Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cement Specimens
2.3. Field Emission Scanning Electron Microscope Analysis
2.4. X-ray Diffraction Analysis
2.5. pH Measurement
2.6. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.7. Flow, Setting Time, Solubility and Disintegration, and Radio-Opacity
2.8. Culture of Human Cementoblast-Like Cells
2.9. Cellular Morphological Analysis and Viable Cell Count
2.10. Immunocytochemistry Analysis
2.11. Gene Expression Experiments
2.12. Alkaline Phosphatase Activity
2.13. Detection of Extracellular Calcium Deposition
2.14. Subcutaneous Implantation and Histological Analysis
2.15. Statistical Analysis
3. Results
3.1. Surface Structures of Cements
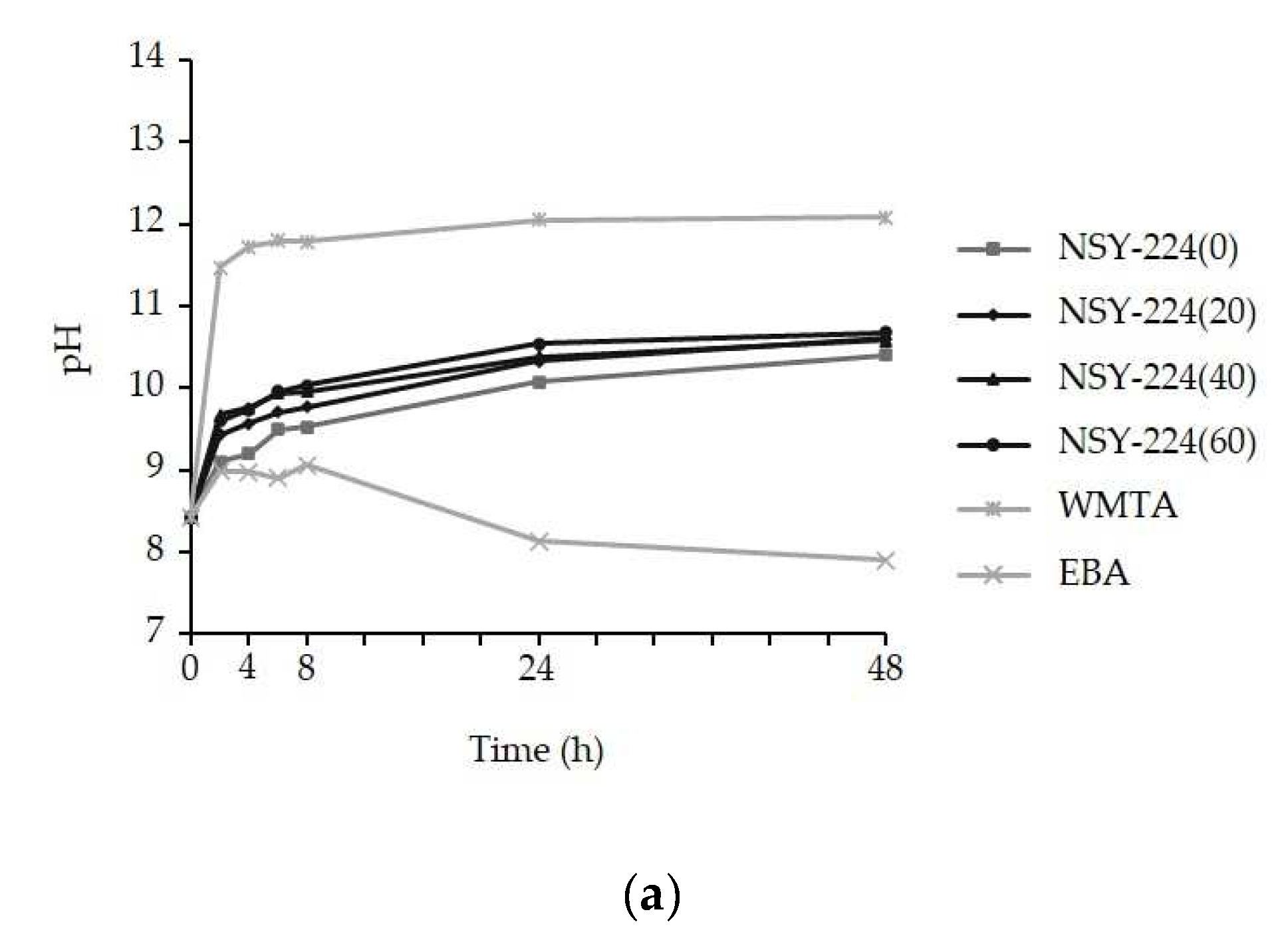
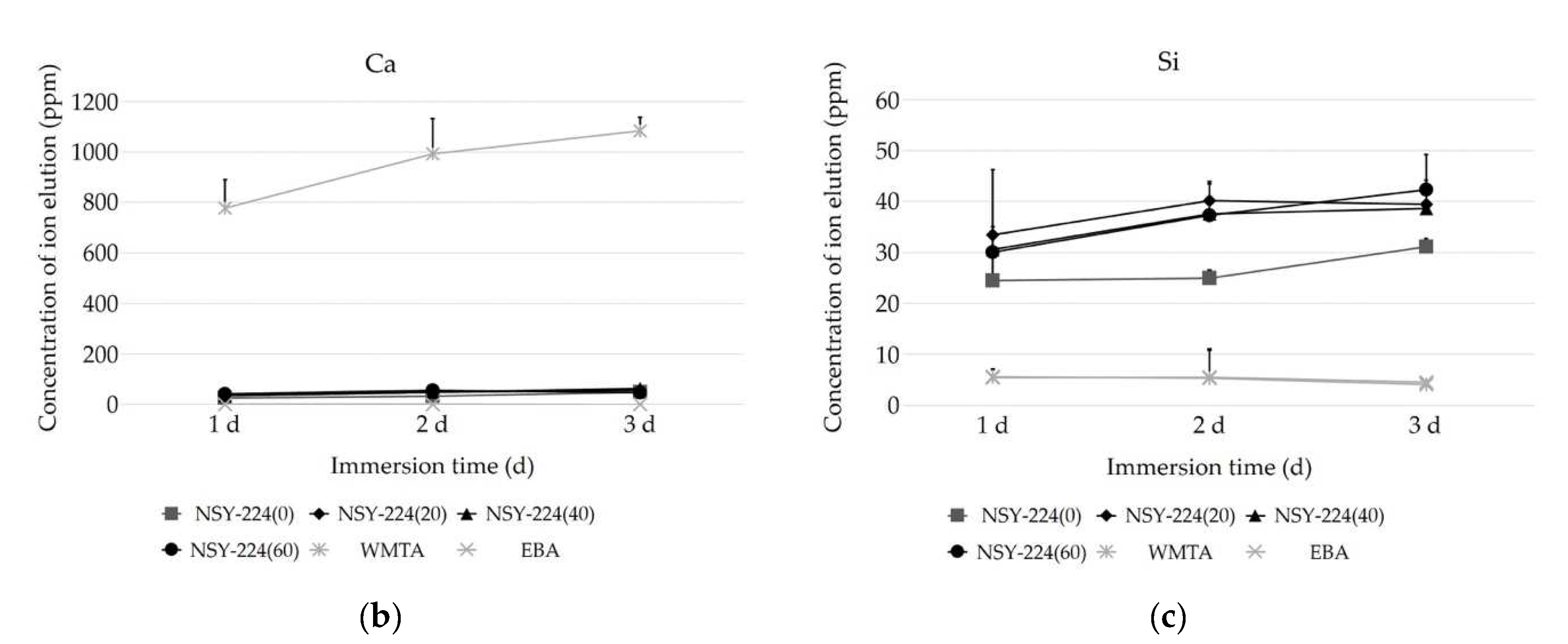
3.2. pH Changes and Ionic Elution of Cements
3.3. ISO Evaluation
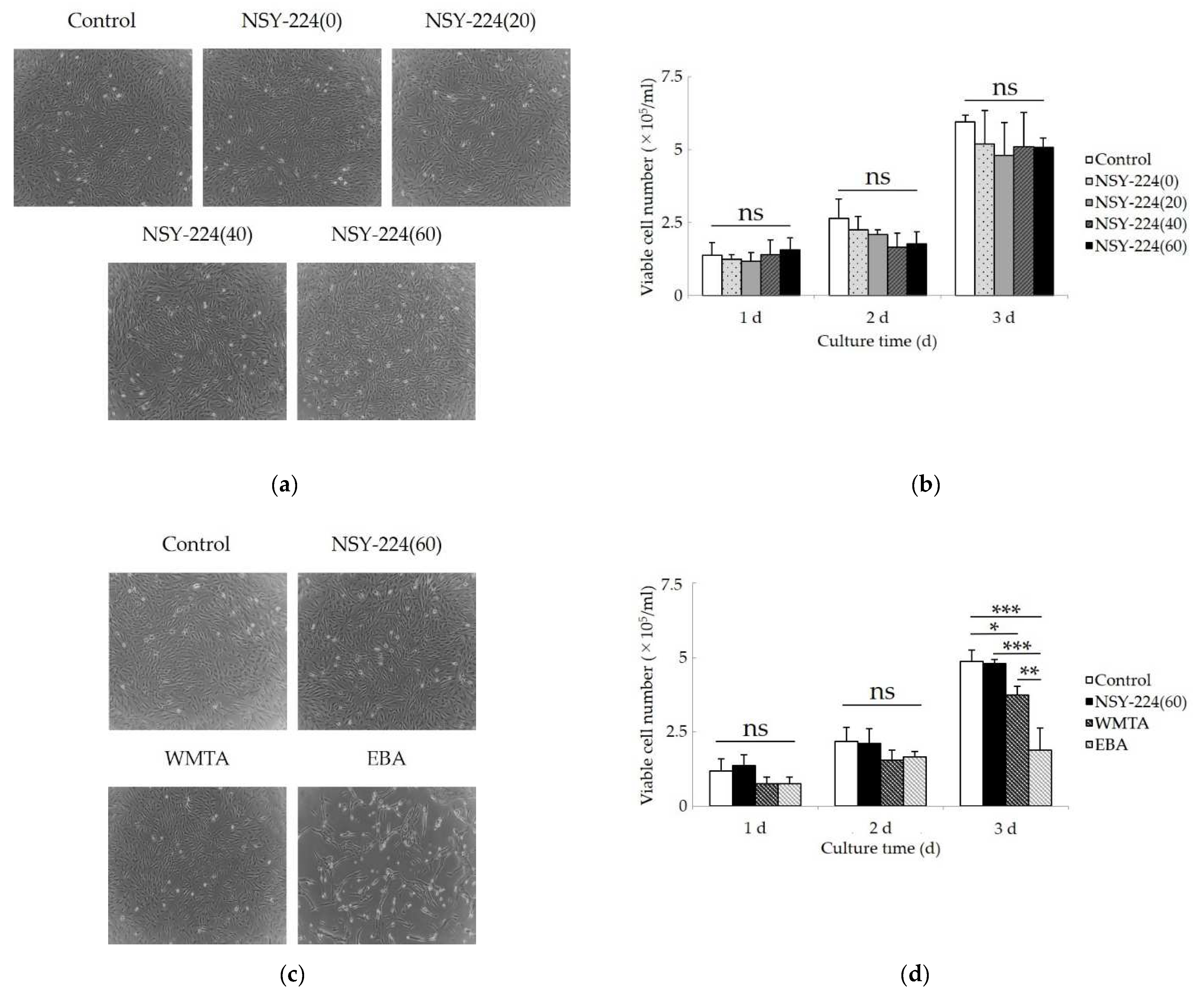
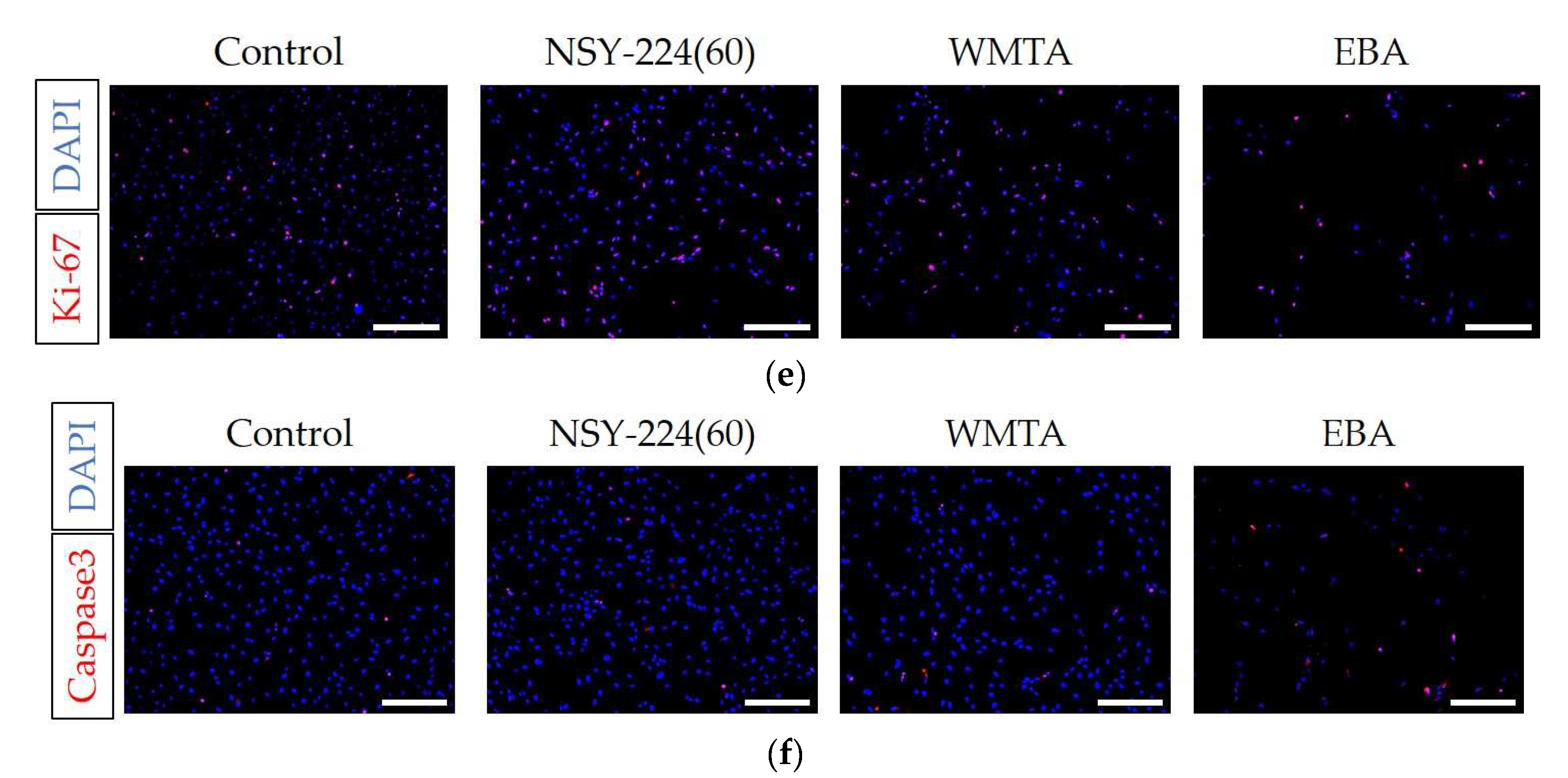
3.4. Effects of Cements on Morphology, Viability, Proliferation, and Apoptosis of HCEMs
3.5. Effects of Cements on Cementoblastic Differentiation and Calcification of HCEMs
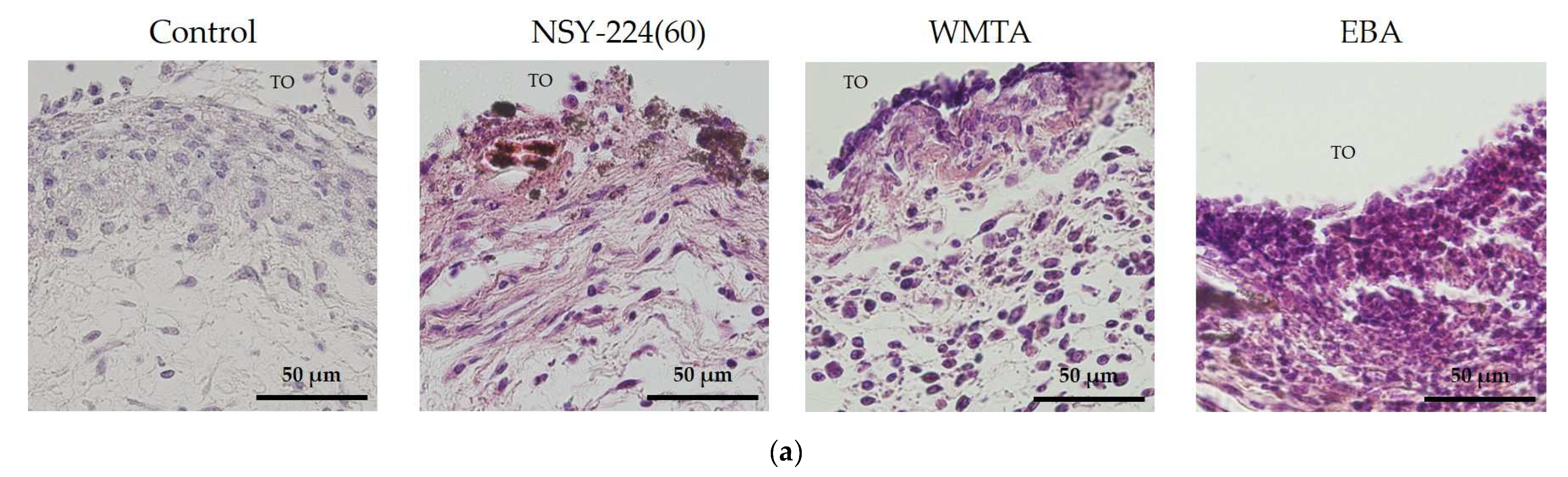
3.6. Effects of Cements on Inflammatory Responses In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingle, J.I.; Bakland, L.K.; Baumgartner, J.C. Ingle’s Endodontics 6; BC Decker: Hamilton, CA, USA, 2008; pp. 1233–1294. [Google Scholar]
- Cohen, S.; Burns, R. Pathways of the Pulp, 6th ed.; Mosby-Year Book: Saint Louis, MO, USA, 1994; pp. 531–568. [Google Scholar]
- Rubinstein, R.A.; Kim, S. Short-Term Observation of the Results of Endodontic Surgery with the Use of a Surgical Operation Microscope and Super-EBA as Root-End Filling Material. J. Endod. 1999, 25, 43–48. [Google Scholar] [CrossRef]
- Marques, J.H.S.; Silva-Sousa, Y.T.C.; Rached-Júnior, F.J.A.; Mazzi-Chaves, J.F.; Miranda, C.E.S.; da Silva, S.R.C.; Steier, L.; Sousa-Neto, M.D. New Methodology to Evaluate Bond Strength of Root-End Filling Materials. Braz. Dent. J. 2015, 26, 288–291. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Yoon, T.-S.; Kim, S.-Y.; Kim, E. Cytotoxicity of Newly Developed Pozzolan Cement and Other Root-End Filling Materials on Human Periodontal Ligament Cell. Restor. Dent. Endod. 2014, 39, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part I: Chemical, Physical, and Antibacterial Properties. J. Endod. 2010, 36, 16–27. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part II: Leakage and Biocompatibility Investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef]
- Parirokh, M.; Torabinejad, M. Mineral Trioxide Aggregate: A Comprehensive Literature Review—Part III: Clinical Applications, Drawbacks, and Mechanism of Action. J. Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Torabinejad, M.; Chivian, N. Clinical Applications of Mineral Trioxide Aggregate. J. Endod. 1999, 25, 197–205. [Google Scholar] [CrossRef]
- Walker, M.P.; Diliberto, A.; Lee, C. Effect of Setting Conditions on Mineral Trioxide Aggregate Flexural Strength. J. Endod. 2006, 32, 334–336. [Google Scholar] [CrossRef]
- Chen, L.; Suh, B.I. Cytotoxicity and Biocompatibility of Resin-Free and Resin-Modified Direct Pulp Capping Materials: A State-of-the-Art Review. Dent. Mater. J. 2017, 36, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. The Story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Höland, W.; Vogel, W.; Naumann, K.; Gummel, J. Interface Reactions between Machinable Bioactive Glass-Ceramics and Bone. J. Biomed. Mater. Res. 1985, 19, 303–312. [Google Scholar] [CrossRef]
- Yin, C.; Okamoto, R.; Kondo, M.; Tanaka, T.; Hattori, H.; Tanaka, M.; Sato, H.; Iino, S.; Koshiro, Y. Electrospinning of Block and Graft Type Silicone Modified Polyurethane Nanofibers. Nanomaterials 2018, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Labban, N.; Wayu, M.B.; Steele, C.M.; Munoz, T.S.; Pollock, J.A.; Case, W.S.; Leopold, M.C. First Generation Amperometric Biosensing of Galactose with Xerogel-Carbon Nanotube Layer-By-Layer Assemblies. Nanomaterials 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.D.; El-Fiqi, A.; Lee, H.-Y.; Singh, R.K.; Kim, D.-A.; Lee, H.-H.; Kim, H.-W. Chitosan–Nanobioactive Glass Electrophoretic Coatings with Bone Regenerative and Drug Delivering Potential. J. Mater. Chem. 2012, 22, 24945–24956. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, N.-H.; Singh, R.K.; Mandakhbayar, N.; Perez, R.A.; Lee, J.-H.; Kim, H.-W. Nanocements Produced from Mesoporous Bioactive Glass Nanoparticles. Biomaterials 2018, 162, 183–199. [Google Scholar] [CrossRef]
- Jung, Y.; Yoon, J.-Y.; Patel, K.D.; Ma, L.; Lee, H.-H.; Kim, J.; Lee, J.-H.; Shin, J. Biological Effects of Tricalcium Silicate Nanoparticle-Containing Cement on Stem Cells from Human Exfoliated Deciduous Teeth. Nanomaterials 2020, 10, 1373. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.-J.; Dev Patel, K.; Campbell Knowles, J.; Lee, J.-H.; Song, M. Physical Properties and Biofunctionalities of Bioactive Root Canal Sealers In Vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Washio, A.; Nakagawa, A.; Nishihara, T.; Maeda, H.; Kitamura, C. Physicochemical Properties of Newly Developed Bioactive Glass Cement and Its Effects on Various Cells. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 373–380. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive Glass-Based Endodontic Sealer as a Promising Root Canal Filling Material without Semisolid Core Materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef] [Green Version]
- Washio, A.; Miura, H.; Morotomi, T.; Ichimaru-Suematsu, M.; Miyahara, H.; Hanada-Miyahara, K.; Yoshii, S.; Murata, K.; Takakura, N.; Akao, E.; et al. Effect of Bioactive Glass-Based Root Canal Sealer on the Incidence of Postoperative Pain after Root Canal Obturation. Int. J. Environ. Res. Public Health 2020, 17, 8857. [Google Scholar] [CrossRef]
- Vincent, B. Physicochemical properties of biomaterials. In Biomaterials for Organ and Tissue Regeneration: New Technologies and Future Prospects; Woodhead Publishing: Cambridge, UK, 2020; pp. 19–32. [Google Scholar]
- Biomadical Equipment Management & Maintenance Programe. Available online: http://nhsrcindia.org/sites/default/files/Biomadical%20Equipment%20Management%20%26%20Maintenance%20Programe.pdf (accessed on 24 May 2021).
- Hanada, K.; Morotomi, T.; Washio, A.; Yada, N.; Matsuo, K.; Teshima, H.; Yokota, K.; Kitamura, C. In Vitro and in Vivo Effects of a Novel Bioactive Glass-Based Cement Used as a Direct Pulp Capping Agent. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, M.; Tahara, H.; Kitagawa, S.; Oka, H.; Kudo, Y.; Sato, S.; Ogawa, I.; Miyaichi, M.; Takata, T. Characterization of Established Cementoblast-like Cell Lines from Human Cementum-Lining Cells in Vitro and in Vivo. Bone 2006, 39, 1035–1042. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rauh, J.; Jacobi, A.; Stiehler, M. Identification of Stable Reference Genes for Gene Expression Analysis of Three-Dimensional Cultivated Human Bone Marrow-Derived Mesenchymal Stromal Cells for Bone Tissue Engineering. Tissue Eng. Part C Methods 2015, 21, 192–206. [Google Scholar] [CrossRef]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Sadr, A.; Yamashita, K.; Tagami, J. CO2 Laser Improves 45S5 Bioglass Interaction with Dentin. J. Dent. Res. 2011, 90, 246–250. [Google Scholar] [CrossRef]
- Bakry, A.S.; Takahashi, H.; Otsuki, M.; Tagami, J. The Durability of Phosphoric Acid Promoted Bioglass-Dentin Interaction Layer. Dent. Mater. 2013, 29, 357–364. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Huang, T.-H.; Kao, C.-T.; Huang, C.-H.; Ding, S.-J. The Effect of a Physiologic Solution PH on Properties of White Mineral Trioxide Aggregate. J. Endod. 2009, 35, 98–101. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.F.; Felippe, M.S.; Felippe, W.T. Biomineralization Ability and Interaction of Mineral Trioxide Aggregate and White Portland Cement with Dentin in a Phosphate-Containing Fluid. J. Endod. 2009, 35, 731–736. [Google Scholar] [CrossRef]
- Gartner, A.H.; Dorn, S.O. Advances in Endodontic Surgery. Dent. Clin. N. Am. 1992, 36, 357–378. [Google Scholar]
- Aqrabawi, J. Sealing Ability of Amalgam, Super EBA Cement, and MTA When Used as Retrograde Filling Materials. Br. Dent. J. 2000, 188, 266–268. [Google Scholar] [CrossRef]
- Grossman, L.I. Solubility of Root Canal Cements. J. Dent. Res. 1978, 57, 927. [Google Scholar] [CrossRef]
- Washington, J.T.; Schneiderman, E.; Spears, R.; Fernandez, C.R.; He, J.; Opperman, L.A. Biocompatibility and Osteogenic Potential of New Generation Endodontic Materials Established by Using Primary Osteoblasts. J. Endod. 2011, 37, 1166–1170. [Google Scholar] [CrossRef]
- Osorio, R.M.; Hefti, A.; Vertucci, F.J.; Shawley, A.L. Cytotoxicity of Endodontic Materials. J. Endod. 1998, 24, 91–96. [Google Scholar] [CrossRef]
- Willershausen, I.; Wolf, T.; Kasaj, A.; Weyer, V.; Willershausen, B.; Marroquin, B.B. Influence of a Bioceramic Root End Material and Mineral Trioxide Aggregates on Fibroblasts and Osteoblasts. Arch. Oral Biol. 2013, 58, 1232–1237. [Google Scholar] [CrossRef]
- Oviir, T.; Pagoria, D.; Ibarra, G.; Geurtsen, W. Effects of Gray and White Mineral Trioxide Aggregate on the Proliferation of Oral Keratinocytes and Cementoblasts. J. Endod. 2006, 32, 210–213. [Google Scholar] [CrossRef]
- Liu, M.; He, L.; Wang, H.; Su, W.; Li, H. Comparison of in Vitro Biocompatibility and Antibacterial Activity of Two Calcium Silicate-Based Materials. J. Mater. Sci. Mater. Med. 2021, 32, 52. [Google Scholar] [CrossRef] [PubMed]
- Hattori-Sanuki, T.; Karakida, T.; Chiba-Ohkuma, R.; Miake, Y.; Yamamoto, R.; Yamakoshi, Y.; Hosoya, N. Characterization of Living Dental Pulp Cells in Direct Contact with Mineral Trioxide Aggregate. Cells 2020, 9, 2336. [Google Scholar] [CrossRef]
- Paranjpe, A.; Zhang, H.; Johnson, J.D. Effects of Mineral Trioxide Aggregate on Human Dental Pulp Cells after Pulp-Capping Procedures. J. Endod. 2010, 36, 1042–1047. [Google Scholar] [CrossRef]
- Samara, A.; Sarri, Y.; Stravopodis, D.; Tzanetakis, G.N.; Kontakiotis, E.G.; Anastasiadou, E. A Comparative Study of the Effects of Three Root-End Filling Materials on Proliferation and Adherence of Human Periodontal Ligament Fibroblasts. J. Endod. 2011, 37, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, T.; Hayashi, M.; Tanabe, N.; Tsuda, H.; Suzuki, Y.; Kawato, T.; Suzuki, N.; Maeno, M.; Ogiso, B. Involvement of the Calcium-Sensing Receptor in Mineral Trioxide Aggregate-Induced Osteogenic Gene Expression in Murine MC3T3-E1 Cells. Dent. Mater. J. 2017, 36, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.R.; Nuutila, K.; Lee, C.C.Y.; Kiwanuka, E.; Singh, M.; Caterson, E.J.; Eriksson, E.; Sørensen, J.A. The External Microenvironment of Healing Skin Wounds. Wound Repair Regen. 2015, 23, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Hazehara-Kunitomo, Y.; Hara, E.S.; Ono, M.; Aung, K.T.; Komi, K.; Pham, H.T.; Akiyama, K.; Okada, M.; Oohashi, T.; Matsumoto, T.; et al. Acidic Pre-Conditioning Enhances the Stem Cell Phenotype of Human Bone Marrow Stem/Progenitor Cells. Int. J. Mol. Sci. 2019, 20, 1097. [Google Scholar] [CrossRef] [Green Version]
- Kruse, C.R.; Singh, M.; Targosinski, S.; Sinha, I.; Sørensen, J.A.; Eriksson, E.; Nuutila, K. The Effect of PH on Cell Viability, Cell Migration, Cell Proliferation, Wound Closure, and Wound Reepithelialization: In Vitro and in Vivo Study. Wound Repair Regen. 2017, 25, 260–269. [Google Scholar] [CrossRef]
- Bonson, S.; Jeansonne, B.G.; Lallier, T.E. Root-End Filling Materials Alter Fibroblast Differentiation. J. Dent. Res. 2004, 83, 408–413. [Google Scholar] [CrossRef]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a Mouse Monoclonal Antibody Reactive with a Human Nuclear Antigen Associated with Cell Proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell Cycle Analysis of a Cell Proliferation-Associated Human Nuclear Antigen Defined by the Monoclonal Antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar]
- Nagata, S. Apoptosis and Clearance of Apoptotic Cells. Annu. Rev. Immunol. 2018, 36, 489–517. [Google Scholar] [CrossRef]
- Shirakawa, T.; Miyawaki, A.; Matsubara, T.; Okumura, N.; Okamoto, H.; Nakai, N.; Rojasawasthien, T.; Morikawa, K.; Inoue, A.; Goto, A.; et al. Daily Oral Administration of Protease-Treated Royal Jelly Protects Against Denervation-Induced Skeletal Muscle Atrophy. Nutrients 2020, 12, 3089. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Yamada, S.; Ota, Y.; Obata, A.; Kasuga, T. Osteoblast-like Cell Responses to Ion Products Released from Magnesium- and Silicate-Containing Calcium Carbonates. Biomed. Mater. Eng. 2017, 28, 47–56. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The Effect of Calcium Ion Concentration on Osteoblast Viability, Proliferation and Differentiation in Monolayer and 3D Culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Obata, A.; Tokuda, S.; Kasuga, T. Enhanced in Vitro Cell Activity on Silicon-Doped Vaterite/Poly(Lactic Acid) Composites. Acta Biomater. 2009, 5, 57–62. [Google Scholar] [CrossRef]
- Chen, C.-L.; Huang, T.-H.; Ding, S.-J.; Shie, M.-Y.; Kao, C.-T. Comparison of Calcium and Silicate Cement and Mineral Trioxide Aggregate Biologic Effects and Bone Markers Expression in MG63 Cells. J. Endod. 2009, 35, 682–685. [Google Scholar] [CrossRef]
- Maeda, H.; Nakano, T.; Tomokiyo, A.; Fujii, S.; Wada, N.; Monnouchi, S.; Hori, K.; Akamine, A. Mineral Trioxide Aggregate Induces Bone Morphogenetic Protein-2 Expression and Calcification in Human Periodontal Ligament Cells. J. Endod. 2010, 36, 647–652. [Google Scholar] [CrossRef]
- Hakki, S.S.; Bozkurt, S.B.; Hakki, E.E.; Belli, S. Effects of Mineral Trioxide Aggregate on Cell Survival, Gene Expression Associated with Mineralized Tissues, and Biomineralization of Cementoblasts. J. Endod. 2009, 35, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M.; Iwasaki, K.; Arzate, H.; Narayanan, A.S.; Izumi, Y.; Morita, I. Cementum Protein 1 (CEMP1) Induces a Cementoblastic Phenotype and Reduces Osteoblastic Differentiation in Periodontal Ligament Cells. J. Cell Physiol. 2012, 227, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Ao, M.; Miyauchi, M.; Abiko, Y.; Takata, T. F-Spondin Regulates the Differentiation of Human Cementoblast-like (HCEM) Cells via BMP7 Expression. Biochem. Biophys. Res. Commun. 2012, 418, 229–233. [Google Scholar] [CrossRef]
- Orimoto, A.; Kurokawa, M.; Handa, K.; Ishikawa, M.; Nishida, E.; Aino, M.; Mitani, A.; Ogawa, M.; Tsuji, T.; Saito, M.; et al. F-Spondin Negatively Regulates Dental Follicle Differentiation through the Inhibition of TGF-β Activity. Arch. Oral Biol. 2017, 79, 7–13. [Google Scholar] [CrossRef]
- Bernstein, A.; Nöbel, D.; Mayr, H.O.; Berger, G.; Gildenhaar, R.; Brandt, J. Histological and Histomorphometric Investigations on Bone Integration of Rapidly Resorbable Calcium Phosphate Ceramics. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 452–462. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Gao, Y.; Huang, Y.; Jiang, X.; Ma, K.; Ling, J. Short-Term Effects of Calcium Ions on the Apoptosis and Onset of Mineralization of Human Dental Pulp Cells in Vitro and in Vivo. Int. J. Mol. Med. 2015, 36, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Viola, N.V.; Guerreiro-Tanomaru, J.M.; da Silva, G.F.; Sasso-Cerri, E.; Tanomaru-Filho, M.; Cerri, P.S. Biocompatibility of an Experimental MTA Sealer Implanted in the Rat Subcutaneous: Quantitative and Immunohistochemical Evaluation. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1773–1781. [Google Scholar] [CrossRef]
- ElReash, A.A.; Hamama, H.; Abdo, W.; Wu, Q.; Zaen El-Din, A.; Xiaoli, X. Biocompatibility of New Bioactive Resin Composite versus Calcium Silicate Cements: An Animal Study. BMC Oral Health 2019, 19, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahi, S.; Rahimi, S.; Lotfi, M.; Yavari, H.; Gaderian, A. A Comparative Study of the Biocompatibility of Three Root-End Filling Materials in Rat Connective Tissue. J. Endod. 2006, 32, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, A.; Ricevuti, G. Leukocyte CD11/CD18 Integrins: Biological and Clinical Relevance. Haematologica 1995, 80, 161–175. [Google Scholar] [PubMed]
- Barford, D.; Flint, A.J.; Tonks, N.K. Crystal Structure of Human Protein Tyrosine Phosphatase 1B. Science 1994, 263, 1397–1404. [Google Scholar] [CrossRef] [PubMed]





| Materials | Code | Compositions * |
|---|---|---|
| Newly developed BG powder | NSY-224 | Calcium silicate glass and calcium hydroxide |
| Nishika Canal Sealer BG | CS-BG | Paste A: bismuth subcarbonate, fatty acid, and silicon dioxide |
| Paste B: calcium silicate glass, magnesium oxide, purified water, silicon dioxide, and others | ||
| White ProRoot MTA | WMTA | Powder: tricalcium silicate, dicalcium silicate, tricalcium aluminate, bismuth oxide, and calcium sulfate |
| Liquid: sterile water | ||
| SuperEBA | EBA | Powder: zinc oxide, alumina, and natural resin |
| Liquid: ortho-ethoxy benzoic acid and eugenol |
| Target Gene | Primer Sequence (5′ to 3′) | |
|---|---|---|
| CEMP-1 | Forward: | ACACTGGTGCCTCCCATACT |
| (NM_001048212) | Reverse: | TCAATAACCCTATCTCTTCACACATC |
| F-SPONDIN | Forward: | TGTCGATGATATTGTAGCTGACC |
| (NM_006108) | Reverse: | CAGGTTTCAGGGGTGTCATC |
| ALP | Forward: | CCGGATGTTACCGAGAGC |
| (X_55958) | Reverse: | GTGGGTCTCTCCGTCCAG |
| β-ACTIN | Forward: | CCAACCGCGAGAAGATGA |
| (NM_001101) | Reverse: | CCAGAGGCGTACAGGGATAG |
| TBP | Forward: | GAACATCATGGATCAGAACAACA |
| (NM_003194) | Reverse: | ATAGGGATTCCGGGAGTCAT |
| HPRT1 | Forward: | TGACCTTGATTTATTTTGCATACC |
| (NM_000194) | Reverse: | CGAGCAAGACGTTCAGTCCT |
| Specimen | NSY-224(0) | NSY-224(20) | NSY-224(40) | NSY-224(60) | WMTA | EBA |
|---|---|---|---|---|---|---|
| Flow | 33.1 mm | 29.7 mm | 22.8 mm | 16.5 mm | 10.6 mm | 33.1 mm |
| Setting time | 150 min | 80 min | 40 min | 8 min | 10 min | 1 min |
| Solubility | 0.6% No disintegration | 0.5% No disintegration | 0.6% No disintegration | 0.5% No disintegration | 2.4% No disintegration | 0.1% No disintegration |
| Radio-opacity | 6 mm Al | 5 mm Al | 5 mm Al | 4 mm Al | 5 mm Al | 5 mm Al |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, K.; Washio, A.; Morotomi, T.; Rojasawasthien, T.; Kokabu, S.; Kitamura, C. Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material. Nanomaterials 2021, 11, 1828. https://doi.org/10.3390/nano11071828
Murata K, Washio A, Morotomi T, Rojasawasthien T, Kokabu S, Kitamura C. Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material. Nanomaterials. 2021; 11(7):1828. https://doi.org/10.3390/nano11071828
Chicago/Turabian StyleMurata, Kazumasa, Ayako Washio, Takahiko Morotomi, Thira Rojasawasthien, Shoichiro Kokabu, and Chiaki Kitamura. 2021. "Physicochemical Properties, Cytocompatibility, and Biocompatibility of a Bioactive Glass Based Retrograde Filling Material" Nanomaterials 11, no. 7: 1828. https://doi.org/10.3390/nano11071828





