Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers
Abstract
:1. Biopolymers, an Inspiration for Designing Smart Surface-Active Polymers
Evolution of Amphipathic Polymers in Biological Systems
2. Natural Biopolymers as Tools for Spontaneous Reconstruction of Biomembrane Assemblies
2.1. Protein-Based Approach
2.2. DNA-Based Amphipathic Polymers
2.3. “Sweet” Sugar-Based Amphiphilic Polymers (SBAPs)
3. Synthetic Polymers for Reconstitution of Membrane Assemblies
3.1. Short Synthetic Amphipathic Polymers (Amphipols)
3.2. Long Amphipathic Polymers
3.3. Derivatization of Amphipathic Polymers
Author Contributions
Funding
Conflicts of Interest
References
- Stupp, S.I.; Zha, R.H.; Palmer, L.C.; Cui, H.; Bitton, R. Self-assembly of biomolecular soft matter. Faraday Discuss. 2013, 166, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, Z.; Xu, B. Bioinspired assembly of small molecules in cell milieu. Chem. Soc. Rev. 2017, 46, 2421–2436. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, L. Polymer science and biology: Structure and dynamics at multiple scales. Faraday Discuss. 2008, 139, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
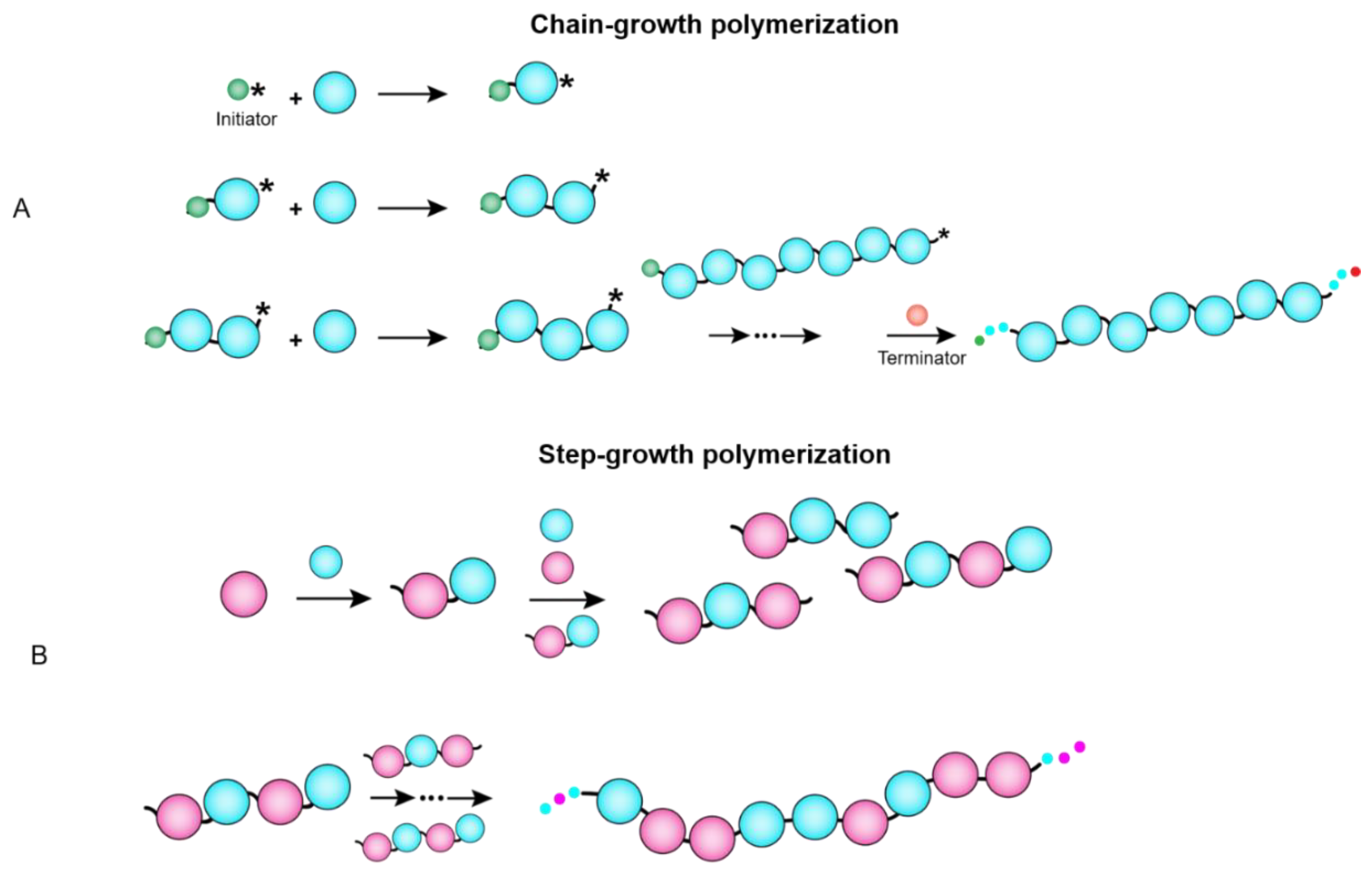
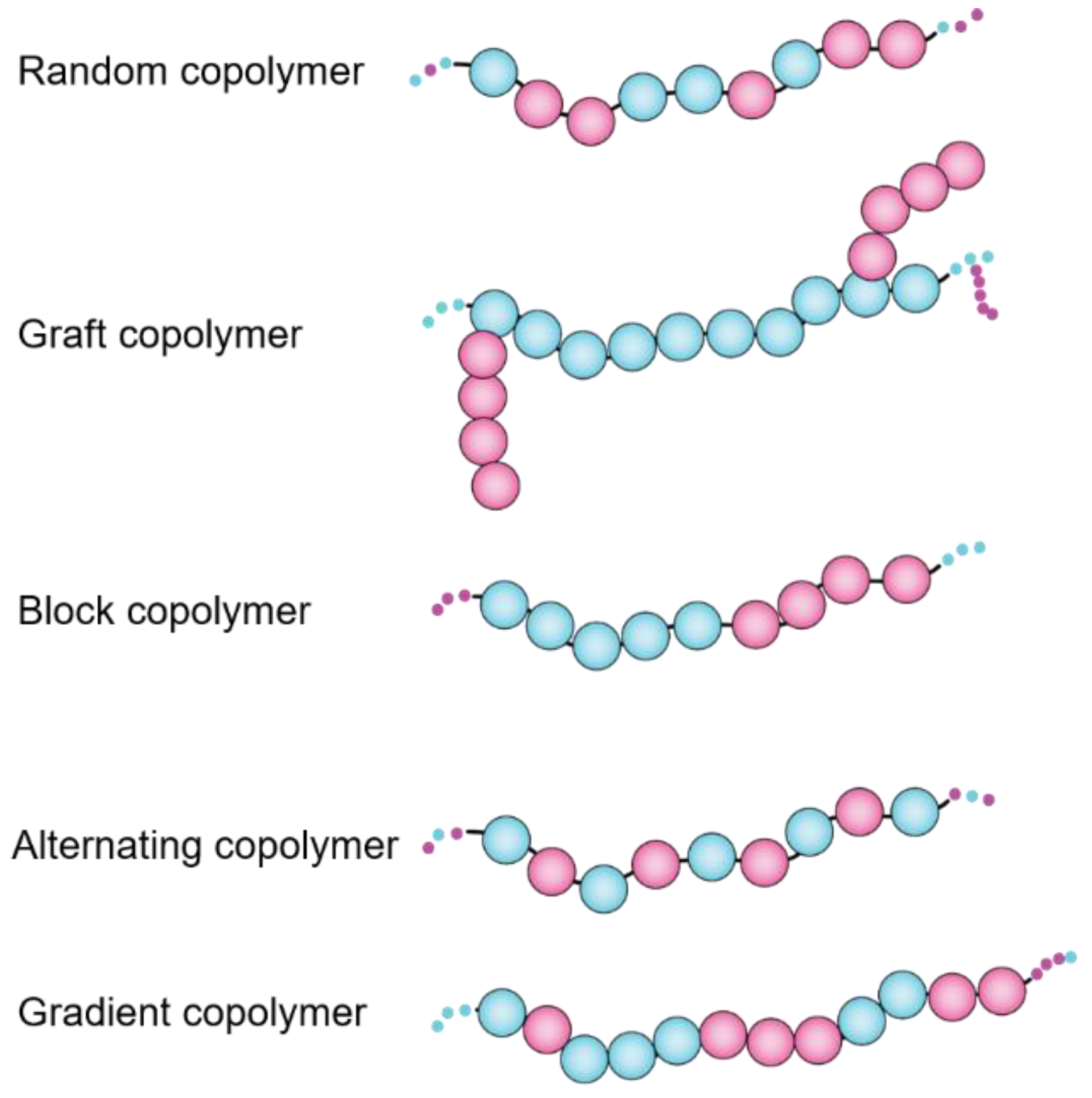
- Huang, J.; Turner, S.R. Recent advances in alternating copolymers: The synthesis, modification, and applications of precision polymers. Polymer 2017, 116, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Friedel, R. Polymer Pioneers—A Popular History of the Science and Technology of Large Molecules. Peter JT Morris. Isis 1987, 78, 275–276. [Google Scholar] [CrossRef]
- Kontopoulou, M.; Wang, W.; Gopakumar, T.; Cheung, C. Effect of composition and comonomer type on the rheology, morphology and properties of ethylene-α-olefin copolymer/polypropylene blends. Polymer 2003, 44, 7495–7504. [Google Scholar] [CrossRef]
- McLean, M.A.; Gregory, M.C.; Sligar, S.G. Nanodiscs: A Controlled Bilayer Surface for the Study of Membrane Proteins. Annu. Rev. Biophys. 2018. [Google Scholar] [CrossRef]
- Rader, D.J. Molecular regulation of HDL metabolism and function: Implications for novel therapies. J. Clin. Investig. 2006, 116, 3090–3100. [Google Scholar] [CrossRef] [Green Version]
- Nath, A.; Atkins, W.M.; Sligar, S.G. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 2007, 46, 2059–2069. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Esmaili, M.; Ghaffari, S.M.; Moosavi-Movahedi, Z.; Atri, M.S.; Sharifizadeh, A.; Farhadi, M.; Yousefi, R.; Chobert, J.-M.; Haertlé, T.; Moosavi-Movahedi, A.A. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT Food Sci. Technol. 2011, 44, 2166–2172. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Mishra, V.K.; Kurtz, J.A.; Cao, D.; Klon, A.E.; Harvey, S.C.; Anantharamaiah, G.M.; Segrest, J.P. Double belt structure of discoidal high density lipoproteins: Molecular basis for size heterogeneity. J. Mol. Biol. 2004, 343, 1293–1311. [Google Scholar] [CrossRef]
- Segrest, J.P.; Jackson, R.L.; Morrisett, J.D.; Gotto, A.M., Jr. A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974, 38, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2002, 2, 853–856. [Google Scholar] [CrossRef]
- Carlson, J.W.; Jonas, A.; Sligar, S.G. Imaging and manipulation of high-density lipoproteins. Biophys. J. 1997, 73, 1184–1189. [Google Scholar] [CrossRef] [Green Version]
- Bayburt, T.H.; Carlson, J.W.; Sligar, S.G. Reconstitution and imaging of a membrane protein in a nanometer-size phospholipid bilayer. J. Struct. Biol. 1998, 123, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, M.A.; Denisov, I.G.; Sligar, S.G. Nanodiscs as a new tool to examine lipid-protein interactions. Methods Mol. Biol. 2013, 974, 415–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sligar, S.G.; Denisov, I.G. Nanodiscs: A toolkit for membrane protein science. Protein Sci. 2021, 30, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Denisov, I.G.; Grinkova, Y.V.; Lazarides, A.A.; Sligar, S.G. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 2004, 126, 3477–3487. [Google Scholar] [CrossRef]
- Grinkova, Y.V.; Denisov, I.G.; Sligar, S.G. Engineering extended membrane scaffold proteins for self-assembly of soluble nanoscale lipid bilayers. Protein Eng. Des. Sel. 2010, 23, 843–848. [Google Scholar] [CrossRef] [Green Version]
- Skar-Gislinge, N.; Simonsen, J.B.; Mortensen, K.; Feidenhans’l, R.; Sligar, S.G.; Lindberg Moller, B.; Bjornholm, T.; Arleth, L. Elliptical structure of phospholipid bilayer nanodiscs encapsulated by scaffold proteins: Casting the roles of the lipids and the protein. J. Am. Chem. Soc. 2010, 132, 13713–13722. [Google Scholar] [CrossRef] [Green Version]
- Alami, M.; Dalal, K.; Lelj-Garolla, B.; Sligar, S.G.; Duong, F. Nanodiscs unravel the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J. 2007, 26, 1995–2004. [Google Scholar] [CrossRef] [Green Version]
- Bayburt, T.H.; Grinkova, Y.V.; Sligar, S.G. Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch. Biochem. Biophys. 2006, 450, 215–222. [Google Scholar] [CrossRef]
- Boldog, T.; Grimme, S.; Li, M.S.; Sligar, S.G.; Hazelbauer, G.L. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc. Natl. Acad. Sci. USA 2006, 103, 11509–11514. [Google Scholar] [CrossRef] [Green Version]
- Carney, C.E.; Lenov, I.L.; Baker, C.J.; MacRenaris, K.W.; Eckermann, A.L.; Sligar, S.G.; Meade, T.J. Nanodiscs as a Modular Platform for Multimodal MR-Optical Imaging. Bioconjug. Chem. 2015, 26, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Zhao, J.; Schatz, G.C.; Sligar, S.G.; Van Duyne, R.P. Screening of Type I and II Drug Binding to Human Cytochrome P450-3A4 in Nanodiscs by Localized Surface Plasmon Resonance Spectroscopy. Anal. Chem. 2009, 81, 3754–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denisov, I.G.; Grinkova, Y.V.; Bayburt, T.H.; Sligar, S.G. Small-angle X-ray scattering study of monodisperse lipid-protein nanodiscs as nanoscale fragments of biological membrane. Biophys. J. 2004, 86, 252A. [Google Scholar]
- Denisov, I.G.; McLean, M.A.; Shaw, A.W.; Grinkova, Y.V.; Sligar, S.G. Thermotropic phase transition in soluble nanoscale lipid bilayers. J. Phys. Chem. B 2005, 109, 15580–15588. [Google Scholar] [CrossRef] [Green Version]
- Nasr, M.L.; Wagner, G. Covalently circularized nanodiscs; challenges and applications. Curr. Opin. Struct. Biol. 2018, 51, 129–134. [Google Scholar] [CrossRef]
- Matsumura, T.; Hu, Z.; Kato, T.; Dreux, M.; Zhang, Y.Y.; Imamura, M.; Hiraga, N.; Juteau, J.M.; Cosset, F.L.; Chayama, K.; et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 2009, 137, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Vaillant, A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antivir. Res. 2016, 133, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Acuna, G.P.; Bucher, M.; Stein, I.H.; Steinhauer, C.; Kuzyk, A.; Holzmeister, P.; Schreiber, R.; Moroz, A.; Stefani, F.D.; Liedl, T. Distance dependence of single-fluorophore quenching by gold nanoparticles studied on DNA origami. ACS Nano 2012, 6, 3189–3195. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Q.; Li, N.; Dai, L.; Liu, Q.; Song, L.; Wang, J.; Li, Y.; Tian, J.; Ding, B. DNA origami as an in vivo drug delivery vehicle for cancer therapy. ACS Nano 2014, 8, 6633–6643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, M.; Hogle, J.M.; Shih, W.M.; Wagner, G.; Nasr, M.L. DNA-corralled nanodiscs for the structural and functional characterization of membrane proteins and viral entry. J. Am. Chem. Soc. 2018, 140, 10639–10643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Faig, A.; Abdelhamid, D.; Uhrich, K. Sugar-based amphiphilic polymers for biomedical applications: From nanocarriers to therapeutics. Acc. Chem. Res. 2014, 47, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. A review of glycosylated carriers for drug delivery. Biomaterials 2012, 33, 4166–4186. [Google Scholar] [CrossRef]
- Oh, T.; Jono, K.; Kimoto, Y.; Hoshino, Y.; Miura, Y. Preparation of multifunctional glycopolymers using double orthogonal reactions and the effect of electrostatic groups on the glycopolymer–lectin interaction. Polym. J. 2019, 51, 1299–1308. [Google Scholar] [CrossRef]
- Ravula, T.; Ramamoorthy, A. Synthesis, Characterization, and Nanodisc formation of Non-ionic Polymers. Angew. Chem. Int. Ed. 2021. [Google Scholar] [CrossRef]
- Popot, J.L.; Althoff, T.; Bagnard, D.; Baneres, J.L.; Bazzacco, P.; Billon-Denis, E.; Catoire, L.J.; Champeil, P.; Charvolin, D.; Cocco, M.J.; et al. Amphipols from A to Z. Annu. Rev. Biophys. 2011, 40, 379–408. [Google Scholar] [CrossRef]
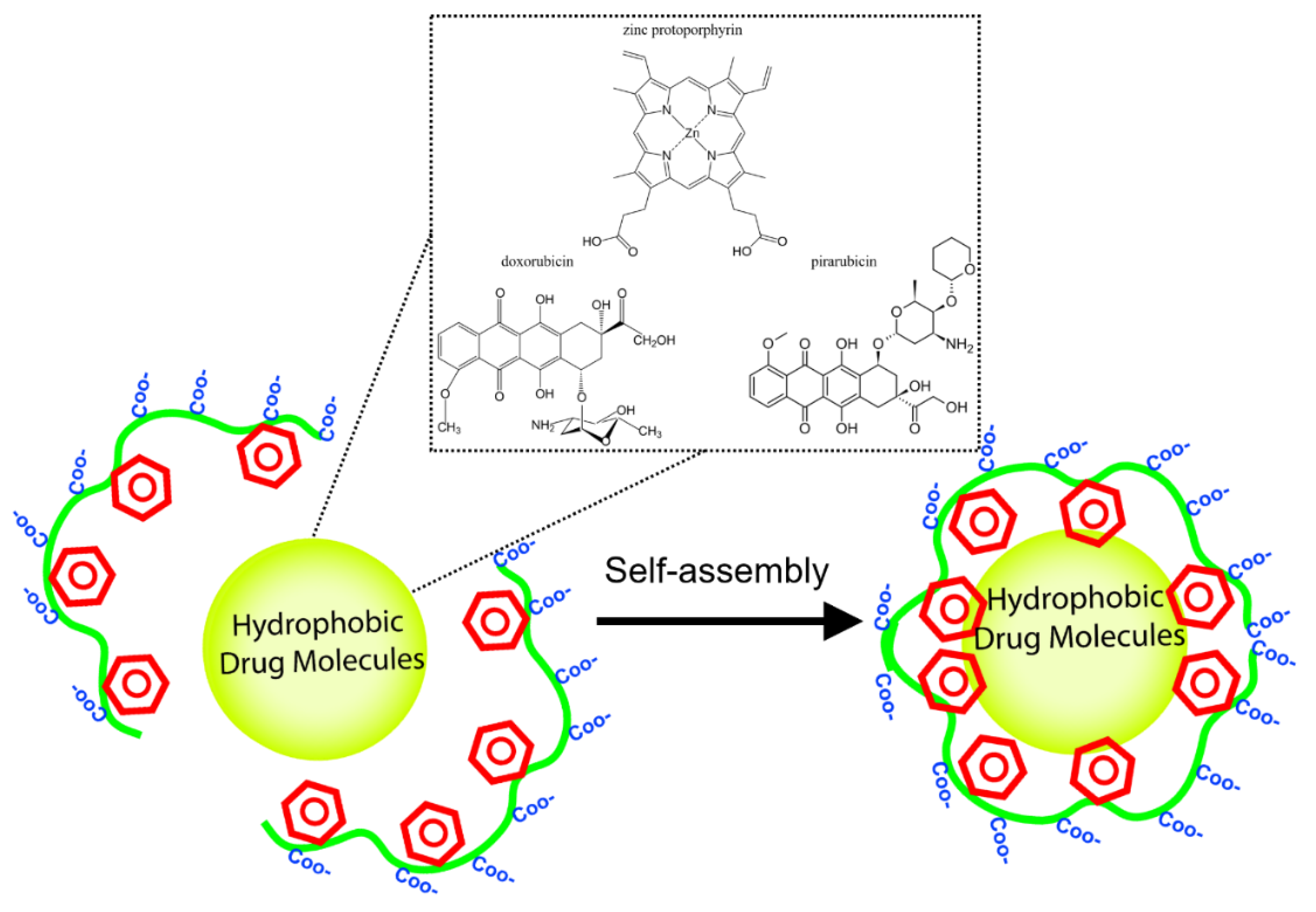
- Iyer, A.K.; Greish, K.; Fang, J.; Murakami, R.; Maeda, H. High-loading nanosized micelles of copoly(styrene-maleic acid)-zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials 2007, 28, 1871–1881. [Google Scholar] [CrossRef]
- Tonge, S.R.; Tighe, B.J. Responsive hydrophobically associating polymers: A review of structure and properties. Adv. Drug Deliv. Rev. 2001, 53, 109–122. [Google Scholar] [CrossRef]
- Greish, K.; Nagamitsu, A.; Fang, J.; Maeda, H. Copoly(styrene-maleic acid)—Pirarubicin micelles: High tumor-targeting efficiency with little toxicity. Bioconjug. Chem. 2005, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Sawa, T.; Fang, J.; Akaike, T.; Maeda, H. SMA-doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J. Control. Release 2004, 97, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. 2010, 1, 149–173. [Google Scholar] [CrossRef] [Green Version]
- Dannhauser, W.; Glaze, W.H.; Dueltgen, R.L.; Ninomiya, K. Evidence from Intrinsic Viscosity and Sedimentation for Hypercoiled Configurations of Styrene-Maleic Acid Copolymer. J. Phys. Chem. 1960, 64, 954–955. [Google Scholar] [CrossRef]
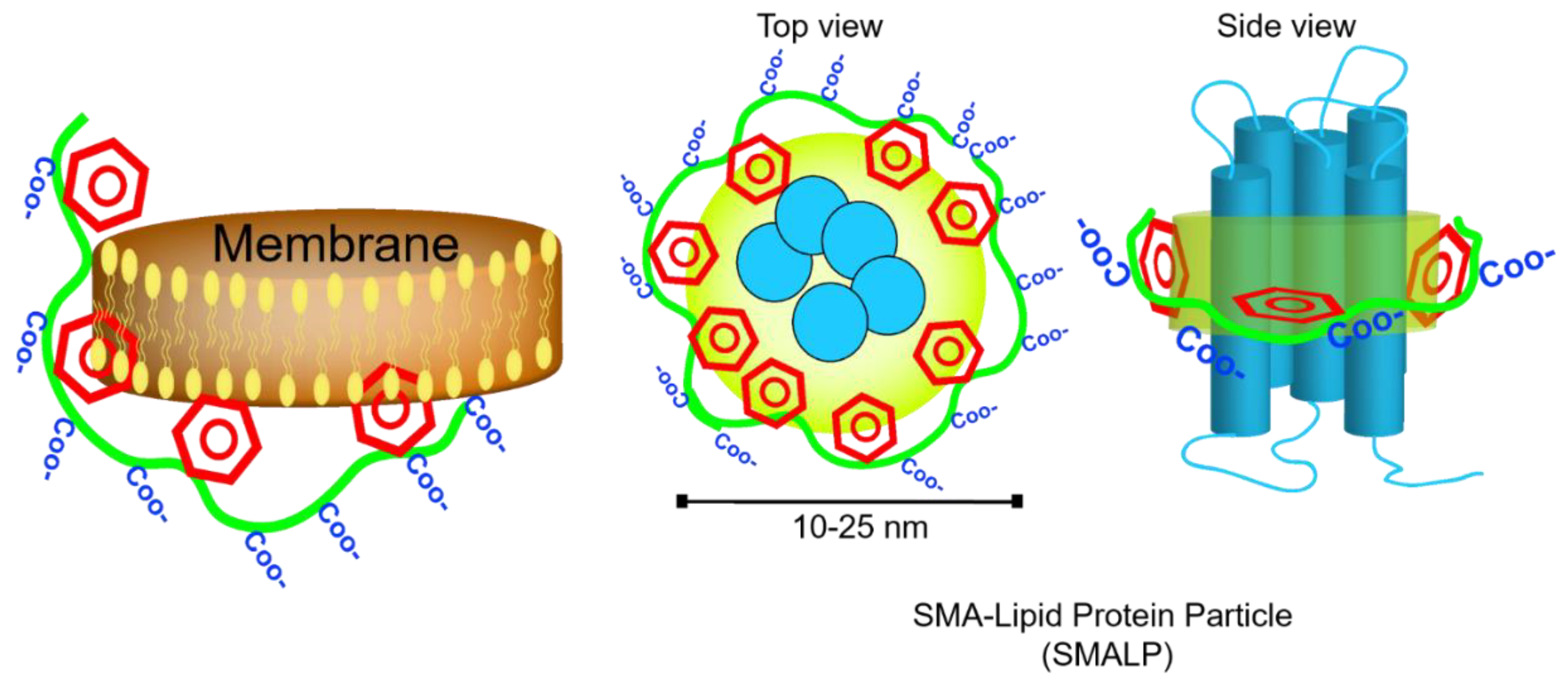
- Orwick, M.C.; Judge, P.J.; Procek, J.; Lindholm, L.; Graziadei, A.; Engel, A.; Grobner, G.; Watts, A. Detergent-free formation and physicochemical characterization of nanosized lipid-polymer complexes: Lipodisq. Angew. Chem. Int. Ed. 2012, 51, 4653–4657. [Google Scholar] [CrossRef]
- Knowles, T.J.; Finka, R.; Smith, C.; Lin, Y.P.; Dafforn, T.; Overduin, M. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. J. Am. Chem. Soc. 2009, 131, 7484–7485. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Larson, R.G. Coarse-grained molecular dynamics studies of the concentration and size dependence of fifth- and seventh-generation PAMAM dendrimers on pore formation in DMPC bilayer. J. Phys. Chem. B 2008, 112, 7778–7784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of Poloxamer Molecules and Poloxamer Micelles Dissolved in Water and Next to Lipid Bilayers: Results from Computer Simulations. J. Phys. Chem. B 2016, 120, 5823–5830. [Google Scholar] [CrossRef]
- Shih, A.Y.; Freddolino, P.L.; Arkhipov, A.; Schulten, K. Assembly of lipoprotein particles revealed by coarse-grained molecular dynamics simulations. J. Struct. Biol. 2007, 157, 579–592. [Google Scholar] [CrossRef]
- Xue, M.M.; Cheng, L.S.; Faustino, I.; Guo, W.L.; Marrink, S.J. Molecular Mechanism of Lipid Nanodisk Formation by Styrene-Maleic Acid Copolymers. Biophys. J. 2018, 115, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Orekhov, P.S.; Bozdaganyan, M.E.; Voskoboynikova, N.; Mulkidjanian, A.Y.; Steinhoff, H.J.; Shaitan, K.V. Styrene/Maleic Acid Copolymers Form SMALPs by Pulling Lipid Patches out of the Lipid Bilayer. Langmuir 2019, 35, 3748–3758. [Google Scholar] [CrossRef]
- Bjørnestad, V.A.; Orwick-Rydmark, M.; Lund, R. Understanding the Structural Pathways for Lipid Nanodisc Formation: How Styrene Maleic Acid Copolymers Induce Membrane Fracture and Disc Formation. Langmuir 2021, 37, 6178–6188. [Google Scholar] [CrossRef] [PubMed]
- Rydmark, M.O.; Christensen, M.K.; Köksal, E.S.; Kantarci, I.; Kustanovich, K.; Yantchev, V.; Jesorka, A.; Gözen, I. Styrene maleic acid copolymer induces pores in biomembranes. Soft Matter 2019, 15, 7934–7944. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Knowles, T.J.; Postis, V.L.; Jamshad, M.; Parslow, R.A.; Lin, Y.-P.; Goldman, A.; Sridhar, P.; Overduin, M.; Muench, S.P. A method for detergent-free isolation of membrane proteins in their local lipid environment. Nat. Protoc. 2016, 11, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Barniol-Xicota, M.; Verhelst, S.H.L. Lipidomic and in-gel analysis of maleic acid co-polymer nanodiscs reveals differences in composition of solubilized membranes. Commun. Biol. 2021, 4, 218. [Google Scholar] [CrossRef]
- Morrison, K.A.; Akram, A.; Mathews, A.; Khan, Z.A.; Patel, J.H.; Zhou, C.; Hardy, D.J.; Moore-Kelly, C.; Patel, R.; Odiba, V.; et al. Membrane protein extraction and purification using styrene-maleic acid (SMA) copolymer: Effect of variations in polymer structure. Biochem. J. 2016, 473, 4349–4360. [Google Scholar] [CrossRef]
- Korotych, O.; Mondal, J.; Gattas-Asfura, K.M.; Hendricks, J.; Bruce, B.D. Evaluation of commercially available styrene-co-maleic acid polymers for the extraction of membrane proteins from spinach chloroplast thylakoids. Eur. Polym. J. 2019, 114, 485–500. [Google Scholar] [CrossRef]
- Scheidelaar, S.; Koorengevel, M.C.; van Walree, C.A.; Dominguez, J.J.; Dorr, J.M.; Killian, J.A. Effect of Polymer Composition and pH on Membrane Solubilization by Styrene-Maleic Acid Copolymers. Biophys. J. 2016, 111, 1974–1986. [Google Scholar] [CrossRef] [Green Version]
- Fiori, M.C.; Jiang, Y.; Altenberg, G.A.; Liang, H. Polymer-encased nanodiscs with improved buffer compatibility. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Ravula, T.; Ramadugu, S.K.; Di Mauro, G.; Ramamoorthy, A. Bioinspired, Size-Tunable Self-Assembly of Polymer-Lipid Bilayer Nanodiscs. Angew. Chem. Int. Ed. 2017, 56, 11466–11470. [Google Scholar] [CrossRef]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Cox, S.J.; Ramamoorthy, A. Formation of pH-Resistant Monodispersed Polymer-Lipid Nanodiscs. Angew. Chem. Int. Ed. 2018, 57, 1342–1345. [Google Scholar] [CrossRef]
- Hall, S.; Tognoloni, C.; Charlton, J.; Bragginton, É.; Rothnie, A.; Sridhar, P.; Wheatley, M.; Knowles, T.; Arnold, T.; Edler, K.; et al. An acid-compatible co-polymer for the solubilization of membranes and proteins into lipid bilayer-containing nanoparticles. Nanoscale 2018, 10, 10609–10619. [Google Scholar] [CrossRef] [Green Version]
- Ravula, T.; Hardin, N.Z.; Ramadugu, S.K.; Ramamoorthy, A. pH Tunable and Divalent Metal Ion Tolerant Polymer Lipid Nanodiscs. Langmuir 2017, 33, 10655–10662. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Toward living radical polymerization. Acc. Chem. Res. 2008, 41, 1133–1142. [Google Scholar] [CrossRef]
- Esmaili, M.; Brown, C.J.; Shaykhutdinov, R.; Acevedo-Morantes, C.; Wang, Y.L.; Wille, H.; Gandour, R.D.; Turner, S.R.; Overduin, M. Homogeneous nanodiscs of native membranes formed by stilbene–maleic-acid copolymers. Nanoscale 2020, 12, 16705–16709. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Autzen, H.E.; Faust, B.; Mann, J.L.; Muir, B.W.; Howard, S.; Postma, A.; Spakowitz, A.J.; Cheng, Y.; Appel, E.A. Lipid Nanodiscs via Ordered Copolymers. Chem 2020, 6, 2782–2795. [Google Scholar] [CrossRef]
- Lindhoud, S.; Carvalho, V.; Pronk, J.W.; Aubin-Tam, M.E. SMA-SH: Modified Styrene-Maleic Acid Copolymer for Functionalization of Lipid Nanodiscs. Biomacromolecules 2016, 17, 1516–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwole, A.O.; Danielczak, B.; Meister, A.; Babalola, J.O.; Vargas, C.; Keller, S. Solubilization of Membrane Proteins into Functional Lipid-Bilayer Nanodiscs Using a Diisobutylene/Maleic Acid Copolymer. Angew. Chem. Int. Ed. 2017, 56, 1919–1924. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, K.; Arakida, J.; Ravula, T.; Ramadugu, S.K.; Sahoo, B.; Kikuchi, J.; Ramamoorthy, A. Spontaneous Lipid Nanodisc Fomation by Amphiphilic Polymethacrylate Copolymers. J. Am. Chem. Soc. 2017, 139, 18657–18663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielczak, B.; Keller, S. Collisional lipid exchange among DIBMA-encapsulated nanodiscs (DIBMALPs). Eur. Polym. J. 2018, 109, 206–213. [Google Scholar] [CrossRef]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Southan, C.; Sharman, J.L.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Alexander, S.P.H.; Buneman, O.P.; Davenport, A.P.; McGrath, J.C.; Peters, J.A.; et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: Towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016, 44, D1054–D1068. [Google Scholar] [CrossRef] [Green Version]
- Xia, D.; Yu, C.A.; Kim, H.; Xia, J.Z.; Kachurin, A.M.; Zhang, L.; Yu, L.; Deisenhofer, J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science 1997, 277, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Benlekbir, S.; Venkatakrishnan, P.; Wang, Y.; Hong, S.; Hosler, J.; Tajkhorshid, E.; Rubinstein, J.L.; Gennis, R.B. Structure of the alternative complex III in a supercomplex with cytochrome oxidase. Nature 2018, 557, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Fu, Z.; Xu, G.G.; Grassucci, R.A.; Zhang, Y.; Frank, J.; Hendrickson, W.A.; Guo, Y. Structure and activity of lipid bilayer within a membrane-protein transporter. Proc. Natl. Acad. Sci. USA 2018, 115, 12985–12990. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Yamaguchi, A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 2002, 419, 587–593. [Google Scholar] [CrossRef]
- Pos, K.M.; Schiefner, A.; Seeger, M.A.; Diederichs, K. Crystallographic analysis of AcrB. FEBS Lett. 2004, 564, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.W.; McDermott, G.; Zgurskaya, H.I.; Nikaido, H.; Koshland, D.E., Jr. Structural basis of multiple drug-binding capacity of the AcrB multidrug efflux pump. Science 2003, 300, 976–980. [Google Scholar] [CrossRef]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Matsumoto, T.; Yamaguchi, A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 2006, 443, 173–179. [Google Scholar] [CrossRef]
- Seeger, M.A.; Schiefner, A.; Eicher, T.; Verrey, F.; Diederichs, K.; Pos, K.M. Structural asymmetry of AcrB trimer suggests a peristaltic pump mechanism. Science 2006, 313, 1295–1298. [Google Scholar] [CrossRef] [Green Version]
- Ball, L.E.; Riley, L.J.; Hadasha, W.; Pfukwa, R.; Smith, C.J.; Dafforn, T.R.; Klumperman, B. Influence of DIBMA Polymer Length on Lipid Nanodisc Formation and Membrane Protein Extraction. Biomacromolecules 2020, 22, 763–772. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, H.; Lape, R.; Greiner, T.; Du, J.; Lu, W.; Sivilotti, L.; Gouaux, E. Mechanism of gating and partial agonist action in the glycine receptor. Cell 2021, 184, 957–968.e21. [Google Scholar] [CrossRef]
- Deng, Y.N.; Kashtoh, H.; Wang, Q.; Zhen, G.X.; Li, Q.Y.; Tang, L.H.; Gao, H.L.; Zhang, C.R.; Qin, L.; Su, M.; et al. Structure and activity of SLAC1 channels for stomatal signaling in leaves. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Flegler, V.J.; Rasmussen, A.; Rao, S.; Wu, N.; Zenobi, R.; Sansom, M.S.; Hedrich, R.; Rasmussen, T.; Böttcher, B. The MscS-like channel YnaI has a gating mechanism based on flexible pore helices. Proc. Natl. Acad. Sci. USA 2020, 117, 28754–28762. [Google Scholar] [CrossRef] [PubMed]
- McDowall, J.S.; Ntai, I.; Hake, J.; Whitley, P.R.; Mason, J.M.; Pudney, C.R.; Brown, D.R. Steady-State Kinetics of a-Synuclein Ferrireductase Activity Identifies the Catalytically Competent Species. Biochemistry 2017, 56, 2497–2505. [Google Scholar] [CrossRef]
- Broecker, J.; Eger, B.T.; Ernst, O.P. Crystallogenesis of Membrane Proteins Mediated by Polymer-Bounded Lipid Nanodiscs. Structure 2017, 25, 384–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr. Sect. F Struct. Biol. 2015, 71, 3–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Pye, V.E.; Caffrey, M. Experimental phasing for structure determination using membrane-protein crystals grown by the lipid cubic phase method. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 104–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bali, A.P.; Sahu, I.D.; Craig, A.F.; Clark, E.E.; Burridge, K.M.; Dolan, M.T.; Dabney-Smith, C.; Konkolewicz, D.; Lorigan, G.A. Structural characterization of styrene-maleic acid copolymer-lipid nanoparticles (SMALPs) using EPR spectroscopy. Chem. Phys. Lipids 2019, 220, 6–13. [Google Scholar] [CrossRef]
- Hite, R.K.; Li, Z.; Walz, T. Principles of membrane protein interactions with annular lipids deduced from aquaporin-0 2D crystals. EMBO J. 2010, 29, 1652–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubec, M.; Bariås, E.; Furse, S.; Govasli, M.L.; George, V.; Turcu, D.; Iashchishyn, I.; Morozova-Roche, L.; Halskau, Ø. Cholesterol is a strong promotor of an α-Synuclein membrane binding mode that accelerates oligomerization. bioRxiv 2019. [Google Scholar] [CrossRef]


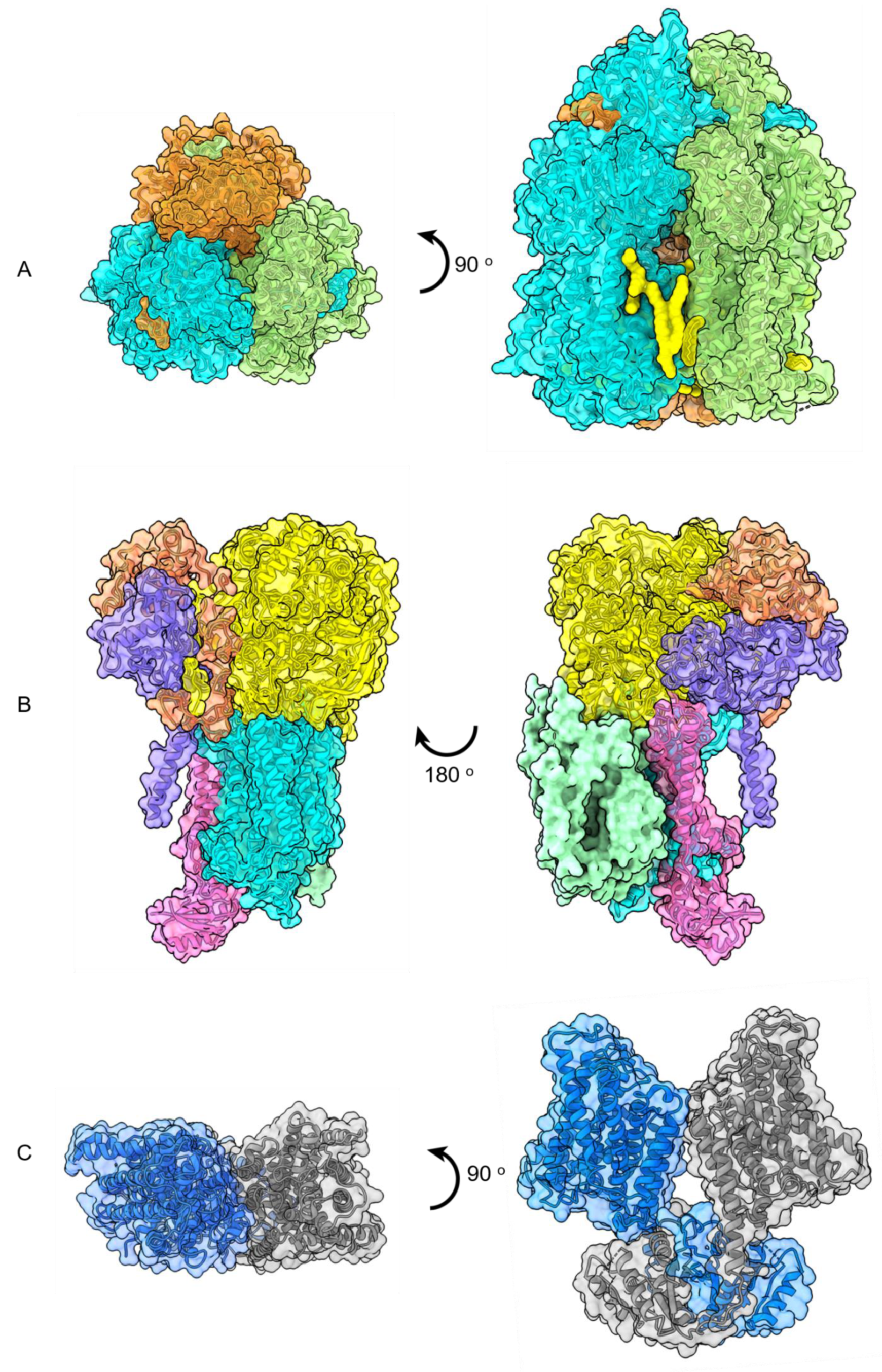
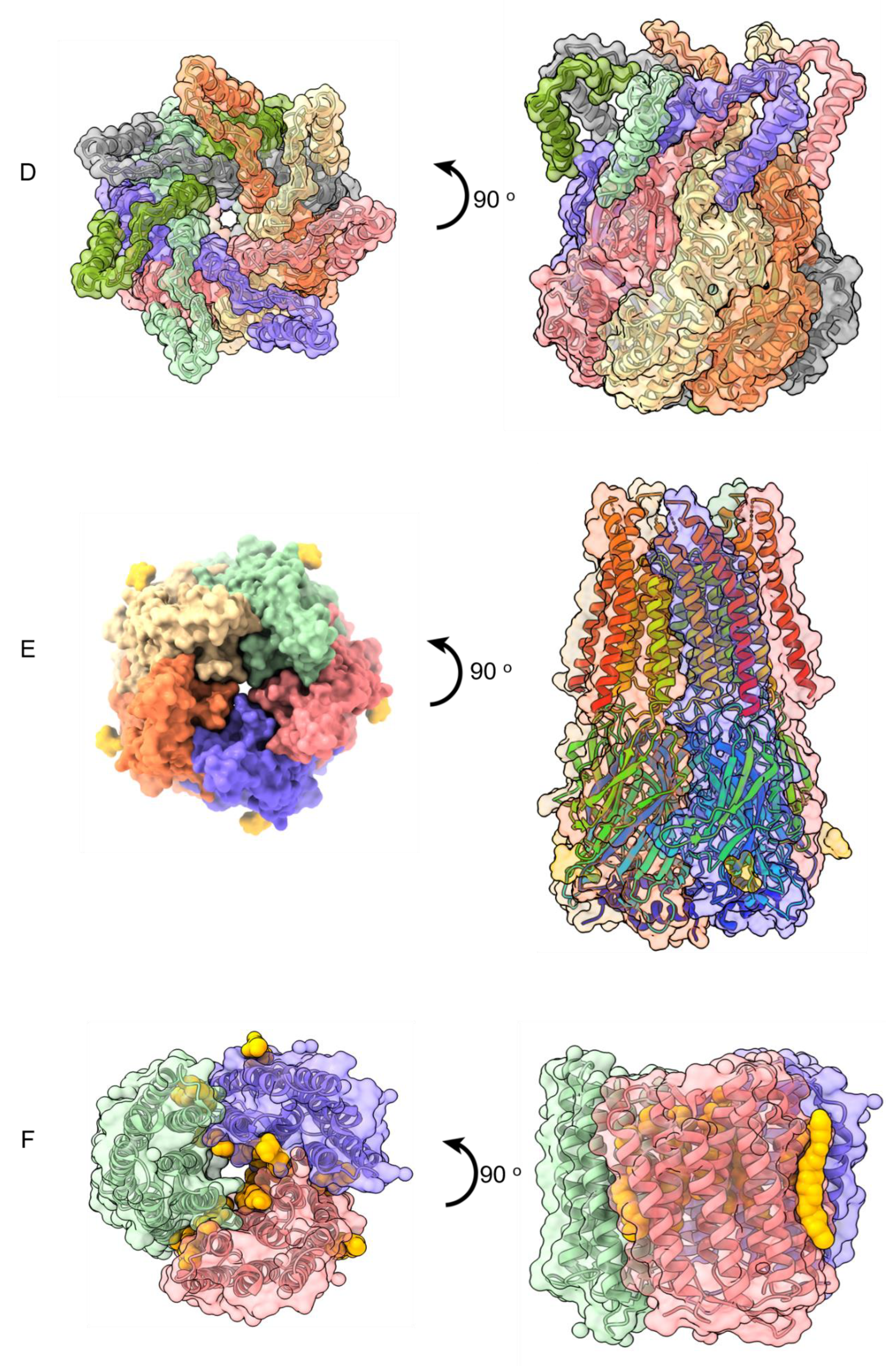
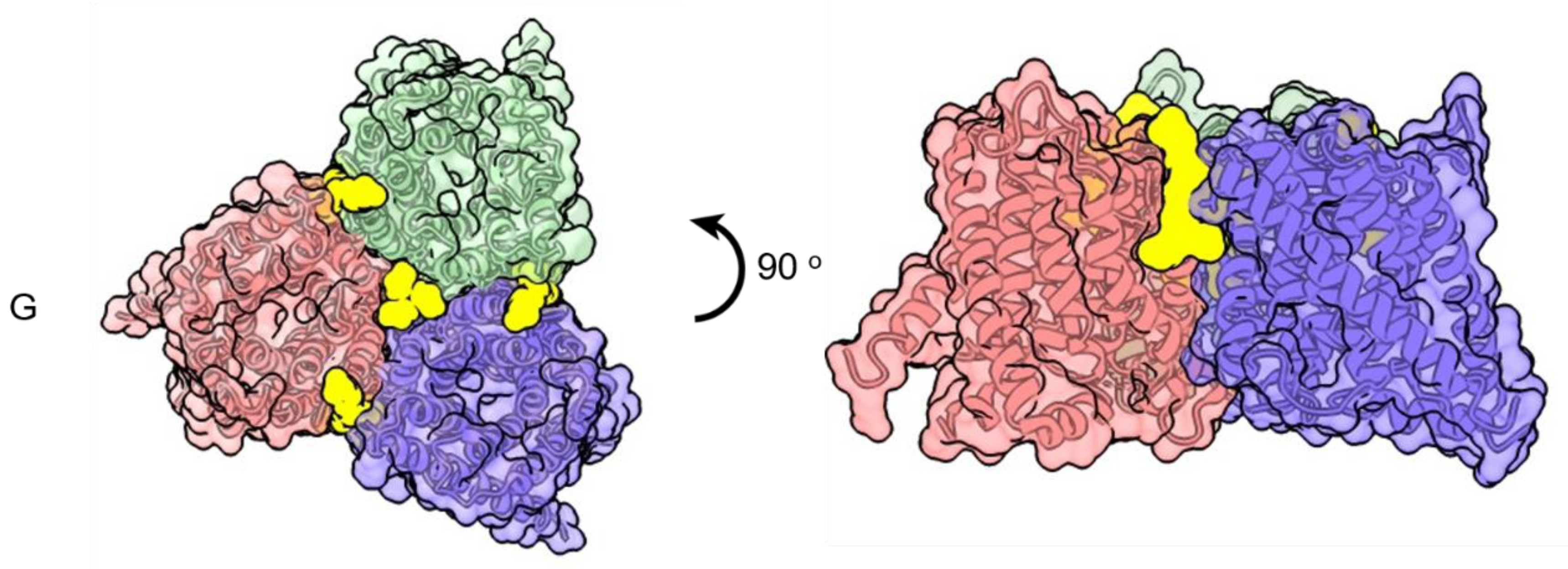
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esmaili, M.; Eldeeb, M.A.; Moosavi-Movahedi, A.A. Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers. Nanomaterials 2021, 11, 1771. https://doi.org/10.3390/nano11071771
Esmaili M, Eldeeb MA, Moosavi-Movahedi AA. Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers. Nanomaterials. 2021; 11(7):1771. https://doi.org/10.3390/nano11071771
Chicago/Turabian StyleEsmaili, Mansoore, Mohamed A. Eldeeb, and Ali Akbar Moosavi-Movahedi. 2021. "Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers" Nanomaterials 11, no. 7: 1771. https://doi.org/10.3390/nano11071771
APA StyleEsmaili, M., Eldeeb, M. A., & Moosavi-Movahedi, A. A. (2021). Current Developments in Native Nanometric Discoidal Membrane Bilayer Formed by Amphipathic Polymers. Nanomaterials, 11(7), 1771. https://doi.org/10.3390/nano11071771







