Multifunctional Tannic Acid-Alendronate Nanocomplexes with Antioxidant, Anti-Inflammatory, and Osteogenic Potency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tannic Acid (TA)-Alendronate (ALN) Nanocomplexes
2.2. Physicochemical Characterization
2.3. In Vitro Cytotoxicity Examination of Each TA-ALN
2.4. Antioxidant Effects
2.4.1. Total Antioxidant Potential
2.4.2. In Vitro Reactive Oxygen Species (ROS) Scavenging Capacity of TA-ALN at Cellular Levels
2.4.3. Defense of Cell Viability under High-ROS Conditions
2.5. Anti-Inflammatory Ability of TA-ALN
2.6. Early Osteogenesis Quantitative Assessment
2.7. Late Osteogenesis Quantitative Assessment
2.8. Assays for Osteogenesis-Related Genes
2.9. Statistical Analysis
3. Results
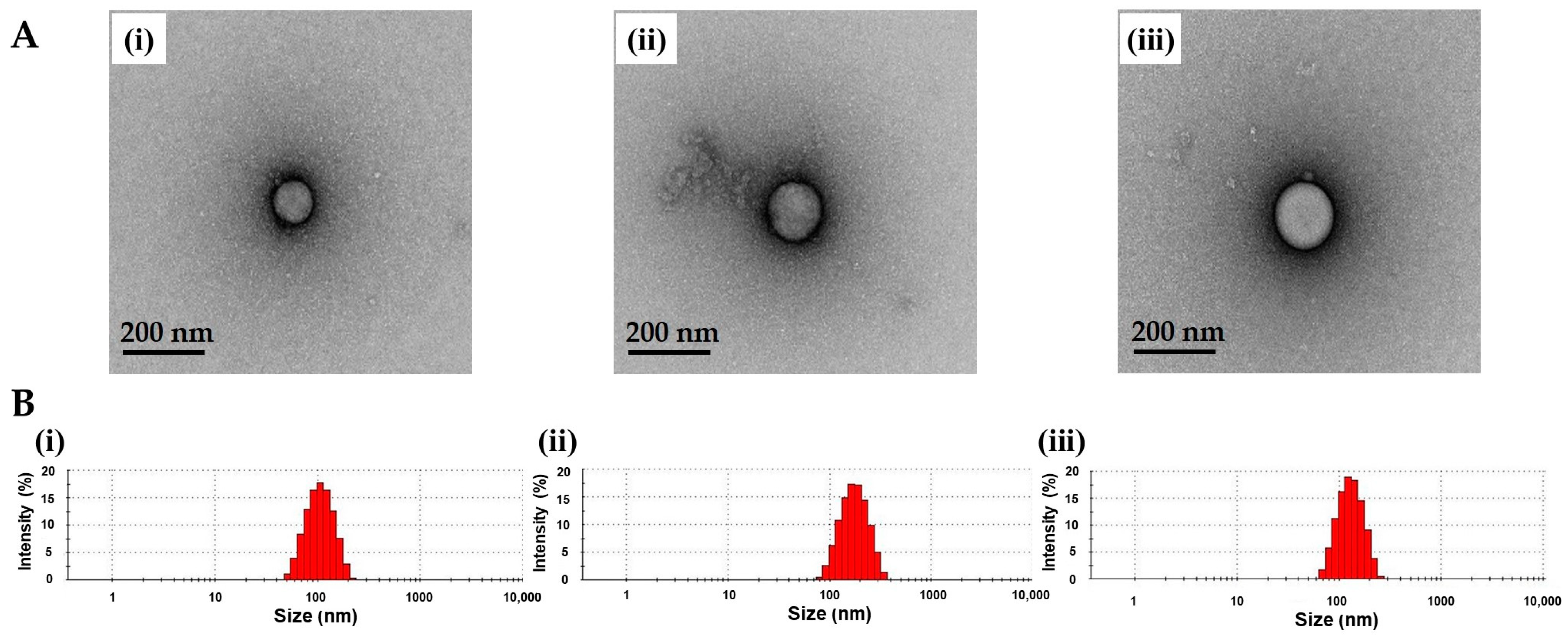
3.1. Characterization of TA-ALN
3.2. Cytotoxic Results of TA-ALN on MC3T3-E1 Cells
3.3. Anti-Oxidant Study
3.3.1. Antioxidant Investigation of TA-ALN
3.3.2. In Vitro ROS Scavenging and Defense Effects of TA-ALN at the Cellular Level
3.4. Anti-Inflammatory Activities of TA-ALN in Inflamed MC3T3-E1 Cells
3.5. Early and Late Osteogenesis Quantitative Assessment
3.6. Measurement of Osteogenesis-Specific Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giljohann, D.A.; Mirkin, C.A. Drivers of biodiagnostic development. Nature 2009, 462, 461–464. [Google Scholar] [CrossRef]
- Colson, Y.L.; Grinstaff, M.W. Biologically responsive polymeric nanoparticles for drug delivery. Adv. Mater. 2012, 24, 3878–3886. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Salamanna, F.; Gambardella, A.; Contartese, D.; Visani, A.; Fini, M. Nano-Based Biomaterials as Drug Delivery Systems Against Osteoporosis: A Systematic Review of Preclinical and Clinical Evidence. Nanomaterials 2021, 11, 530. [Google Scholar] [CrossRef]
- Wang, D.; Miller, S.C.; Kopeckova, P.; Kopecek, J. Bone-targeting macromolecular therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 1049–1076. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.H.; Jo, H.S.; Choi, S.; Song, H.G.; Kim, H.J.; Kim, K.N.; Kim, S.E.; Park, K. Lactoferrin-Anchored Tannylated Mesoporous Silica Nanomaterials for Enhanced Osteo-Differentiation Ability. Pharmaceutics 2020, 13, 30. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Meng, F.F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic Acid-Mediated Surface Functionalization of Polymeric Nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. Int. J. Mol. Sci. 2018, 19, 3602. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.N.; Gu, J.; Jia, S.; Guan, Y.; Zhang, Y. Zero-order release of polyphenolic drugs from dynamic, hydrogen-bonded LBL films. Soft Matter 2016, 12, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhou, B.; Li, J.; Xu, W.; Liu, S.; Li, Y.; Chen, Y.; Li, B. Supramolecular design of coordination bonding architecture on zein nanoparticles for pH-responsive anticancer drug delivery. Colloids Surf. B Biointerfaces 2015, 136, 1224–1233. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater 2013, 101, 560–567. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N.; Silan, C. Inherently antioxidant and antimicrobial tannic acid release from poly(tannic acid) nanoparticles with controllable degradability. Colloids Surf. B Biointerfaces 2016, 142, 334–343. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Hatami, E.; Wagh, S.; Dan, N.; Tripathi, M.K.; Khan, S.; Hafeez, B.B.; Meibohm, B.; Chauhan, S.C.; et al. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J. Colloid Interface Sci. 2019, 535, 133–148. [Google Scholar] [CrossRef]
- Huang, H.; Li, P.; Liu, C.L.; Ma, H.L.; Huang, H.; Lin, Y.C.; Wang, C.; Yang, Y.L. pH-Responsive nanodrug encapsulated by tannic acid complex for controlled drug delivery. RSC Adv. 2017, 7, 2829–2835. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Kozlovskaya, V.; Zavgorodnya, O.; Martinez-Lopez, C.; Catledge, S.; Kharlampieva, E. Encapsulation of anticancer drug by hydrogen-bonded multilayers of tannic acid. Soft Matter 2014, 10, 9237–9247. [Google Scholar] [CrossRef]
- Asadi, E.; Abdouss, M.; Leblanc, R.M.; Ezzati, N.; Wilson, J.N.; Azodi-Deilami, S. In vitro/in vivo study of novel anti-cancer, biodegradable cross-linked tannic acid for fabrication of 5-fluorouracil-targeting drug delivery nano-device based on a molecular imprinted polymer. RSC Adv. 2016, 6, 37308–37318. [Google Scholar] [CrossRef]
- Song, B.; Yang, L.; Han, L.; Jia, L. Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing. Materials 2019, 12, 1803. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.Y.; Liu, Y.M.; Chang, R.; Xing, R.R.; Yan, X.H. Supramolecular Photothermal Nanomaterials as an Emerging Paradigm toward Precision Cancer Therapy. Adv. Funct. Mater. 2019, 29, 1806877. [Google Scholar] [CrossRef]
- Lin, D.H.; Xing, B.S. Tannic acid adsorption and its role for stabilizing carbon nanotube suspensions. Environ. Sci. Technol. 2008, 42, 5917–5923. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Jackson, J.K.; Letchford, K. The Effective Solubilization of Hydrophobic Drugs Using Epigallocatechin Gallate or Tannic Acid-Based Formulations. J. Pharm. Sci.-Us 2016, 105, 3143–3152. [Google Scholar] [CrossRef]
- Lu, R.F.; Zhang, X.Q.; Cheng, X.X.; Zhang, Y.G.; Zan, X.J.; Zhang, L.T. Medical Applications Based on Supramolecular Self-Assembled Materials From Tannic Acid. Front. Chem. 2020, 8, 871. [Google Scholar] [CrossRef]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Development of bisphosphonates. Breast Cancer Res. 2002, 4, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.P.; Davison, K.S.; Olszynski, W.P.; Beattie, K.A.; Adachi, J.D. A critical review of brand and generic alendronate for the treatment of osteoporosis. Springerplus 2013, 2, 550. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Hisa, I.; Seino, S.; Kaji, H. Alendronate Induces Mineralization in Mouse Osteoblastic MC3T3-E1 Cells: Regulation of Mineralization-Related Genes. Exp. Clin. Endocr. Diab. 2010, 118, 719–723. [Google Scholar] [CrossRef]
- von Knoch, F.; Jaquiery, C.; Kowalsky, M.; Schaeren, S.; Alabre, C.; Martin, I.; Rubash, H.E.; Shanbhag, A.S. Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 2005, 26, 6941–6949. [Google Scholar] [CrossRef]
- Wang, C.Z.; Chen, S.M.; Chen, C.H.; Wang, C.K.; Wang, G.J.; Chang, J.K.; Ho, M.L. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials 2010, 31, 8674–8683. [Google Scholar] [CrossRef]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Kim, H.J.; Park, K.; Song, H.R. 3D printed alendronate-releasing poly(caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed. Mater. 2016, 11, 055005. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.E.; Yun, Y.P.; Choi, S.W.; Jeon, D.I.; Kim, H.J.; Park, K.; Song, H.R. Osteogenesis and new bone formation of alendronate-immobilized porous PLGA microspheres in a rat calvarial defect model. J. Ind. Eng. Chem. 2017, 52, 277–286. [Google Scholar] [CrossRef]
- Shim, K.S.; Kim, H.J.; Kim, S.E.; Park, K. Simple surface biofunctionalization of biphasic calcium phosphates for improving osteogenic activity and bone tissue regeneration. J. Ind. Eng. Chem. 2018, 68, 220–228. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Shetty, A.B.; Hatami, E.; Chowdhury, P.; Yallapu, M.M. Pectin-Tannic Acid Nano-Complexes Promote the Delivery and Bioactivity of Drugs in Pancreatic Cancer Cells. Pharmaceutics 2020, 12, 285. [Google Scholar] [CrossRef] [Green Version]
- Hatami, E.; Nagesh, P.K.B.; Chowdhury, P.; Chauhan, S.C.; Jaggi, M.; Samarasinghe, A.E.; Yallapu, M.M. Tannic Acid-Lung Fluid Assemblies Promote Interaction and Delivery of Drugs to Lung Cancer Cells. Pharmaceutics 2018, 10, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandna, S.; Thakur, N.S.; Reddy, Y.N.; Kaur, R.; Bhaumik, J. Engineering Lignin Stabilized Bimetallic Nanocomplexes: Structure, Mechanistic Elucidation, Antioxidant, and Antimicrobial Potential. ACS Biomater. Sci. Eng. 2019, 5, 3212–3227. [Google Scholar] [CrossRef]
- Jung, S.Y.; Hwang, H.; Jo, H.S.; Choi, S.; Kim, H.J.; Kim, S.E.; Park, K. Tannylated Calcium Carbonate Materials with Antacid, Anti-Inflammatory, and Antioxidant Effects. Int. J. Mol. Sci. 2021, 22, 4614. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Kato, J.; Moss, J. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 18964–18969. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar] [CrossRef]
- Kim, S.E.; Choi, S.; Hong, J.Y.; Shim, K.S.; Kim, T.H.; Park, K.; Lee, S.H. Accelerated Osteogenic Differentiation of MC3T3-E1 Cells by Lactoferrin-Conjugated Nanodiamonds through Enhanced Anti-Oxidant and Anti-Inflammatory Effects. Nanomaterials 2020, 10, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Yoon, J.S.; Lee, J.Y.; Kim, H.J.; Park, K.; Kim, S.E. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohyd. Polym. 2019, 209, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Yoon, S.; Kim, W.J. Tannic acid-based nanogel as an efficient anti-inflammatory agent. Biomater. Sci.-Uk 2020, 8, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Suh, D.H.; Yun, Y.P.; Lee, J.Y.; Park, K.; Chung, J.Y.; Lee, D.W. Local delivery of alendronate eluting chitosan scaffold can effectively increase osteoblast functions and inhibit osteoclast differentiation. J. Mater. Sci. Mater. Med. 2012, 23, 2739–2749. [Google Scholar] [CrossRef]
- Yun, Y.P.; Kim, S.J.; Lim, Y.M.; Park, K.; Kim, H.J.; Jeong, S.I.; Kim, S.E.; Song, H.R. The effect of alendronate-loaded polycarprolactone nanofibrous scaffolds on osteogenic differentiation of adipose-derived stem cells in bone tissue regeneration. J. Biomed. Nanotechnol. 2014, 10, 1080–1090. [Google Scholar] [CrossRef]
- Choi, S.; Noh, S.H.; Lim, C.O.; Kim, H.J.; Jo, H.S.; Min, J.S.; Park, K.; Kim, S.E. Icariin-Functionalized Nanodiamonds to Enhance Osteogenic Capacity In Vitro. Nanomaterials 2020, 10, 2071. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Lee, D.W.; Kang, E.Y.; Jeong, W.J.; Lee, B.; Jeong, M.S.; Kim, H.J.; Park, K.; Song, H.R. Alendronate-Eluting Biphasic Calcium Phosphate (BCP) Scaffolds Stimulate Osteogenic Differentiation. BioMed Res. Int. 2015, 2015, 320713. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Jo, H.-S.; Song, H.; Kim, H.-J.; Oh, J.-K.; Cho, J.-W.; Park, K.; Kim, S.-E. Multifunctional Tannic Acid-Alendronate Nanocomplexes with Antioxidant, Anti-Inflammatory, and Osteogenic Potency. Nanomaterials 2021, 11, 1812. https://doi.org/10.3390/nano11071812
Choi S, Jo H-S, Song H, Kim H-J, Oh J-K, Cho J-W, Park K, Kim S-E. Multifunctional Tannic Acid-Alendronate Nanocomplexes with Antioxidant, Anti-Inflammatory, and Osteogenic Potency. Nanomaterials. 2021; 11(7):1812. https://doi.org/10.3390/nano11071812
Chicago/Turabian StyleChoi, Somang, Han-Saem Jo, Heegyeong Song, Hak-Jun Kim, Jong-Keon Oh, Jae-Woo Cho, Kyeongsoon Park, and Sung-Eun Kim. 2021. "Multifunctional Tannic Acid-Alendronate Nanocomplexes with Antioxidant, Anti-Inflammatory, and Osteogenic Potency" Nanomaterials 11, no. 7: 1812. https://doi.org/10.3390/nano11071812
APA StyleChoi, S., Jo, H.-S., Song, H., Kim, H.-J., Oh, J.-K., Cho, J.-W., Park, K., & Kim, S.-E. (2021). Multifunctional Tannic Acid-Alendronate Nanocomplexes with Antioxidant, Anti-Inflammatory, and Osteogenic Potency. Nanomaterials, 11(7), 1812. https://doi.org/10.3390/nano11071812