 41, 199–207. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, J.; Wang, L.; Xue, Q. Frictional dependence of graphene and carbon nanotube in diamond-like carbon/ionic liquids hybrid films in vacuum. Carbon N. Y. 2014, 80, 734–745. [Google Scholar] [CrossRef]
- Khare, V.; Pham, M.Q.; Kumari, N.; Yoon, H.S.; Kim, C.S.; Park, J.I.; Ahn, S.H. Graphene-ionic liquid based hybrid nanomaterials as novel lubricant for low friction and wear. ACS Appl. Mater. Interfaces 2013, 5, 4063–4075. [Google Scholar] [CrossRef]
- Gusain, R.; Mungse, H.P.; Kumar, N.; Ravindran, T.R.; Pandian, R.; Sugimura, H.; Khatri, O.P. Covalently attached graphene–ionic liquid hybrid nanomaterials: Synthesis, characterization and tribological application. J. Mater. Chem. A 2016, 4, 926–937. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Melvin, A.A.; Joshi, S.; Dash, S.; Tyagi, A.K. Effective Noncovalent Functionalization of Poly(ethylene glycol) to Reduced Graphene Oxide Nanosheets through γ-Radiolysis for Enhanced Lubrication. J. Phys. Chem. C 2016, 120, 2139–2148. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent Functionalization of Fluorinated Graphene and Subsequent Application as Water-based Lubricant Additive. ACS Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar] [CrossRef]
- Salvatore, G.A.; Münzenrieder, N.; Kinkeldei, T.; Petti, L.; Zysset, C.; Strebel, I.; Büthe, L.; Tröster, G. Wafer-scale design of lightweight and transparent electronics that wraps around hairs. Nat. Commun. 2014, 5, 2982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, S.; Zhu, Y. Nanomaterial-enabled stretchable conductors: Strategies, materials and devices. Adv. Mater. 2015, 27, 1480–1511. [Google Scholar] [CrossRef]
- Zheng, Q.; Lee, J.; Shen, X.; Chen, X.; Kim, J.-K. Graphene-based wearable piezoresistive physical sensors. Mater. Today 2020, 36, 158–179. [Google Scholar] [CrossRef]
- Manna, R.; Srivastava, S.K. Fabrication of functionalized graphene filled carboxylated nitrile rubber nanocomposites as flexible dielectric materials. Mater. Chem. Front. 2017, 1, 780–788. [Google Scholar] [CrossRef]
- Wu, S.; Li, J.; Zhang, G.; Yao, Y.; Li, G.; Sun, R.; Wong, C. Ultrafast Self-Healing Nanocomposites via Infrared Laser and Their Application in Flexible Electronics. ACS Appl. Mater. Interfaces 2017, 9, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. A covalently cross-linked reduced functionalized graphene oxide/polyurethane composite based on Diels–Alder chemistry and its potential application in healable flexible electronics. J. Mater. Chem. C 2017, 5, 220–228. [Google Scholar] [CrossRef]
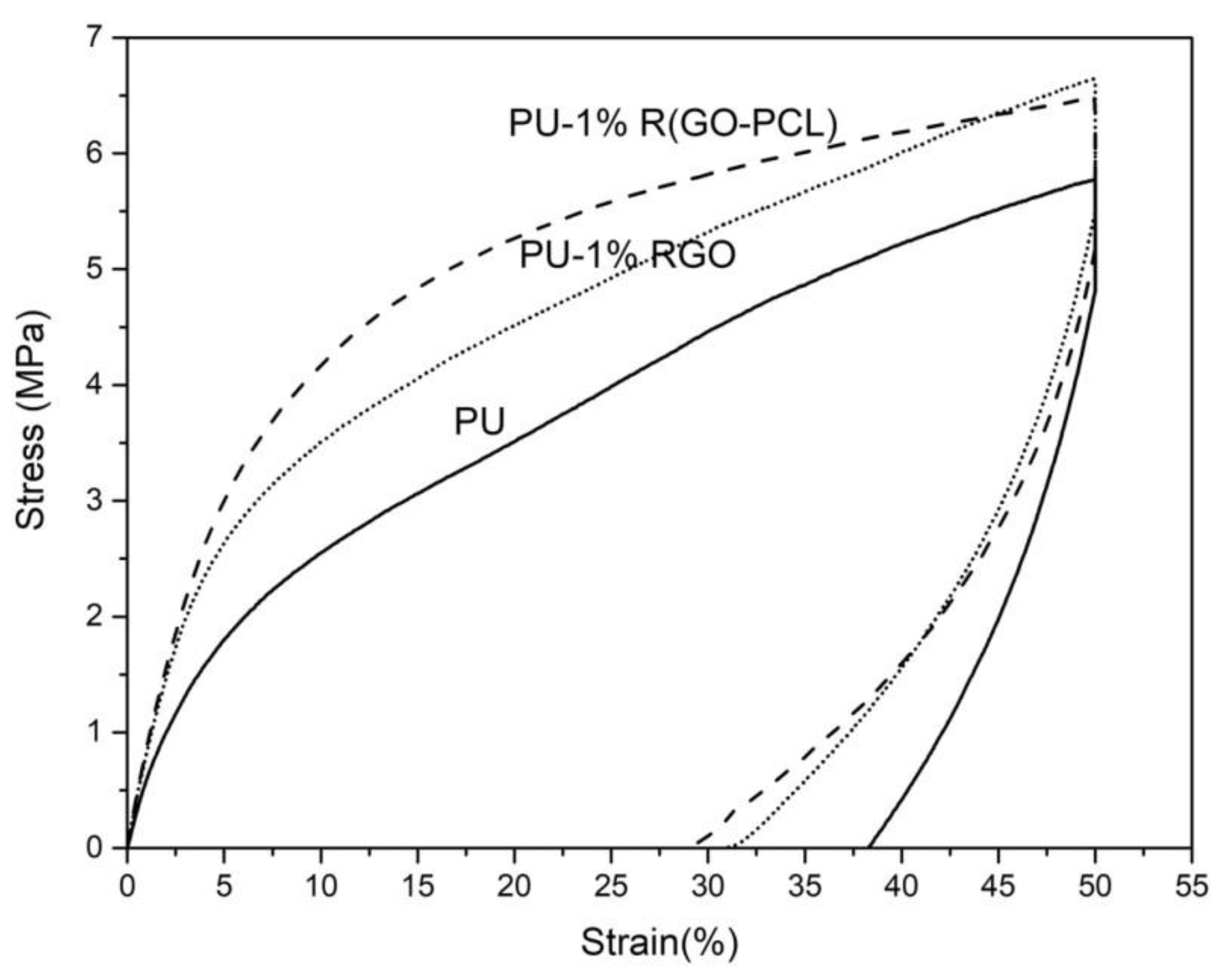
- Lotfi Mayan Sofla, R.; Rezaei, M.; Babaie, A. Investigation of the effect of graphene oxide functionalization on the physical, mechanical and shape memory properties of polyurethane/reduced graphene oxide nanocomposites. Diam. Relat. Mater. 2019, 95, 195–205. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, L.; Zou, Y.; Song, L.; Dong, N.; Wang, J. Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites. Nanomaterials 2019, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Li, W.; Xiao, X.; Ye, X.; Chen, W. Synthesis and optical limiting properties of graphene oxide/bimetallic nanoparticles. Optik 2016, 127, 1792–1796. [Google Scholar] [CrossRef]
- Xu, X.; Li, P.; Zhang, L.; Liu, X.; Zhang, H.L.; Shi, Q.; He, B.; Zhang, W.; Qu, Z.; Liu, P. Covalent Functionalization of Graphene by Nucleophilic Addition Reaction: Synthesis and Optical-Limiting Properties. Chem. Asian J. 2017, 12, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Nancy, P.; Jose, J.; Joy, N.; Valluvadasan, S.; Philip, R.; Antoine, R.; Thomas, S.; Kalarikkal, N. Fabrication of Silver-Decorated Graphene Oxide Nanohybrids via Pulsed Laser Ablation with Excellent Antimicrobial and Optical Limiting Performance. Nanomaterials 2021, 11, 880. [Google Scholar] [CrossRef]
- Petris, A.; Vasiliu, I.C.; Gheorghe, P.; Iordache, A.M.; Ionel, L.; Rusen, L.; Iordache, S.; Elisa, M.; Trusca, R.; Ulieru, D.; et al. Graphene Oxide-Based Silico-Phosphate Composite Films for Optical Limiting of Ultrashort Near-Infrared Laser Pulses. Nanomaterials 2020, 10, 1638. [Google Scholar] [CrossRef]
- Stathis, A.; Stavrou, M.; Papadakis, I.; Obratzov, I.; Couris, S. Enhancing and Tuning the Nonlinear Optical Response and Wavelength-Agile Strong Optical Limiting Action of N-octylamine Modified Fluorographenes. Nanomaterials 2020, 10, 2319. [Google Scholar] [CrossRef]
- Oluwafemi, O.S.; Anyik, J.L.; Zikalala, N.E.; Sakho, E.H.M. Biosynthesis of silver nanoparticles from water hyacinth plant leaves extract for colourimetric sensing of heavy metals. Nano Struct. Nano Objects 2019, 20, 100387. [Google Scholar] [CrossRef]
- Perry, A.; Green, S.J.; Horsell, D.W.; Hornett, S.M.; Wood, M.E. A pyrene-appended spiropyran for selective photo-switchable binding of Zn(II): UV–visible and fluorescence spectroscopy studies of binding and non-covalent attachment to graphene, graphene oxide and carbon nanotubes. Tetrahedron 2015, 71, 6776–6783. [Google Scholar] [CrossRef] [Green Version]
- Vinayan, B.P.; Schwarzburger, N.I.; Fichtner, M. Synthesis of a nitrogen rich (2D-1D) hybrid carbon nanomaterial using a MnO2 nanorod template for high performance Li-ion battery applications. J. Mater. Chem. A 2015, 3, 6810–6818. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Wan, C.; Zhang, Y. Non-covalent functionalization of graphene oxide by pyrene-block copolymers for enhancing physical properties of poly(methyl methacrylate). RSC Adv. 2015, 5, 79947–79955. [Google Scholar] [CrossRef]
- Maity, N.; Mandal, A.; Nandi, A.K. Synergistic interfacial effect of polymer stabilized graphene via non-covalent functionalization in poly(vinylidene fluoride) matrix yielding superior mechanical and electronic properties. Polymers 2016, 88, 79–93. [Google Scholar] [CrossRef]
- Li, P.; Qu, Z.; Chen, X.; Huo, X.; Zheng, X.; Wang, D.; Yang, W.; Ji, L.; Liu, P.; Xu, X. Soluble graphene composite with aggregation-induced emission feature: Non-covalent functionalization and application in explosive detection. J. Mater. Chem. C 2017, 5, 6216–6223. [Google Scholar] [CrossRef]
- Kaur, P.; Shin, M.-S.; Sharma, N.; Kaur, N.; Joshi, A.; Chae, S.-R.; Park, J.-S.; Kang, M.-S.; Sekhon, S.S. Non-covalent functionalization of graphene with poly(diallyl dimethylammonium) chloride: Effect of a non-ionic surfactant. Int. J. Hydrogen Energy 2015, 40, 1541–1547. [Google Scholar] [CrossRef]
- Wang, H.; Bi, S.-G.; Ye, Y.-S.; Xue, Y.; Xie, X.-L.; Mai, Y.-W. An effective non-covalent grafting approach to functionalize individually dispersed reduced graphene oxide sheets with high grafting density, solubility and electrical conductivity. Nanoscale 2015, 7, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.Y.; Yeom, Y.S.; Seo, H.Y.; Kumar, P.; Lee, A.S.; Baek, K.-Y.; Yoon, H.G. Ionic block copolymer doped reduced graphene oxide supports with ultra-fine Pd nanoparticles: Strategic realization of ultra-accelerated nanocatalysis. J. Mater. Chem. A 2015, 3, 20471–20476. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, J.; Bao, F.; Jiang, S.; Zhang, X.; Ma, R. Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction. RSC Adv. 2015, 5, 42754–42761. [Google Scholar] [CrossRef]
- Ji, S.; Kim, S.J.; Song, W.; Myung, S.; Heo, J.; Lim, J.; An, K.-S.; Lee, S.S. Work function-tunable transparent electrodes based on all graphene-based materials for organic–graphene photodetectors. RSC Adv. 2016, 6, 19372–19376. [Google Scholar] [CrossRef]
- Ji, S.; Min, B.K.; Kim, S.K.; Myung, S.; Kang, M.; Shin, H.S.; Song, W.; Heo, J.; Lim, J.; An, K.S.; et al. Work function engineering of graphene oxide via covalent functionalization for organic field-effect transistors. Appl. Surf. Sci. 2017, 419, 252–258. [Google Scholar] [CrossRef]
- Ren, X.; Hu, Z.; Hu, H.; Qiang, R.; Li, L.; Li, Z.; Yang, Y.; Zhang, Z.; Wu, H. Noncovalently-functionalized reduced graphene oxide sheets by water-soluble methyl green for supercapacitor application. Mater. Res. Bull. 2015, 70, 215–221. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Khanra, P.; Samanta, P.; Koo, H.; Murmu, N.C.; Kuila, T. Non-covalent functionalization of reduced graphene oxide using sulfanilic acid azocromotrop and its application as a supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 7323–7331. [Google Scholar] [CrossRef]
- Khanra, P.; Uddin, M.E.; Kim, N.H.; Kuila, T.; Lee, S.H.; Lee, J.H. Electrochemical performance of reduced graphene oxide surface-modified with 9-anthracene carboxylic acid. RSC Adv. 2015, 5, 6443–6451. [Google Scholar] [CrossRef]
- Hu, H.; Hu, Z.; Ren, X.; Yang, Y.; Qiang, R.; An, N.; Wu, H. Non-covalent functionalization of graphene with bisphenol a for high-performance supercapacitors. Chin. J. Chem. 2015, 33, 199–206. [Google Scholar] [CrossRef]
- An, N.; Zhang, F.; Hu, Z.; Li, Z.; Li, L.; Yang, Y.; Guo, B.; Lei, Z. Non-covalently functionalizing a graphene framework by anthraquinone for high-rate electrochemical energy storage. RSC Adv. 2015, 5, 23942–23951. [Google Scholar] [CrossRef]
- Mohammadi, A.; Peighambardoust, S.J.; Entezami, A.A.; Arsalani, N. High performance of covalently grafted poly(o-methoxyaniline) nanocomposite in the presence of amine-functionalized graphene oxide sheets (POMA/f-GO) for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 5776–5787. [Google Scholar] [CrossRef]
- Zhu, J.; Zhuang, X.; Yang, J.; Feng, X.; Hirano, S. Graphene-coupled nitrogen-enriched porous carbon nanosheets for energy storage. J. Mater. Chem. A 2017, 5, 16732–16739. [Google Scholar] [CrossRef]
- Trigueiro, J.P.C.; Lavall, R.L.; Silva, G.G. Supercapacitors based on modified graphene electrodes with poly(ionic liquid). J. Power Sources 2014, 256, 264–273. [Google Scholar] [CrossRef]
- Mao, L.; Li, Y.; Chi, C.; On Chan, H.S.; Wu, J. Conjugated polyfluorene imidazolium ionic liquids intercalated reduced graphene oxide for high performance supercapacitor electrodes. Nano Energy 2014, 6, 119–128. [Google Scholar] [CrossRef]
- Wan, Y.J.; Yang, W.H.; Yu, S.H.; Sun, R.; Wong, C.P.; Liao, W.H. Covalent polymer functionalization of graphene for improved dielectric properties and thermal stability of epoxy composites. Compos. Sci. Technol. 2016, 122, 27–35. [Google Scholar] [CrossRef]
- Bag, S.; Samanta, A.; Bhunia, P.; Raj, C.R. Rational functionalization of reduced graphene oxide with imidazolium-based ionic liquid for supercapacitor application. Int. J. Hydrogen Energy 2016, 41, 22134–22143. [Google Scholar] [CrossRef]
- Yuan, K.; Xu, Y.; Uihlein, J.; Brunklaus, G.; Shi, L.; Heiderhoff, R.; Que, M.; Forster, M.; Chassé, T.; Pichler, T.; et al. Straightforward Generation of Pillared, Microporous Graphene Frameworks for Use in Supercapacitors. Adv. Mater. 2015, 27, 6714–6721. [Google Scholar] [CrossRef]
- Li, X.; Faghri, A. Review and advances of direct methanol fuel cells (DMFCs) part I: Design, fabrication, and testing with high concentration methanol solutions. J. Power Sources 2013, 226, 223–240. [Google Scholar] [CrossRef]
- Hoseini, S.J.; Bahrami, M.; Maddahfar, M.; Hashemi Fath, R.; Roushani, M. Polymerization of graphene oxide nanosheet by using of aminoclay: Electrocatalytic activity of its platinum nanohybrids. Appl. Organomet. Chem. 2018, 32, e3894. [Google Scholar] [CrossRef] [Green Version]
- Wen, C.; Gao, X.; Huang, T.; Wu, X.; Xu, L.; Yu, J.; Zhang, H.; Zhang, Z.; Han, J.; Ren, H. Reduced graphene oxide supported chromium oxide hybrid as high efficient catalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 11099–11107. [Google Scholar] [CrossRef]
- Liu, K.; Song, Y.; Chen, S. Oxygen reduction catalyzed by nanocomposites based on graphene quantum dots-supported copper nanoparticles. Int. J. Hydrogen Energy 2016, 41, 1559–1567. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.K.; Yang, C.; Zhou, Z.X.; Liu, X.W.; Li, Y. Active 3D Pd/graphene aerogel catalyst for hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2016, 41, 15225–15235. [Google Scholar] [CrossRef]
- Yun, M.; Choe, J.E.; You, J.M.; Ahmed, M.S.; Lee, K.; Üstündaǧ, Z.; Jeon, S. High catalytic activity of electrochemically reduced graphene composite toward electrochemical sensing of Orange II. Food Chem. 2015, 169, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Ahmed, M.S.; Jeon, S. Covalent functionalization of graphene with 1,5-diaminonaphthalene and ultrasmall palladium nanoparticles for electrocatalytic oxygen reduction. Int. J. Hydrogen Energy 2017, 42, 2061–2070. [Google Scholar] [CrossRef]
- Vinoth, R.; Babu, S.G.; Bharti, V.; Gupta, V.; Navaneethan, M.; Bhat, S.V.; Muthamizhchelvan, C.; Ramamurthy, P.C.; Sharma, C.; Aswal, D.K.; et al. Ruthenium based metallopolymer grafted reduced graphene oxide as a new hybrid solar light harvester in polymer solar cells. Sci. Rep. 2017, 7, 43133. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xu, X.; Li, Z.; Yang, F.; Zhang, L.; Li, Y.; Wang, A.; Chen, S. Construction of efficient counter electrodes for dye-sensitized solar cells: Fe2O3nanoparticles anchored onto graphene frameworks. Carbon N. Y. 2016, 96, 947–954. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wang, C.C.; Lu, S.Y. Graphene aerogels as a highly efficient counter electrode material for dye-sensitized solar cells. Carbon N. Y. 2013, 54, 291–299. [Google Scholar] [CrossRef]
- Tao, L.; Huo, Z.; Dai, S.; Ding, Y.; Zhu, J.; Zhang, C.; Zhang, B.; Yao, J.; Nazeeruddin, M.K.; Grätzel, M. Stable Quasi-Solid-State Dye-Sensitized Solar Cells Using Novel Low Molecular Mass Organogelators and Room-Temperature Molten Salts. J. Phys. Chem. C 2014, 118, 16718–16726. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Xue, B.; Wang, Z.; Meng, Q.; Chen, L. Solid-state composite electrolyte LiI/3-hydroxypropionitrile/SiO2 for dye-sensitized solar cells. J. Am. Chem. Soc. 2005, 127, 6394–6401. [Google Scholar] [CrossRef]
- Brennan, L.J.; Barwich, S.T.; Satti, A.; Faure, A.; Gun’ko, Y.K. Graphene–ionic liquid electrolytes for dye sensitised solar cells. J. Mater. Chem. A 2013, 1, 8379. [Google Scholar] [CrossRef] [Green Version]
- Kowsari, E.; Chirani, M.R. High efficiency dye-sensitized solar cells with tetra alkyl ammonium cation-based ionic liquid functionalized graphene oxide as a novel additive in nanocomposite electrolyte. Carbon N. Y. 2017, 118, 384–392. [Google Scholar] [CrossRef]
- Sun, X.; He, B.; Zhu, J.; Zhu, R.; Chen, H.; Duan, Y.; Tang, Q. Multifunctional brominated graphene oxide boosted charge extraction for high-efficiency and stable all-inorganic CsPbBr3 perovskite solar cells. Chem. Eng. J. 2021, 412, 128727. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Gao, K.; Chen, N.; Sun, X.; Ti, D.; Bai, C.; Cui, R.; Qu, L. Graphene quantum dots for energy storage and conversion: From fabrication to applications. Mater. Chem. Front. 2020, 4, 421–436. [Google Scholar] [CrossRef]
- Mahalingam, S.; Manap, A.; Omar, A.; Low, F.W.; Afandi, N.F.; Chia, C.H.; Rahim, N.A. Functionalized graphene quantum dots for dye-sensitized solar cell: Key challenges, recent developments and future prospects. Renew. Sustain. Energy Rev. 2021, 144, 110999. [Google Scholar] [CrossRef]
- Kolpak, A.M.; Grossman, J.C. Azobenzene-functionalized carbon nanotubes as high-energy density solar thermal fuels. Nano Lett. 2011, 11, 3156–3162. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Feng, Y.; Cao, C.; Li, M.; Liu, E.; Li, S.; Qin, C.; Hu, W.; Feng, W. A high energy density azobenzene/graphene hybrid: A nano-templated platform for solar thermal storage. J. Mater. Chem. A 2015, 3, 11787–11795. [Google Scholar] [CrossRef]
- Rudd, P.M.; Elliott, T.; Cresswell, P.; Wilson, I.A.; Dwek, R.A. Glycosylation and the immune system. Science 2001, 291, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hage, D.S. Glycoform analysis of alpha1-acid glycoprotein by capillary electrophoresis. J. Chromatogr. A 2016, 1475, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Lequin, R.M. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [Green Version]
- Stavenhagen, K.; Plomp, R.; Wuhrer, M. Site-Specific Protein N- and O-Glycosylation Analysis by a C18-Porous Graphitized Carbon-Liquid Chromatography-Electrospray Ionization Mass Spectrometry Approach Using Pronase Treated Glycopeptides. Anal. Chem. 2015, 87, 11691–11699. [Google Scholar] [CrossRef]
- Yang, Y.; Boysen, R.I.; Chowdhury, J.; Alam, A.; Hearn, M.T.W. Analysis of peptides and protein digests by reversed phase high performance liquid chromatography-electrospray ionisation mass spectrometry using neutral pH elution conditions. Anal. Chim. Acta 2015, 872, 84–94. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Gao, M.; Zhang, X.; Yang, P. Multilayer Hydrophilic Poly(phenol-formaldehyde resin)-Coated Magnetic Graphene for Boronic Acid Immobilization as a Novel Matrix for Glycoproteome Analysis. ACS Appl. Mater. Interfaces 2015, 7, 16011–16017. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef]
- Luo, J.; Cong, J.; Liu, J.; Gao, Y.; Liu, X. A facile approach for synthesizing molecularly imprinted graphene for ultrasensitive and selective electrochemical detecting 4-nitrophenol. Anal. Chim. Acta 2015, 864, 74–84. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Zhu, H.; Pan, G.; Qiu, F.; Meng, M.; Yao, J.; Yuan, D. Graphene oxide based molecularly imprinted polymers with double recognition abilities: The combination of covalent boronic acid and traditional non-covalent monomers. Chem. Eng. J. 2016, 290, 220–231. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Cong, J.; Luo, J.; Liu, X. Selective and sensitive glycoprotein detection via a biomimetic electrochemical sensor based on surface molecular imprinting and boronate-modified reduced graphene oxide. Sens. Actuators B Chem. 2018, 259, 1–9. [Google Scholar] [CrossRef]
- Eissa, S.; Jimenez, G.C.; Mahvash, F.; Guermoune, A.; Tlili, C.; Szkopek, T.; Zourob, M.; Siaj, M. Functionalized CVD monolayer graphene for label-free impedimetric biosensing. Nano Res. 2015, 8, 1698–1709. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Park, J.Y. Gold Nanoparticles Assembled Chemically Functionalized Reduced Graphene Oxide Supported Electrochemical Immunosensor for Ultra-Sensitive Prostate Cancer Detection. J. Electrochem. Soc. 2017, 164, B234–B239. [Google Scholar] [CrossRef]
- Bonanni, A.; Del Valle, M. Use of nanomaterials for impedimetric DNA sensors: A review. Anal. Chim. Acta 2010, 678, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Bonanni, A.; Pumera, M. Graphene Platform for Hairpin-DNA- Based Impedimetric Genosensing. ACS Nano 2011, 5, 2356–2361. [Google Scholar] [CrossRef]
- Urbanová, V.; Holá, K.; Bourlinos, A.B.; Čépe, K.; Ambrosi, A.; Loo, A.H.; Pumera, M.; Karlický, F.; Otyepka, M.; Zbořil, R. Thiofluorographene-hydrophilic graphene derivative with semiconducting and genosensing properties. Adv. Mater. 2015, 27, 2305–2310. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, L.; Fan, R.; Ge, X.; Sun, Y. Water-dispersible triethylenetetramine-functionalized graphene: Preparation, characterization and application as an amperometric glucose sensor. Mater. Sci. Eng. C 2016, 68, 308–316. [Google Scholar] [CrossRef]
- You, J.-M.; Han, H.S.; Jeon, S. Synthesis of Electrochemically Reduced Graphene Oxide Bonded to Thiodiazole-Pd and Applications to Biosensor. J. Nanosci. Nanotechnol. 2015, 15, 5691–5698. [Google Scholar] [CrossRef]
- Rao Vusa, C.S.; Manju, V.; Berchmans, S.; Arumugam, P. Electrochemical amination of graphene using nanosized PAMAM dendrimers for sensing applications. RSC Adv. 2016, 6, 33409–33418. [Google Scholar] [CrossRef]
- Geetha Bai, R.; Muthoosamy, K.; Tuvikene, R.; Nay Ming, H.; Manickam, S. Highly Sensitive Electrochemical Biosensor Using Folic Acid-Modified Reduced Graphene Oxide for the Detection of Cancer Biomarker. Nanomaterials 2021, 11, 1272. [Google Scholar] [CrossRef] [PubMed]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Associations of elevated nonesterified fatty acids and β-hydroxybutyrate concentrations with early lactation reproductive performance and milk production in transition dairy cattle in the northeastern United States. J. Dairy Sci. 2010, 93, 1596–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrow, N.J.; Sanghera, G.S.; Walters, S.J.; Watkin, J.L. Development of a commercial amperometric biosensor electrode for the ketone D-3-hydroxybutyrate. Biosens. Bioelectron. 2005, 20, 1617–1625. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.H.; Liu, C.C. An electrochemical biosensor of the ketone 3-β-hydroxybutyrate for potential diabetic patient management. Sens. Actuators B Chem. 2008, 129, 818–825. [Google Scholar] [CrossRef]
- Khorsand, F.; Darziani Azizi, M.; Naeemy, A.; Larijani, B.; Omidfar, K. An electrochemical biosensor for 3-hydroxybutyrate detection based on screen-printed electrode modified by coenzyme functionalized carbon nanotubes. Mol. Biol. Rep. 2013, 40, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Veerapandian, M.; Hunter, R.; Neethirajan, S. Ruthenium dye sensitized graphene oxide electrode for on-farm rapid detection of beta-hydroxybutyrate. Sens. Actuators B Chem. 2016, 228, 180–184. [Google Scholar] [CrossRef]
- Mao, H.; Ji, C.; Liu, M.; Sun, Y.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.-M. Hydrophilic polymer/polypyrrole/graphene oxide nanosheets with different performances in electrocatalytic applications to simultaneously determine dopamine and ascorbic acid. RSC Adv. 2016, 6, 111632–111639. [Google Scholar] [CrossRef]
- Olsen, G.; Ulstrup, J.; Chi, Q. Crown-Ether Derived Graphene Hybrid Composite for Membrane-Free Potentiometric Sensing of Alkali Metal Ions. ACS Appl. Mater. Interfaces 2016, 8, 37–41. [Google Scholar] [CrossRef]
- Suhag, D.; Sharma, A.K.; Patni, P.; Garg, S.K.; Rajput, S.K.; Chakrabarti, S.; Mukherjee, M. Hydrothermally functionalized biocompatible nitrogen doped graphene nanosheet based biomimetic platforms for nitric oxide detection. J. Mater. Chem. B 2016, 4, 4780–4789. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, H.; Xu, Y.; Yang, W.; Liu, A.; Shan, F.; Cao, M.; Liu, J. Graphene nanodots encaged 3-D gold substrate as enzyme loading platform for the fabrication of high performance biosensors. Sens. Actuators B Chem. 2015, 220, 1186–1195. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X.; Li, M.; Fan, Y.; Sun, R. Humidity sensor based on reduced graphene oxide/lignosulfonate composite thin-film. Sensors Actuators B Chem. 2018, 255, 1569–1576. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett 2011, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Zhang, L.; Liu, M.; Zhang, Z. Biomedical applications of graphene. Theranostics 2012, 2, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Giallombardo, D.; Botta, L.; Bondì, M.L.; Agnello, S. Synthesis and self-assembly of a PEGylated-graphene aerogel. Compos. Sci. Technol. 2016, 128, 193–200. [Google Scholar] [CrossRef]
- Kavitha, T.; Kang, I.K.; Park, S.Y. Poly(4-vinyl pyridine)-grafted graphene oxide for drug delivery and antimicrobial applications. Polym. Int. 2015, 64, 1660–1666. [Google Scholar] [CrossRef]
- Zhang, Q.; Chi, H.; Tang, M.; Chen, J.; Li, G.; Liu, Y.; Liu, B. Mixed surfactant modified graphene oxide nanocarriers for DOX delivery to cisplatin-resistant human ovarian carcinoma cells. RSC Adv. 2016, 6, 87258–87269. [Google Scholar] [CrossRef]
- Xie, M.; Lei, H.; Zhang, Y.; Xu, Y.; Shen, S.; Ge, Y.; Li, H.; Xie, J. Non-covalent modification of graphene oxide nanocomposites with chitosan/dextran and its application in drug delivery. RSC Adv. 2016, 6, 9328–9337. [Google Scholar] [CrossRef]
- Shim, G.; Lee, J.; Kim, J.; Lee, H.-J.; Kim, Y.B.; Oh, Y.-K. Functionalization of nano-graphenes by chimeric peptide engineering. RSC Adv. 2015, 5, 49905–49913. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, H.; Xu, Q.; Long, Z.; Kou, X.; Zhang, X.; Zhao, M.; Mi, J.; Li, Z. Three-dimensional DNA/graphene assemblies with favorable stability. Sci. Adv. Mater. 2017, 9, 1146–1150. [Google Scholar] [CrossRef]
- Wang, P.; Wang, X.; Tang, Q.; Chen, H.; Zhang, Q.; Jiang, H.; Wang, Z. Functionalized graphene oxide against U251 glioma cells and its molecular mechanism. Mater. Sci. Eng. C 2020, 116, 111187. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Botta, L.; Gulino, E.F.; Gulli, D. Tunable release of Chlorhexidine from Polycaprolactone-based filaments containing graphene nanoplatelets. Eur. Polym. J. 2019, 110, 221–232. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Maio, A.; Gallo, G. PLA graphene nanoplatelets nanocomposites: Physical properties and release kinetics of an antimicrobial agent. Compos. Part. B Eng. 2017, 109, 138–146. [Google Scholar] [CrossRef]
- Patil, P.S.; Fountas-Davis, N.; Huang, H.; Evancho-Chapman, M.M.; Fulthon, J.A.; Shriver, L.P.; Leipzig, N.D. Fluorinated Methacrylamide Chitosan Hydrogels Enhance Collagen Synthesis in Wound Healing through Increased Oxygen Availability. Acta Biomater. 2016, 36, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [Green Version]
- Farris, A.L.; Rindone, A.N.; Grayson, W.L. Oxygen delivering biomaterials for tissue engineering. J. Mater. Chem. B 2016, 4, 3422–3432. [Google Scholar] [CrossRef]
- Dalvi, V.H.; Rossky, P.J. Molecular origins of fluorocarbon hydrophobicity. Proc. Natl. Acad. Sci. USA 2010, 107, 13603–13607. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Morais, D.S.; Rodrigues, M.A.; Silva, T.I.; Lopes, M.A.; Santos, M.; Santos, J.D.; Botelho, C.M. Development and characterization of novel alginate-based hydrogels as vehicles for bone substitutes. Carbohydr. Polym. 2013, 95, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Caridade, S.G.; Vale, A.C.; Cunha, E.; Sousa, M.P.; Mano, J.F.; Paiva, M.C.; Alves, N.M. Biomedical films of graphene nanoribbons and nanoflakes with natural polymers. RSC Adv. 2017, 7, 27578–27594. [Google Scholar] [CrossRef] [Green Version]
- Scaffaro, R.; Botta, L.; Lopresti, F.; Maio, A.; Sutera, F. Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 2017, 24, 447–478. [Google Scholar] [CrossRef]
- Wu, D.; Bäckström, E.; Hakkarainen, M. Starch Derived Nanosized Graphene Oxide Functionalized Bioactive Porous Starch Scaffolds. Macromol. Biosci. 2017, 17, 1600397. [Google Scholar] [CrossRef]
- Sarvari, R.; Sattari, S.; Massoumi, B.; Agbolaghi, S.; Beygi-Khosrowshahi, Y.; Kahaie-Khosrowshahi, A. Composite electrospun nanofibers of reduced graphene oxide grafted with poly(3-dodecylthiophene) and poly(3-thiophene ethanol) and blended with polycaprolactone. J. Biomater. Sci. Polym. Ed. 2017, 28, 1740–1761. [Google Scholar] [CrossRef] [PubMed]
- Massoumi, B.; Ghandomi, F.; Abbasian, M.; Eskandani, M.; Jaymand, M. Surface functionalization of graphene oxide with poly(2-hydroxyethyl methacrylate)-graft-poly(ε-caprolactone) and its electrospun nanofibers with gelatin. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1000. [Google Scholar] [CrossRef]
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, 055005. [Google Scholar] [CrossRef]
- Benkli, Y.E.; Can, M.F.; Turan, M.; Çelik, M.S. Modification of organo-zeolite surface for the removal of reactive azo dyes in fixed-bed reactors. Water Res. 2005, 39, 487–493. [Google Scholar] [CrossRef]
- Meena Sundari, P.; Meenambal, T. A comparative study on the adsorptive efficiency of low-cost adsorbents for the removal of methylene blue from its aqueous solution. Desalin. Water Treat. 2015, 57, 18851–18860. [Google Scholar] [CrossRef]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Maio, A.; Gammino, M.; Fortunato Gulino, E.; Megna, B.; Fara, P.; Scaffaro, R. Rapid One-Step Fabrication of Graphene Oxide-Decorated Polycaprolactone Three-Dimensional Templates for Water Treatment. ACS Appl. Polym. Mater. 2020, 2, 4993–5005. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Wu, C.H.; Wu, J.Y. Adsorption of direct dyes from aqueous solutions by carbon nanotubes: Determination of equilibrium, kinetics and thermodynamics parameters. J. Colloid Interface Sci. 2008, 327, 308–315. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.S.; Bukhari, A.A.; Khaled, M.; Alharthi, M.; Fettouhi, M.; Abuilaiwi, F.A. Removal of Chromium (III) from Water by Using Modified and Nonmodified Carbon Nanotubes. J. Nanomater. 2010, 2010, 232378. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl. Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Tehrani, A.D.D.; Adeli, M. Bioconjugated graphene oxide hydrogel as an effective adsorbent for cationic dyes removal. Ecotoxicol. Environ. Saf. 2018, 147, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Coutinho, J.A.P.; Fernandes, A.M.; Freire, M.G. Complete removal of textile dyes from aqueous media using ionic-liquid-based aqueous two-phase systems. Sep. Purif. Technol. 2014, 128, 58–66. [Google Scholar] [CrossRef]
- Gharehbaghi, M.; Shemirani, F. A Novel Method for Dye Removal: Ionic Liquid-Based Dispersive Liquid-Liquid Extraction (IL-DLLE). Clean Soil Air Water 2012, 40, 290–297. [Google Scholar] [CrossRef]
- Gao, H.; Kan, T.; Zhao, S.; Qian, Y.; Cheng, X.; Wu, W.; Wang, X.; Zheng, L. Removal of anionic azo dyes from aqueous solution by functional ionic liquid cross-linked polymer. J. Hazard. Mater. 2013, 261, 83–90. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, H.; Wang, W.J.; Li, B.G.; Zhu, S. Collectable and Recyclable Mussel-Inspired Poly(ionic liquid)-Based Sorbents for Ultrafast Water Treatment. ACS Sustain. Chem. Eng. 2017, 5, 2829–2835. [Google Scholar] [CrossRef]
- Marullo, S.; Rizzo, C.; Dintcheva, N.T.; Giannici, F.; D’Anna, F. Ionic liquids gels: Soft materials for environmental remediation. J. Colloid Interface Sci. 2018, 517, 182–193. [Google Scholar] [CrossRef]
- Cheng, N.; Hu, Q.; Guo, Y.; Wang, Y.; Yu, L. Efficient and selective removal of dyes using imidazolium-based supramolecular gels. ACS Appl. Mater. Interfaces 2015, 7, 10258–10265. [Google Scholar] [CrossRef]
- Zambare, R.; Song, X.; Bhuvana, S.; Antony Prince, J.S.; Nemade, P. Ultrafast Dye Removal Using Ionic Liquid-Graphene Oxide Sponge. ACS Sustain. Chem. Eng. 2017, 5, 6026–6035. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Loizidou, M.D.; Grigoropoulou, H.P. Equilibrium and kinetic ion exchange studies of Pb2+, Cr3+, Fe3+ and Cu2+ on natural clinoptilolite. Water Res. 2002, 36, 2784–2792. [Google Scholar] [CrossRef]
- Feng, M.L.; Sarma, D.; Qi, X.H.; Du, K.Z.; Huang, X.Y.; Kanatzidis, M.G. Efficient Removal and Recovery of Uranium by a Layered Organic-Inorganic Hybrid Thiostannate. J. Am. Chem. Soc. 2016, 138, 12578–12585. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Wat. Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on Chitosan/Sulfydryl-functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef]
- Pan, N.; Li, L.; Ding, J.; Wang, R.; Jin, Y.; Xia, C. A Schiff base/quaternary ammonium salt bifunctional graphene oxide as an efficient adsorbent for removal of Th(IV)/U(VI). J. Colloid Interface Sci. 2017, 508, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A review of graphene-based separation membrane: Materials, characteristics, preparation and applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Fouladivanda, M.; Karimi-Sabet, J.; Abbasi, F.; Moosavian, M.A. Step-by-step improvement of mixed-matrix nanofiber membrane with functionalized graphene oxide for desalination via air-gap membrane distillation. Sep. Purif. Technol. 2021, 256, 117809. [Google Scholar] [CrossRef]
- Goel, P.; Mandal, P.; Bhuvanesh, E.; Shahi, V.K.; Chattopadhyay, S. Temperature resistant cross-linked brominated poly phenylene oxide-functionalized graphene oxide nanocomposite anion exchange membrane for desalination. Sep. Purif. Technol. 2021, 255, 117730. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Yin, M.-J.; Zhao, Q.; Jin, C.-G.; Wang, N.; Ji, S.; Ritt, C.L.; Elimelech, M.; An, Q.-F. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotechnol. 2021, 16, 337–343. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.-B.; Yu, H.; Liu, J.Z.; Easton, C.D.; Wang, Z.; Hu, Y.; Xie, Z.; Wu, H.-A.; Zhang, X.; et al. Ultrafast water evaporation through graphene membranes with subnanometer pores for desalination. J. Memb. Sci. 2021, 621, 118934. [Google Scholar] [CrossRef]
- Erken, E.; Yıldız, Y.; Kilbaş, B.; Şen, F. Synthesis and Characterization of Nearly Monodisperse Pt Nanoparticles for C 1 to C 3 Alcohol Oxidation and Dehydrogenation of Dimethylamine-borane (DMAB). J. Nanosci. Nanotechnol. 2016, 16, 5944–5950. [Google Scholar] [CrossRef] [PubMed]
- Baskaya, G.; Esirden, İ.; Erken, E.; Sen, F.; Kaya, M. Synthesis of 5-Substituted-1H-Tetrazole Derivatives Using Monodisperse Carbon Black Decorated Pt Nanoparticles as Heterogeneous Nanocatalysts. J. Nanosci. Nanotechnol. 2017, 17, 1992–1999. [Google Scholar] [CrossRef]
- Sun, L.-B.; Liu, X.-Q.; Zhou, H.-C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymers 2013, 54, 5087–5103. [Google Scholar] [CrossRef] [Green Version]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef]
- Porahmad, N.; Baharfar, R. Graphene oxide covalently functionalized with an organic superbase as highly efficient and durable nanocatalyst for green Michael addition reaction. Res. Chem. Intermed. 2018, 44, 305–323. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Hashemi Fath, R.; Hoseini, S.J. Covalently cyclopalladium(II) complex/reduced-graphene oxide as the effective catalyst for the Suzuki–Miyaura reaction at room temperature. J. Organomet. Chem. 2017, 828, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Nancy, P.; Nair, A.K.; Antoine, R.; Thomas, S.; Kalarikkal, N. In Situ Decoration of Gold Nanoparticles on Graphene Oxide via Nanosecond Laser Ablation for Remarkable Chemical Sensing and Catalysis. Nanomaterials 2019, 9, 1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Ding, S.; Xiao, W.; Peng, Y.; Deng, S.; Zhang, N. 3-Aminopropyl-triethoxysilane Functionalized Graphene Oxide: A Highly Efficient and Recyclable Catalyst for Knoevenagel Condensation. Catal. Lett. 2015, 145, 1000–1007. [Google Scholar] [CrossRef]
- Achary, L.S.K.; Kumar, A.; Rout, L.; Kunapuli, S.V.S.; Dhaka, R.S.; Dash, P. Phosphate functionalized graphene oxide with enhanced catalytic activity for Biginelli type reaction under microwave condition. Chem. Eng. J. 2018, 331, 300–310. [Google Scholar] [CrossRef]
- Roeser, J.; Kailasam, K.; Thomas, A. Covalent triazine frameworks as heterogeneous catalysts for the synthesis of cyclic and linear carbonates from carbon dioxide and epoxides. ChemSusChem 2012, 5, 1793–1799. [Google Scholar] [CrossRef]
- Watile, R.A.; Deshmukh, K.M.; Dhake, K.P.; Bhanage, B.M. Efficient synthesis of cyclic carbonate from carbon dioxide using polymer anchored diol functionalized ionic liquids as a highly active heterogeneous catalyst. Catal. Sci. Technol. 2012, 2, 1051. [Google Scholar] [CrossRef]
- Tharun, J.; Hwang, Y.; Roshan, R.; Ahn, S.; Kathalikkattil, A.C.; Park, D.-W. A novel approach of utilizing quaternized chitosan as a catalyst for the eco-friendly cycloaddition of epoxides with CO2. Catal. Sci. Technol. 2012, 2, 1674. [Google Scholar] [CrossRef]
- Xu, J.; Xu, M.; Wu, J.; Wu, H.; Zhang, W.-H.; Li, Y.-X. Graphene oxide immobilized with ionic liquids: Facile preparation and efficient catalysis for solvent-free cycloaddition of CO 2 to propylene carbonate. RSC Adv. 2015, 5, 72361–72368. [Google Scholar] [CrossRef]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 2015, 111, 5345–5403. [Google Scholar] [CrossRef]
- Hostert, L.; Blaskievicz, S.F.; Fonsaca, J.E.S.; Domingues, S.H.; Zarbin, A.J.G.; Orth, E.S. Imidazole-derived graphene nanocatalysts for organophosphate destruction: Powder and thin film heterogeneous reactions. J. Catal. 2017, 356, 75–84. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Patel, V.; Gajera, H.; Gupta, A.; Manocha, L.; Madamwar, D. Synthesis of ethyl caprylate in organic media using Candida rugosa lipase immobilized on exfoliated graphene oxide: Process parameters and reusability studies. Biochem. Eng. J. 2015, 95, 62–70. [Google Scholar] [CrossRef]
- Dronov, R.; Kurth, D.G.; Möhwald, H.; Scheller, F.W.; Lisdat, F. A self-assembled cytochrome c/xanthine oxidase multilayer arrangement on gold. Electrochim. Acta 2007, 53, 1107–1113. [Google Scholar] [CrossRef]
- Patila, M.; Kouloumpis, A.; Gournis, D.; Rudolf, P.; Stamatis, H. Laccase-functionalized graphene oxide assemblies as efficient nanobiocatalysts for oxidation reactions. Sensors 2016, 16, 287. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, G.; Jing, L.; Peng, X.; Li, Y. Facile self-assembly of magnetite nanoparticles on three-dimensional graphene oxide-chitosan composite for lipase immobilization. Biochem. Eng. J. 2015, 98, 75–83. [Google Scholar] [CrossRef]
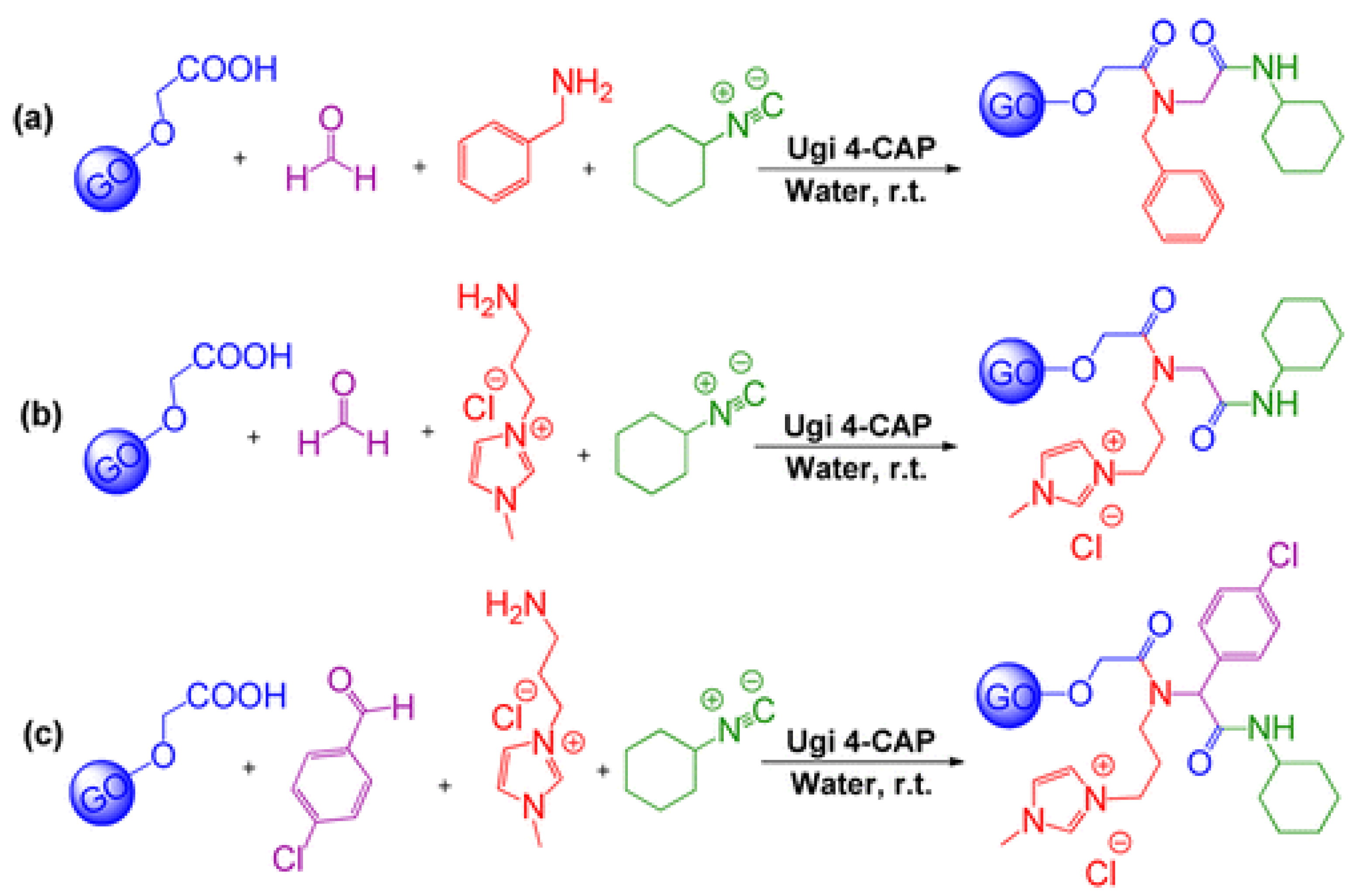
- Rezaei, A.; Akhavan, O.; Hashemi, E.; Shamsara, M. Ugi Four-Component Assembly Process: An Efficient Approach for One-Pot Multifunctionalization of Nanographene Oxide in Water and Its Application in Lipase Immobilization. Chem. Mater. 2016, 28, 3004–3016. [Google Scholar] [CrossRef]
- Besharati Vineh, M.; Saboury, A.A.; Poostchi, A.A.; Rashidi, A.M.; Parivar, K. Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int. J. Biol. Macromol. 2018, 106, 1314–1322. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maio, A.; Pibiri, I.; Morreale, M.; Mantia, F.P.L.; Scaffaro, R. An Overview of Functionalized Graphene Nanomaterials for Advanced Applications. Nanomaterials 2021, 11, 1717. https://doi.org/10.3390/nano11071717
Maio A, Pibiri I, Morreale M, Mantia FPL, Scaffaro R. An Overview of Functionalized Graphene Nanomaterials for Advanced Applications. Nanomaterials. 2021; 11(7):1717. https://doi.org/10.3390/nano11071717
Chicago/Turabian StyleMaio, Andrea, Ivana Pibiri, Marco Morreale, Francesco Paolo La Mantia, and Roberto Scaffaro. 2021. "An Overview of Functionalized Graphene Nanomaterials for Advanced Applications" Nanomaterials 11, no. 7: 1717. https://doi.org/10.3390/nano11071717






