Study on the Effect of Nanoparticle Used in Nano-Fluid Flooding on Droplet–Interface Electro-Coalescence
Abstract
:1. Introduction
1.1. Droplet–Interface Coalescence
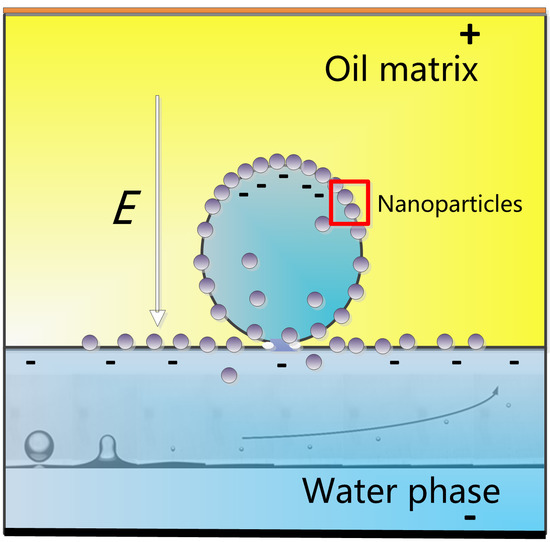
1.2. Influence of NPs on Droplet–Interface Coalescence
2. Experimental Set-Up and Procedure
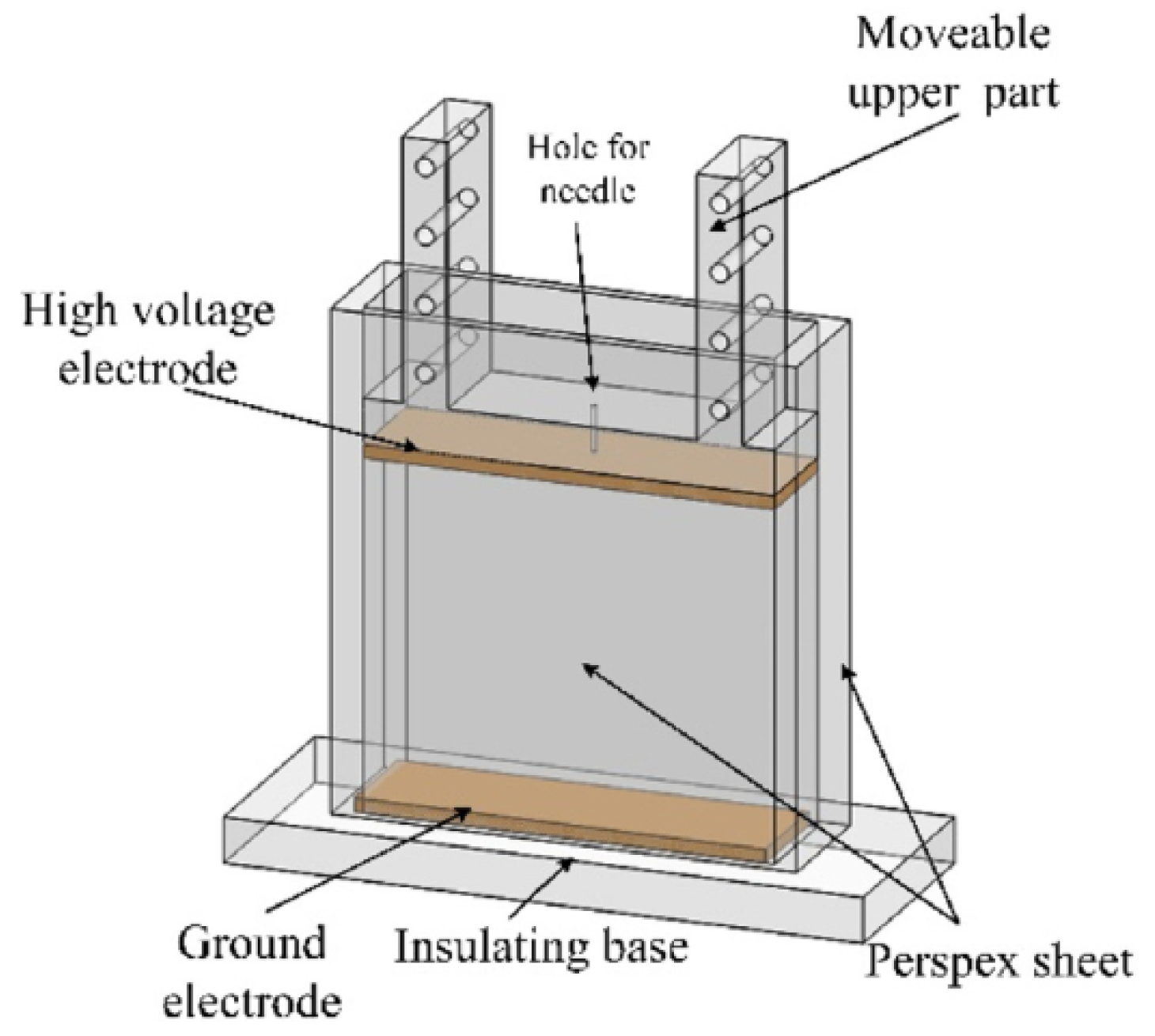
2.1. Experimental Cell
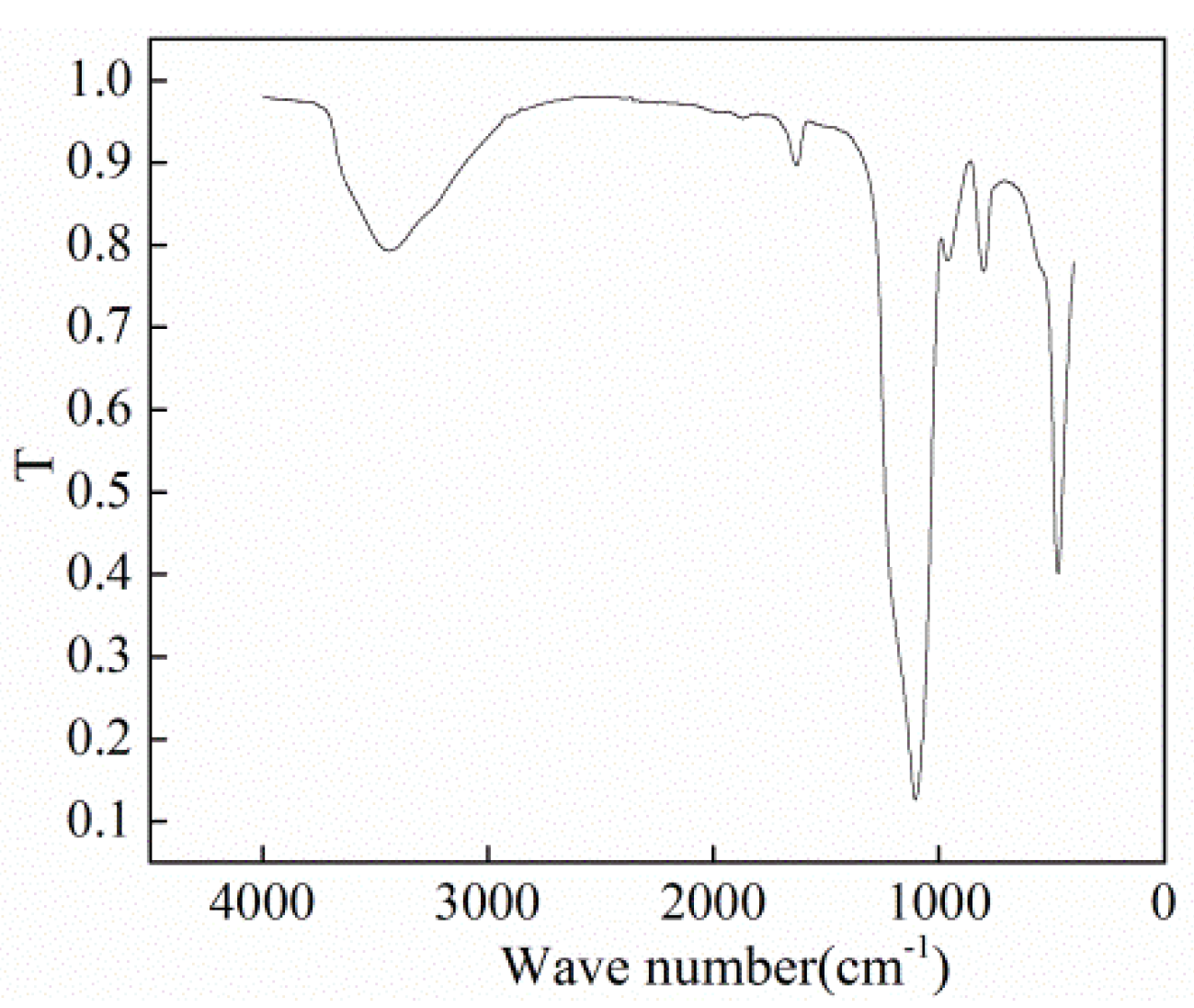
2.2. Preparation of Experimental Liquids
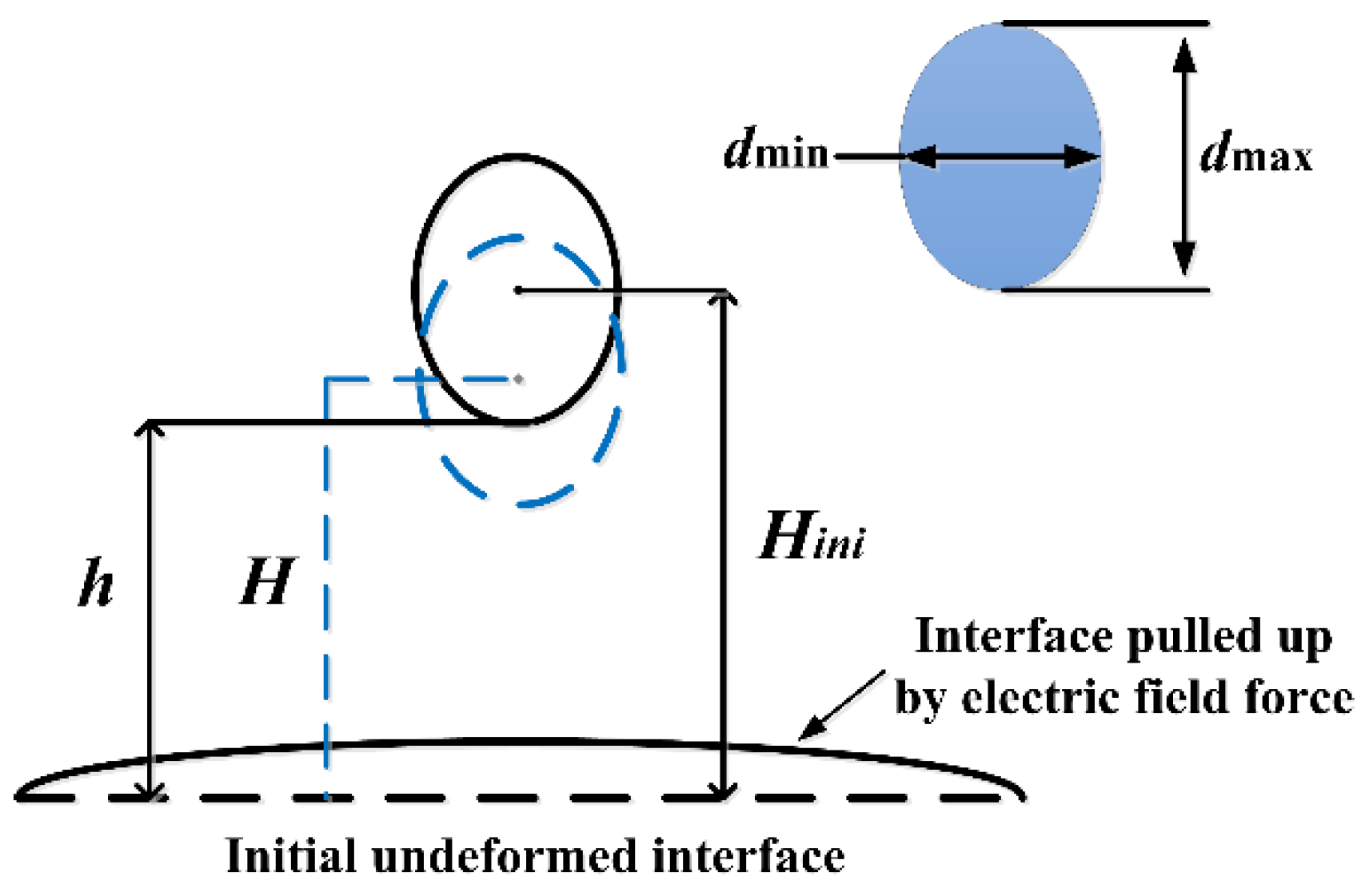
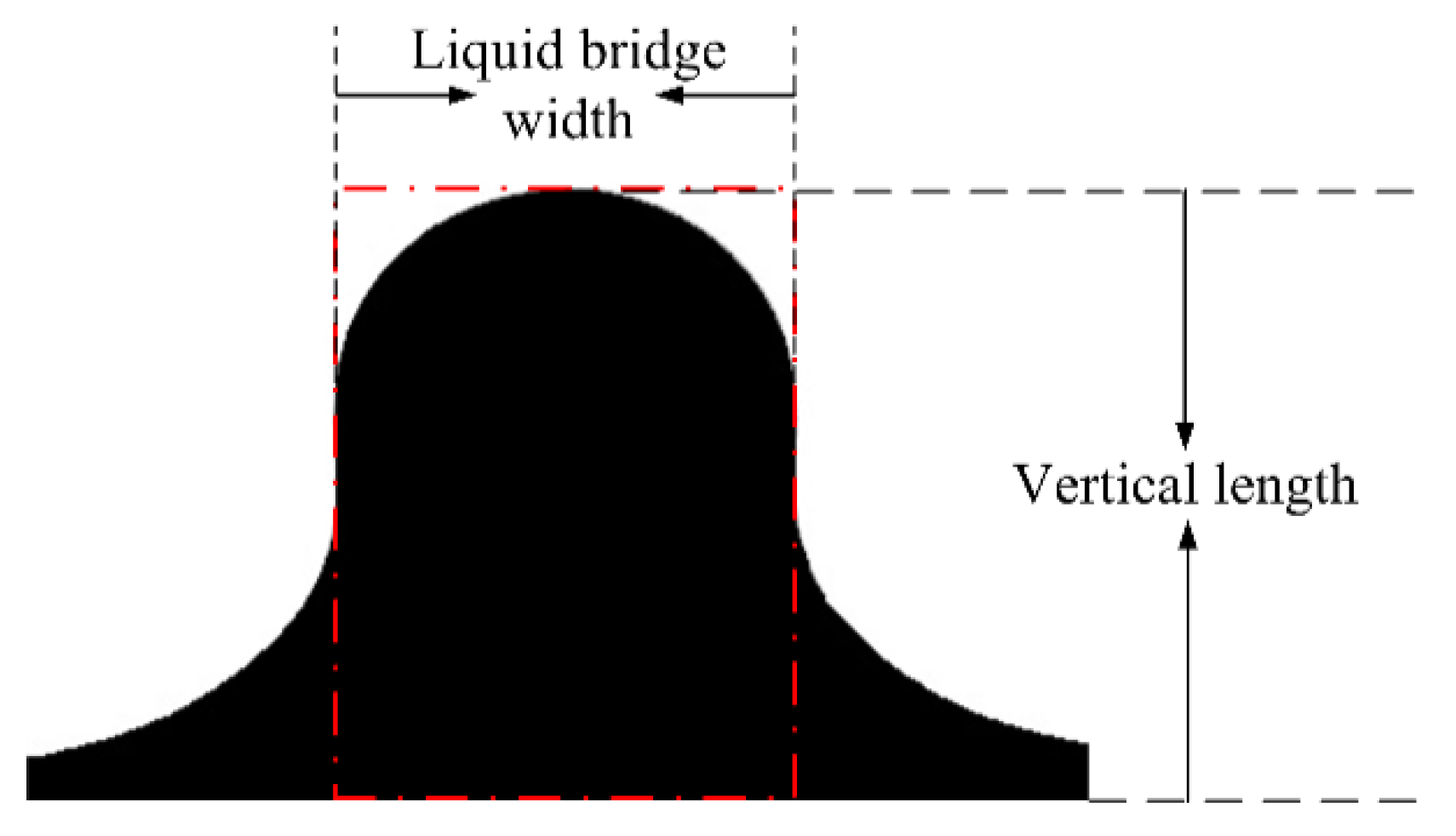
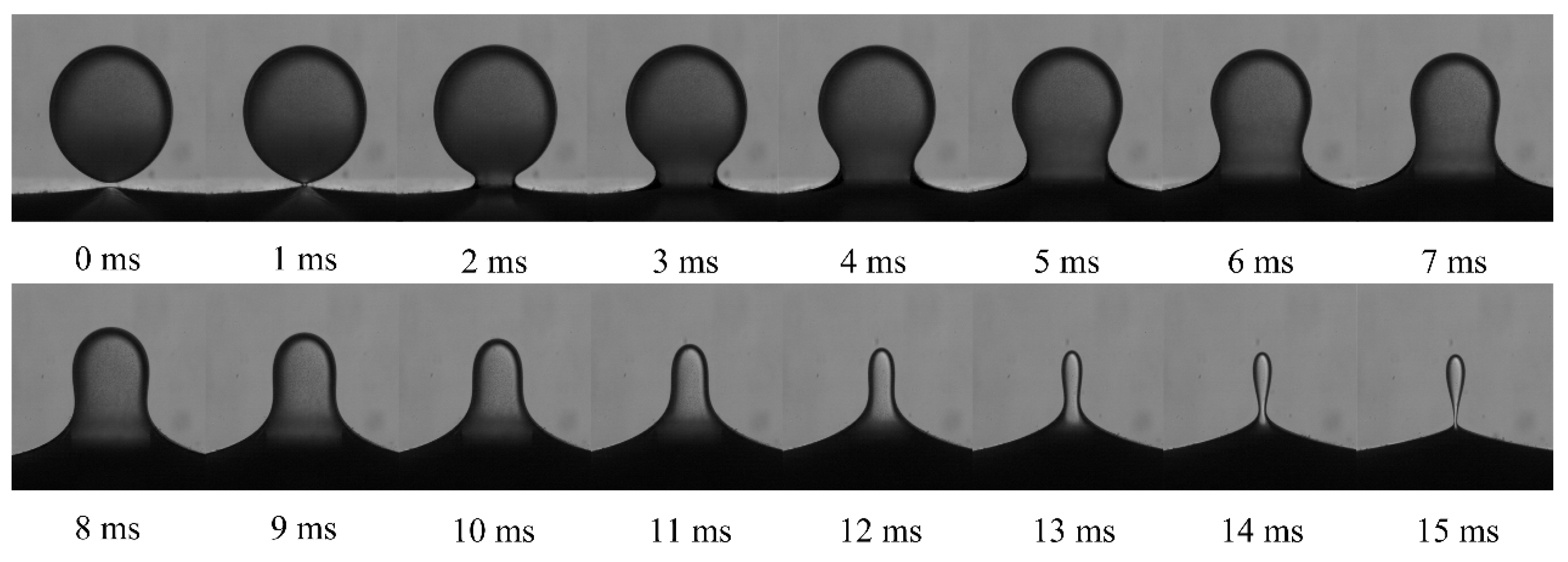
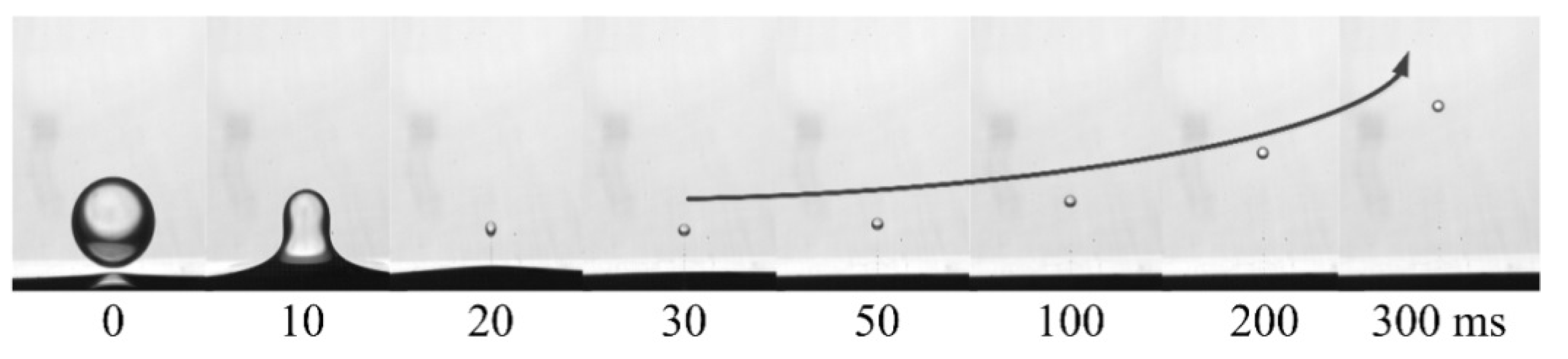
2.3. Data Extraction and Processing
3. Theory
4. Results and Discussion
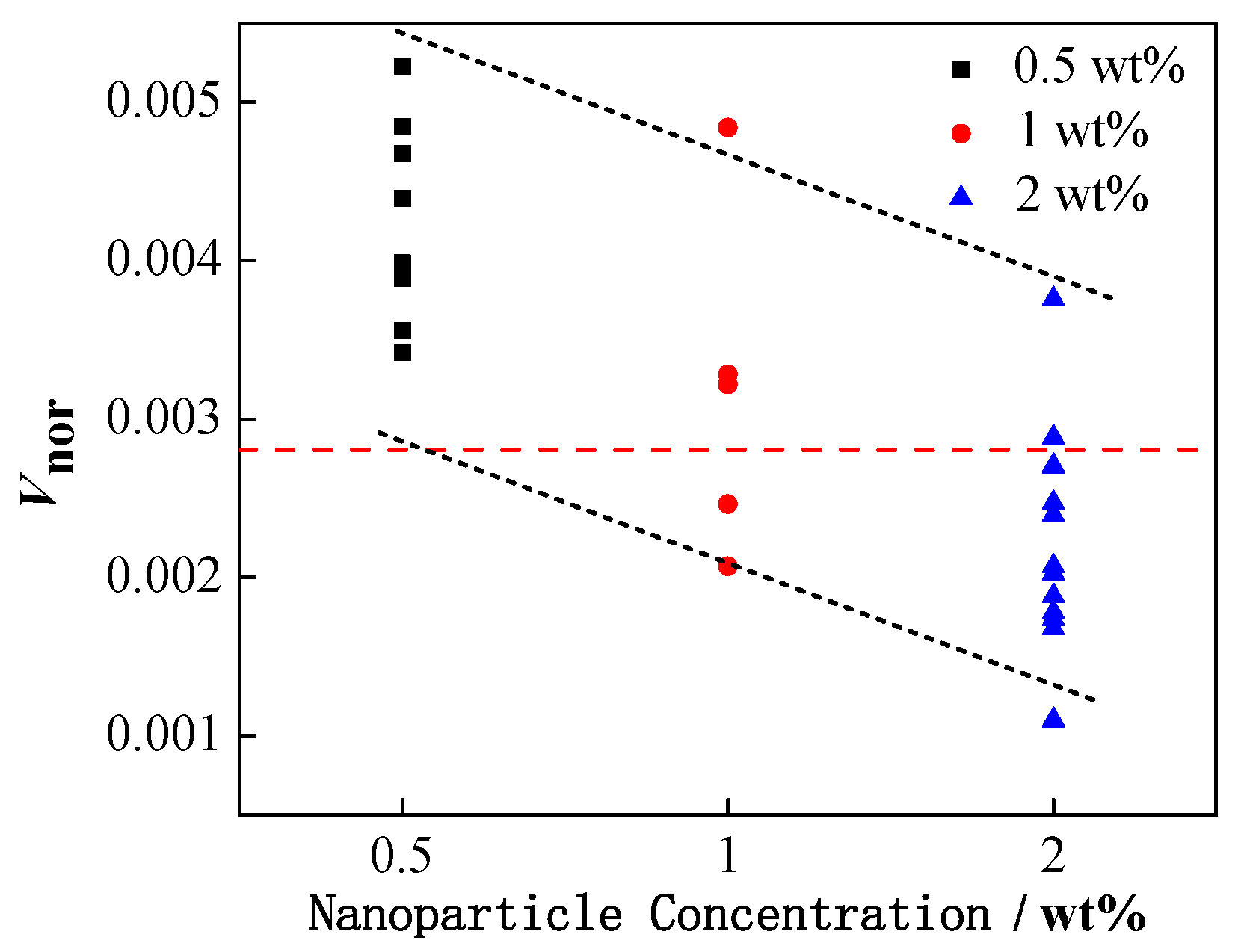
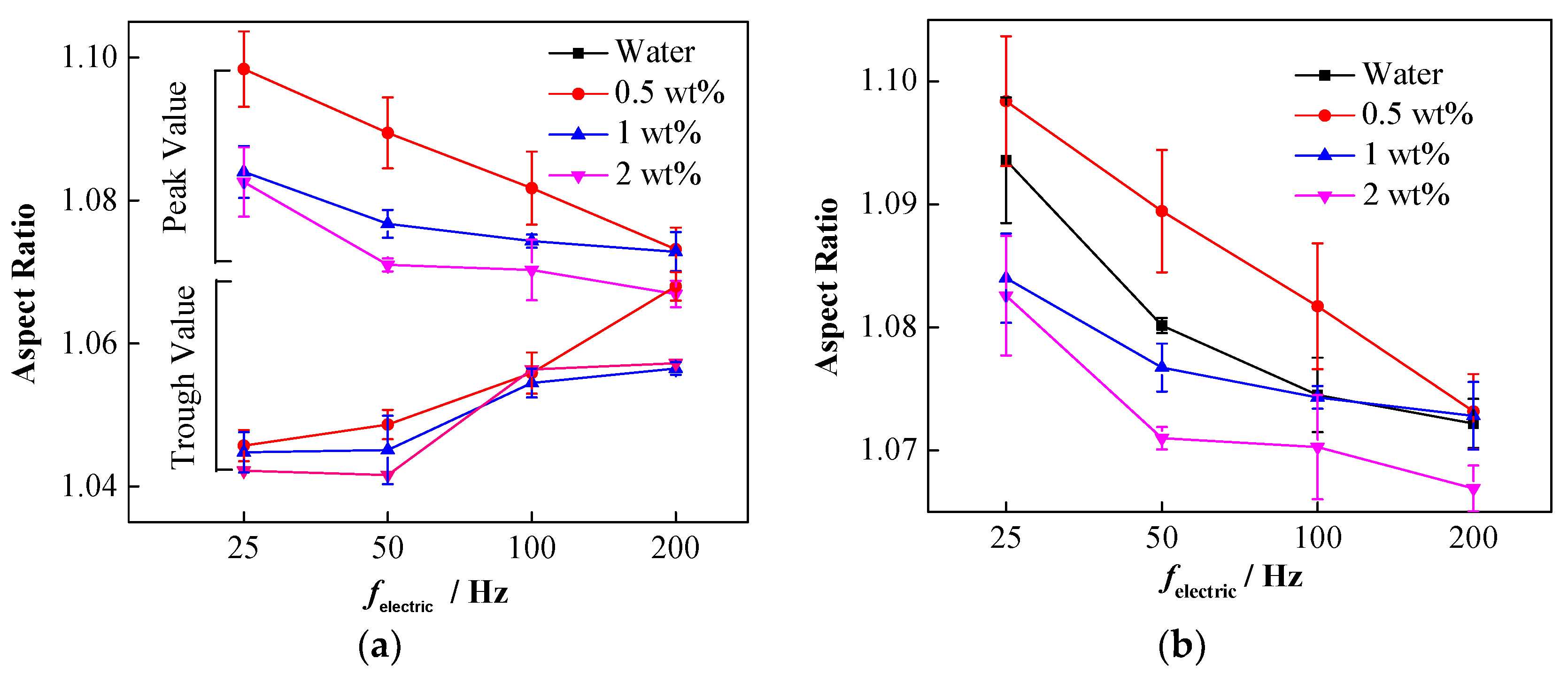
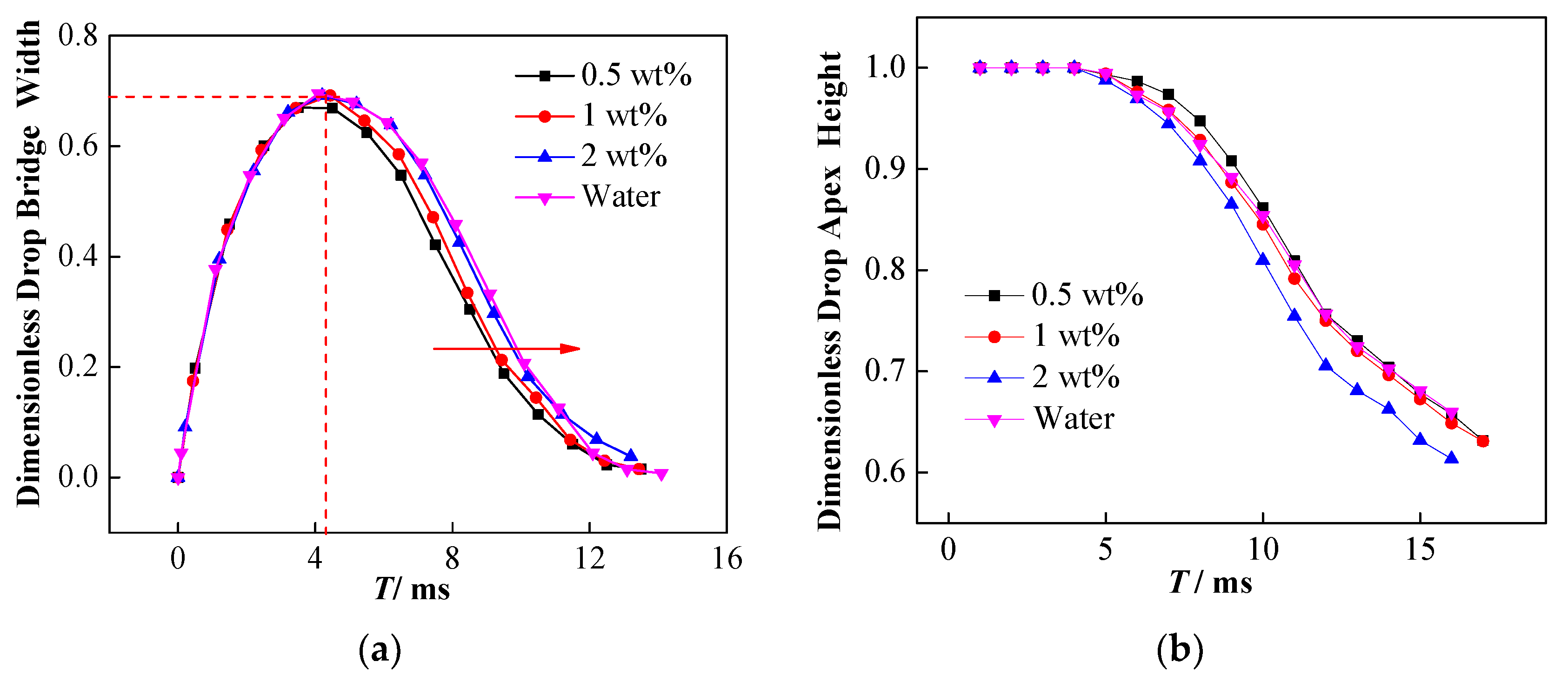
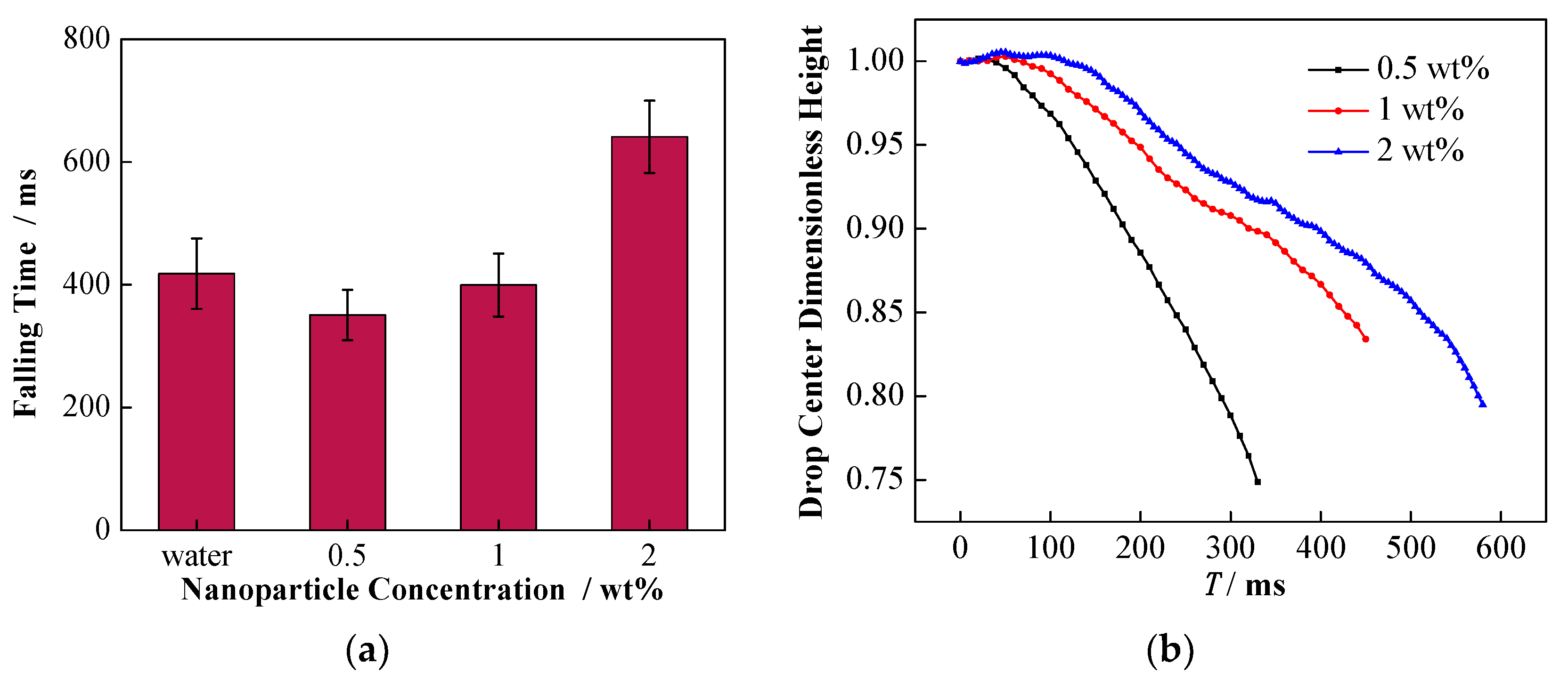
4.1. Effect of Interfacial Tension
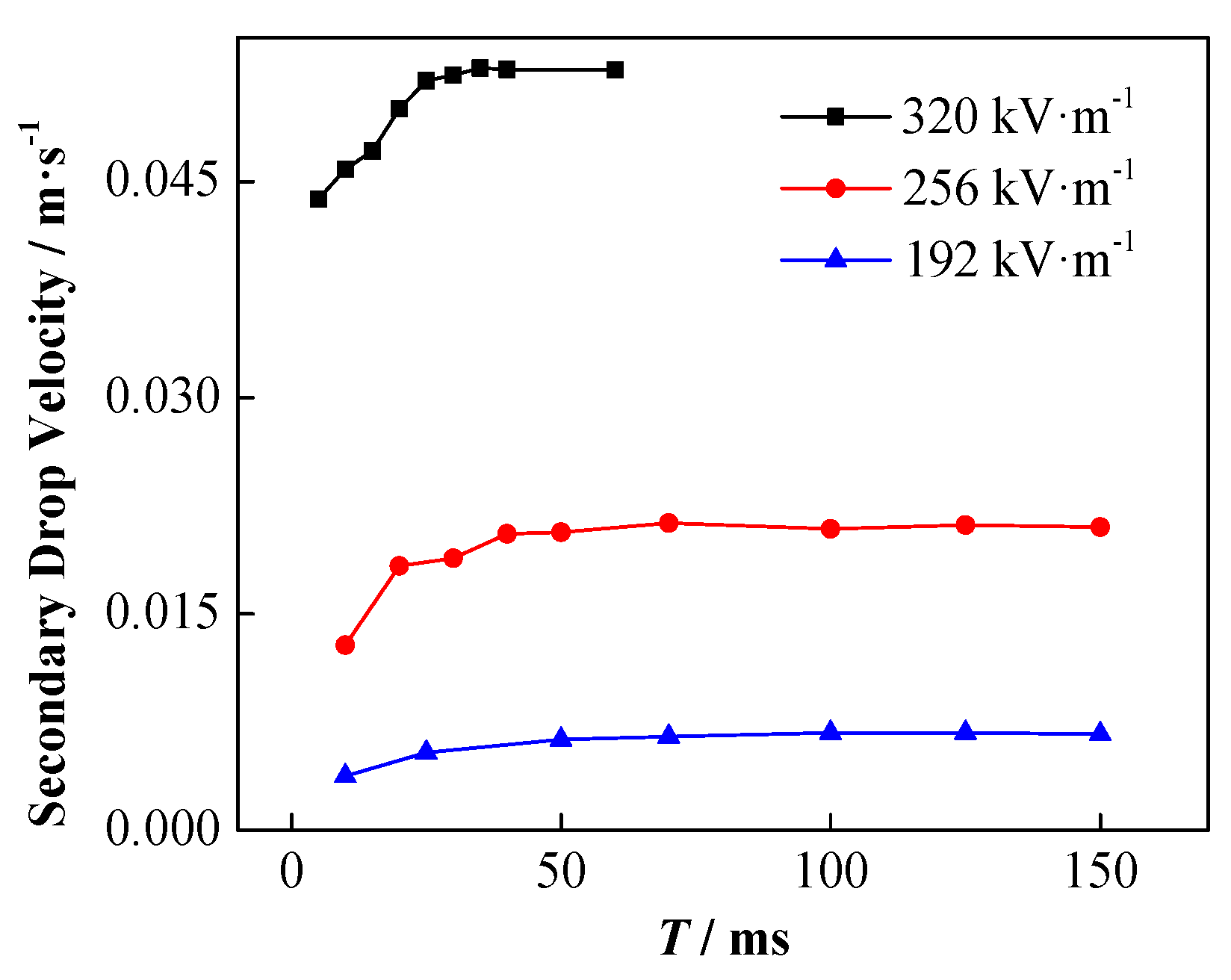
4.2. Effect of Conductivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sofla, S.; James, L.A.; Zhang, Y. Insight into the stability of hydrophilic silica nanoparticles in seawater for enhanced oil recovery implications. Fuel 2018, 216, 559–571. [Google Scholar] [CrossRef]
- Cohen, C.; Giles, R.; Sergeyeva, V.; Mittal, N.; Tabeling, P.; Zerrouki, D.; Baudry, J.; Bibette, J.; Bremond, N. Parallelised production of fine and calibrated emulsions by coupling flow-focusing technique and partial wetting phenomenon. Microfluid. Nanofluid. 2014, 17, 959–966. [Google Scholar] [CrossRef]
- Youssif, M.I.; El-Maghraby, R.M.; Saleh, S.; Elgibaly, A. Silica nanofluid flooding for enhanced oil recovery in sandstone rocks. Egypt. J. Pet. 2017, 27, 105–110. [Google Scholar] [CrossRef]
- Ragab, A.M.S.; Hannora, A.E. An experimental investigation of silica nano particles for enhanced oil recovery applications. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015. [Google Scholar] [CrossRef]
- Hu, Z.; Azmi, S.M.; Raza, G.; Glover, P.W.J.; Wen, D. Nanoparticle-assisted water-flooding in berea sandstones. Energy Fuels 2016, 30, 2791–2804. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Mhatre, S.; Vivacqua, V.; Ghadiri, M.; Abdullah, A.; Al-Marri, M.; Hassanpour, A.; Hewakandamby, B.; Azzopardi, B.; Kermani, B. Electrostatic phase separation: A review. Chem. Eng. Res. Des. 2015, 96, 177–195. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, L.; Cui, X.; Chen, J.; Zeng, H. Intermolecular and surface forces at solid/oil/water/gas interfaces in petroleum production. J. Colloid Interface Sci. 2019, 537, 505–519. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, D.; Shi, L.; Wu, H.; Cao, Y.; He, Y.; Xie, T. Properties of nano-fluids and their applications in enhanced oil recovery: A comprehensive review. Energy Fuel 2020, 34, 1202–1218. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; Mantilla, C.A.; Berg, F.G.A.V.D.; Zeng, H. Interaction mechanism of oil-in-water emulsions with asphaltenes determined using droplet probe AFM. Langmuir 2016, 32, 2302–2310. [Google Scholar] [CrossRef] [PubMed]
- Ragab, A.M.S.; Hannora, A.E. A comparative investigation of nano particle effects for improved oil recovery—Experimental work. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 11–14 October 2015. [Google Scholar] [CrossRef]
- Tarek, M. Investigating nano-fluid mixture effects to enhance oil recovery. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar] [CrossRef] [Green Version]
- Rondón, M.; Bouriat, P.; Lachaise, J.; Salager, J.-L. Breaking of water-in-crude oil emulsions. 1. Physicochemical phenomenology of demulsifier action. Energy Fuels 2008, 20, 1600–1604. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A.O.; Williams, T.J. Electrostatic enhancement of coalescence of water droplets in oil: A review of the current understanding. J. Chem. Eng. 2001, 84, 173–192. [Google Scholar] [CrossRef]
- Yang, D.; Xu, M.; He, L.; Luo, X.; Lü, Y.; Yan, H.; Tian, C. The influence and optimisation of electrical parameters for enhanced coalescence under pulsed DC electric field in a cylindrical electrostatic coalesce. Chem. Eng. Sci. 2015, 138, 71–85. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M.; Sharif, A. Electrostatic and hydrodynamic separation of aqueous droplets in a flowing viscous oil. Chem. Eng. Process. 2002, 41, 649–657. [Google Scholar] [CrossRef]
- Drelich, J. The effect of electric field pulsation frequency on breaking water-in-oil emulsions. Fuel Process. Technol. 1992, 31, 105–113. [Google Scholar] [CrossRef]
- Mhatre, S.; Thaokar, R. Electrocoalescence in non-uniform electric fields: An experimental study. Chem. Eng. Process 2015, 96, 28–38. [Google Scholar] [CrossRef]
- Mhatre, S.; Simon, S.; Sjöblom, J. Shape evolution of a water drop in asphaltene solution under weak DC electric fields. Chem. Eng. Res. Des. 2019, 141, 540–549. [Google Scholar] [CrossRef]
- Taylor, G. Disintegration of water droplets in an electric field. Proc. R. Soc. Lond. 1964, 280, 383–397. [Google Scholar]
- Chiesa, M.; Ingebrigtsen, S.; Melheim, J.; Hemmingsen, P.; Hansen, E.; Hestad, Ø. Investigation of the role of viscosity on electrocoalescence of water droplets in oil. Sep. Purif. Technol. 2006, 50, 267–277. [Google Scholar] [CrossRef]
- Yang, D.; Ghadiri, M.; Sun, Y.; He, L.; Luo, X.; Lü, Y. Critical electric field strength for partial coalescence of droplets on oil–water interface under DC electric field. Chem. Eng. Res. Des. 2018, 136, 83–93. [Google Scholar] [CrossRef]
- Blanchette, F.; Bigioni, T.P. Partial coalescence of droplets at liquid interfaces. Nat. Phys. 2006, 2, 254–257. [Google Scholar] [CrossRef]
- Charles, G.E.; Mason, S.G. The mechanism of partial coalescence of liquid droplets at liquid/liquid interfaces. J. Colloid Sci. 1960, 15, 105–122. [Google Scholar] [CrossRef]
- Charles, G.E.; Mason, S.G. The coalescence of liquid droplets with flat liquid/liquid interface. J. Colloid Sci. 1960, 15, 236–267. [Google Scholar] [CrossRef]
- Chen, X.; Mandre, S.; Feng, J.J. Partial coalescence between a droplet and a liquid-liquid interface. Phys. Fluids 2006, 18, 051705. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, J.D.; Carmigniani, R.; Kannan, A.; Burton, J.C.; Nagel, S.R. Coalescence of bubbles and droplets in an outer fluid. Nat. Commun. 2014, 5, 3182. [Google Scholar] [CrossRef] [Green Version]
- Mittal, N.; Cohen, C.; Bibette, J. Dynamics of step-emulsification: From a single to a collection of emulsion droplet generators. Phys. Fluids 2014, 26, 501. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Catastrophic phase inversion of water-in-oil emulsions stabilized by hydroplethobic silica. Langmuir 2000, 16, 2539–2547. [Google Scholar] [CrossRef]
- Vignati, E.; Piazza, R.; Lockhart, T.P. Pickering emulsions: Interfacial tension, colloidal layer morphology, and trapped-particle motion. Langmuir 2003, 19, 6650–6656. [Google Scholar] [CrossRef]
- Bremond, N.; Thiam, A.R.; Bibette, J. Decompressing emulsion droplets favors coalescence. Phys. Rev. Lett. 2008, 100, 2. [Google Scholar] [CrossRef]
- Liu, Z.; Chan, S.T.; Faizi, H.A.; Roberts, R.C.; Shum, H.C. Droplet-based electro-coalescence for probing threshold disjoining pressure. Lab. A Chip 2015, 15, 2018–2024. [Google Scholar] [CrossRef] [Green Version]
- Flatté, M.E.; Kornyshev, A.A.; Urbakh, M. Understanding voltage-induced localization of nanoparticles at a liquid–liquid interface. J. Phys. Condens. Matter 2008, 20, 073102. [Google Scholar] [CrossRef] [Green Version]
- Musinski, L.; Liu, T.; Gilchrist, B.; Gallimore, A.; Keidar, M. Nanoparticle electric propulsion: Experimental results. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9−12 July 2006; p. 4803. [Google Scholar]
- Russell, T.P.; Cui, M.; Emrick, T. Stabilizing Liquid Droplets of Arbitrary Shape by the Interfacial Jamming of Nanoparticles. U.S. Patent 9,878,296, 30 January 2018. [Google Scholar]
- Chen, G.; Tan, P.; Chen, S.; Huang, J.; Wen, W.; Xu, L. Coalescence of pickering emulsion droplets induced by an electric field. Phys. Rev. Lett. 2013, 110, 064502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, A.B.; Caggioni, M.; Ergun, R.; Hartel, R.W.; Spicer, P.T. Arrested coalescence in Pickering emulsions. Soft Matter 2011, 7, 7710–7716. [Google Scholar] [CrossRef]
- Harbottle, D.; Bueno, P.; Isaksson, R.; Kretzschmar, I. Coalescence of particle-laden droplets with a planar oil–water interface. J. Colloid Interface Sci. 2011, 362, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Connington, K.W.; Miskin, M.Z.; Lee, T.; Jaeger, H.M.; Morris, J.F. Lattice Boltzmann simulations of particle-laden liquid bridges: Effects of volume fraction and wettability. Int. J. Multiphas Flow. 2015, 76, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Sun, Y.; He, L.; Luo, X.; Lü, Y.; Yin, H.; Xia, X.; Zhang, H. Coalescence characteristics of silica nanoparticle-laden droplets with a planar interface under direct current electric field. Chem. Eng. Res. Des. 2018, 140, 128–141. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, D.; Sun, H.; Wu, H.; Chang, Q.; Shi, L.; Cao, Y.; He, Y.; Xie, T. Experimental study on the falling and coalescence characteristics of droplets under alternating electric fields. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125136. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; He, L.; Luo, X.; Lü, Y.; Qiao, H.; Liu, J.; Liu, S. The droplet-interface coalescence characteristics of water containing nanoparticles in oil under electric fields of different waveforms. Chem. Eng. Process. 2018, 132, 89–97. [Google Scholar] [CrossRef]
- Davis, R.H.; Schonberg, J.A.; Rallison, J.M. The lubrication force between two viscous drops. Phys. Fluids A 1989, 1, 77–81. [Google Scholar] [CrossRef]
- Klingenberg, D.J.; Swol, F.V.; Zukoski, C.F. The small shear rate response of electrorheological suspensions. I. Simulation in the point–dipole limit. J. Chem. Phys. 1991, 94, 6170–6178. [Google Scholar] [CrossRef]
- Siu, L.Y.; Wan, J.K.T.; Yu, W.K. Interparticle force in polydisperse electrorheological fluids: Beyond the dipole approximation. Comput. Phys. Commun. 2001, 142, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, M.; Melheim, J.; Pedersen, A.; Ingebrigtsen, S.; Berg, G. Forces acting on water droplets falling in oil under the influence of an electric field: Numerical predictions versus experimental observations. Eur. J. Mech. B Fluids 2005, 24, 717–732. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Tan, S.H.; Nguyen, N.T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. J. Phys. D Appl. Phys. 2008, 41, 085502. [Google Scholar] [CrossRef]
- Moosavi, M.; Goharshadi, E.K.; Youssefi, A. Fabrication, characterization, and measurement of some physicochemical properties of ZnO nanofluids. Int. J. Heat Fluid Flow. 2010, 31, 599–605. [Google Scholar] [CrossRef]
- Tanvir, S. Surface tension of nanofluid-type fuels containing suspended nanomaterials. Nanoscale Res. Lett. 2012, 7, 226. [Google Scholar]
- Vafaei, S.; Purkayastha, A.; Jain, A.; Ramanath, G.; Borca-Tasciuc, T. The effect of nanoparticles on the liquid–gas surface tension of Bi2Te3 nanofluids. Nanotechnology 2009, 20, 185702. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.C.; Johnson, D. Interfacial tension measurements of colloidal suspensions: An explanation of colloidal particle-driven interfacial flows. Adv. Space Res. 2003, 32, 149–153. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants-similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Clegg, P.S.; Herzig, E.M.; Schofield, A.; Horozov, T.S.; Binks, B.P.; Cates, M.E.; Poon, W.C.K. Colloid-stabilized emulsions: Behaviour as the interfacial tension is reduced. J. Phys. Condens. Matter 2005, 17, 3433–3438. [Google Scholar] [CrossRef]
- Tambe, D.E.; Sharma, M.M. Factors controlling the stability of colloid-stabilized emulsions: III. Measurement of the rheological properties of colloid-laden interfaces. J. Colloid Interface Sci. 1995, 171, 456–462. [Google Scholar] [CrossRef]
- Lishchuk, S.V. Effective surface dilatational viscosity of highly concentrated particle-laden interfaces. Phys. Rev. E 2014, 90, 053005. [Google Scholar] [CrossRef] [PubMed]
- Mousavichoubeh, M.; Shariaty-Niassar, M.; Ghadiri, M. The effect of interfacial tension on secondary droplet formation in electro-coalescence of water droplets in oil. Chem. Eng. Sci. 2011, 66, 5330–5337. [Google Scholar] [CrossRef]
- Weheliye, W.H.; Dong, T.; Angeli, P. Effect of surfactants on droplet coalescence at liquid/liquid interfaces. Chem. Eng. Sci. 2016, 161, 215–227. [Google Scholar] [CrossRef]
- Ariyapadi, S.; Balachandar, R.; Berruti, F. Effect of surfactant on the characteristics of a droplet-laden jet. Chem. Eng. Process. 2004, 43, 547–553. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M. Motion, deformation and break-up of aqueous droplets in oils under high electric field strengths. Chem. Eng. Process. 2003, 42, 259–272. [Google Scholar] [CrossRef]
- Szakáll, M.; Diehl, K.; Mitra, S.K.; Borrmann, S. A wind tunnel study on the shape, oscil-lation, and internal circulation of large raindroplets with sizes between 2.5 and 7.5 mm. J. Atmos. Sci. 2009, 66, 150–157. [Google Scholar] [CrossRef]
- Vivacqua, V.; Ghadiri, M.; Abdullah, A.; Hassanpour, A.; Al-Marri, M.; Azzopardi, B.; Hewakandamby, B.; Kermani, B. Analysis of partial electrocoalescence by Level-Set and finite element methods. Chem. Eng. Res. Des. 2016, 114, 180–189. [Google Scholar] [CrossRef]
- Midtgård, O.M. Electrostatic field theory and circuit analysis in the design of coalescers with pulsed dc voltage. Chem. Eng. J. 2009, 151, 168–175. [Google Scholar] [CrossRef]
- Hamlin, B.S.; Creasey, J.C.; Ristenpart, W.D. Electrically tunable partial coalescence of oppositely charged drops. Phys. Rev. Lett. 2012, 109, 094501. [Google Scholar] [CrossRef] [PubMed] [Green Version]




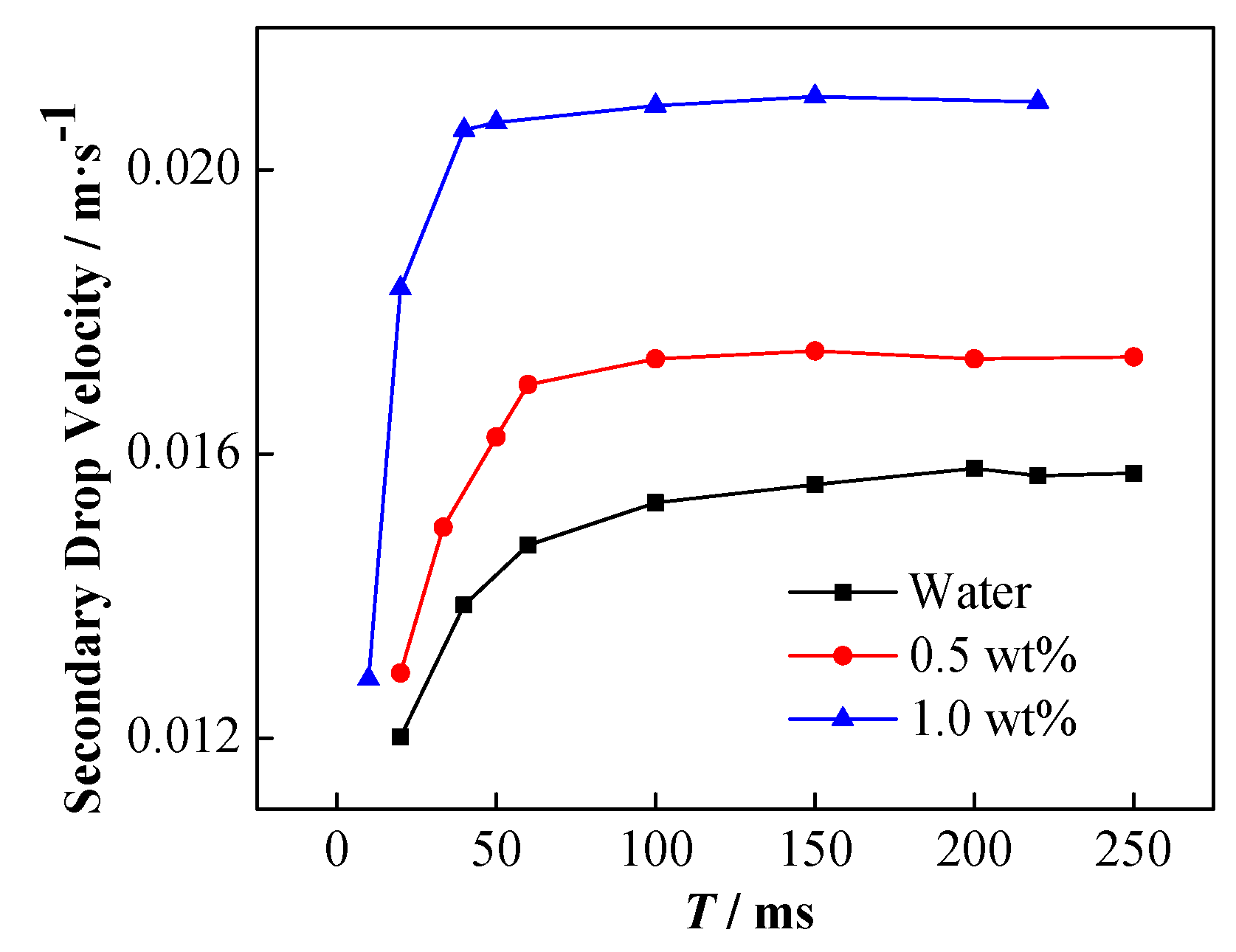
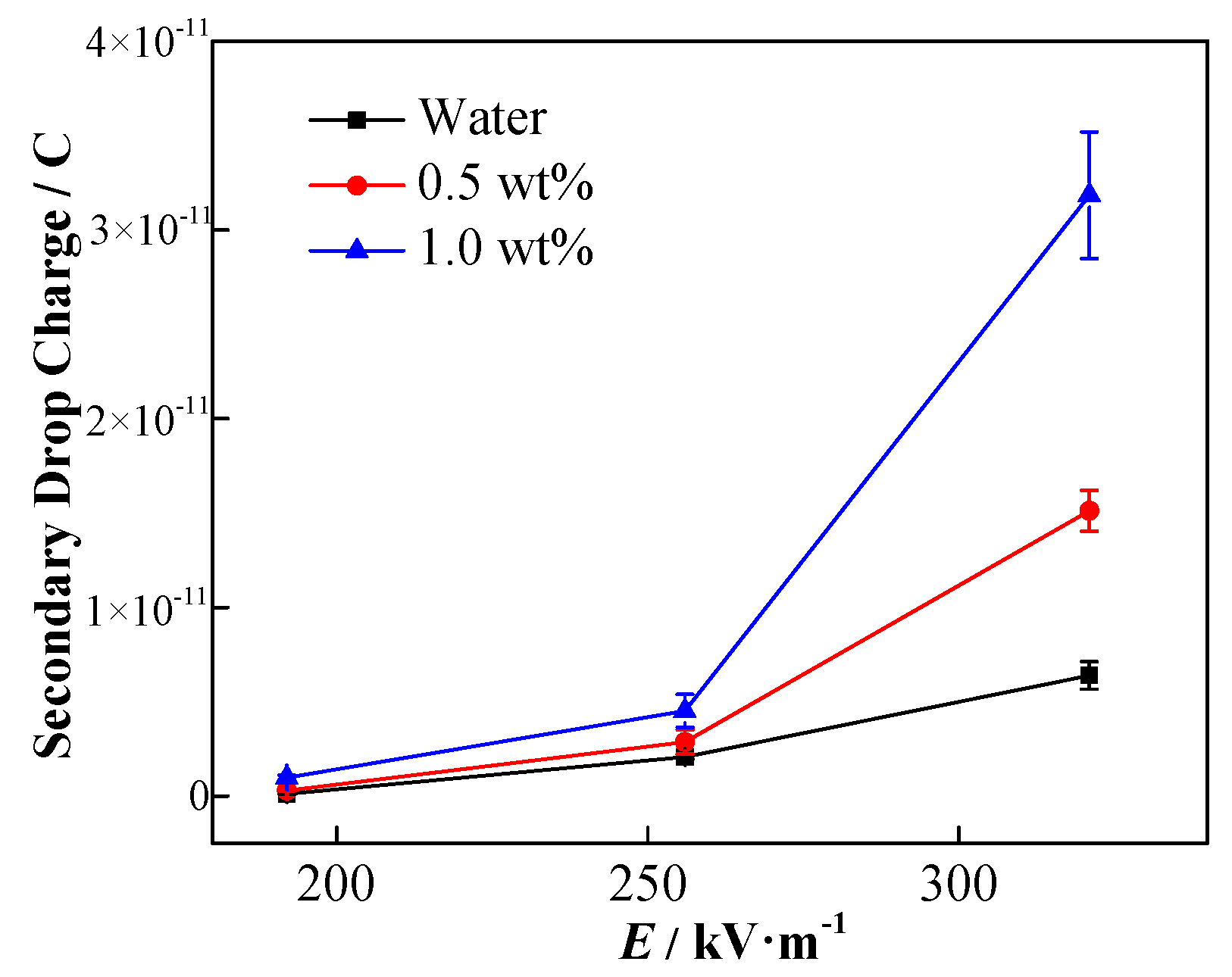
| Conductivity (μS·cm−1) | Viscosity (mPa·s) | Density (kg·m−3) | Interfacial Tension (mN·m−1) | |
|---|---|---|---|---|
| Deionized water | 1.57 | 1.046 | 1000 | 35.68 |
| Dimethyl silicone oil | 2.3 × 10−7 | 156.1 | 963 | |
| 0.5 wt% | 31.2 | 1.089 | 1015 | 4.56 |
| 1 wt% | 58.3 | 1.124 | 1016 | 9.57 |
| 2 wt% | 108.6 | 1.212 | 1020 | 34.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Sun, H.; Chang, Q.; Sun, Y.; He, L. Study on the Effect of Nanoparticle Used in Nano-Fluid Flooding on Droplet–Interface Electro-Coalescence. Nanomaterials 2021, 11, 1764. https://doi.org/10.3390/nano11071764
Yang D, Sun H, Chang Q, Sun Y, He L. Study on the Effect of Nanoparticle Used in Nano-Fluid Flooding on Droplet–Interface Electro-Coalescence. Nanomaterials. 2021; 11(7):1764. https://doi.org/10.3390/nano11071764
Chicago/Turabian StyleYang, Donghai, Huayao Sun, Qing Chang, Yongxiang Sun, and Limin He. 2021. "Study on the Effect of Nanoparticle Used in Nano-Fluid Flooding on Droplet–Interface Electro-Coalescence" Nanomaterials 11, no. 7: 1764. https://doi.org/10.3390/nano11071764