Atomic Arrangements of Graphene-like ZnO
Abstract
:1. Introduction
2. Materials and Methods
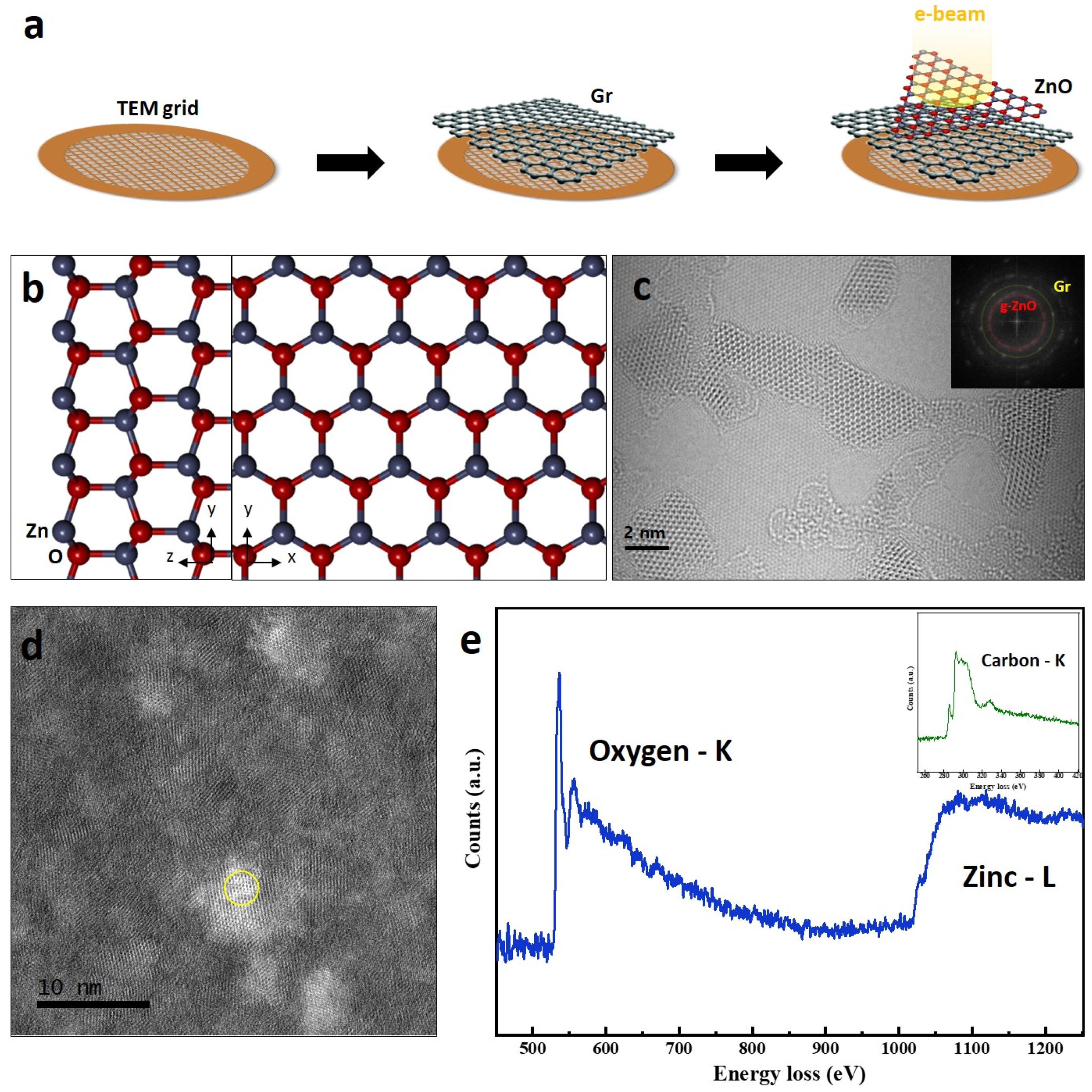
2.1. Preparation of the Specimen
2.2. ARTEM Observations and STEM-EELS Spectra
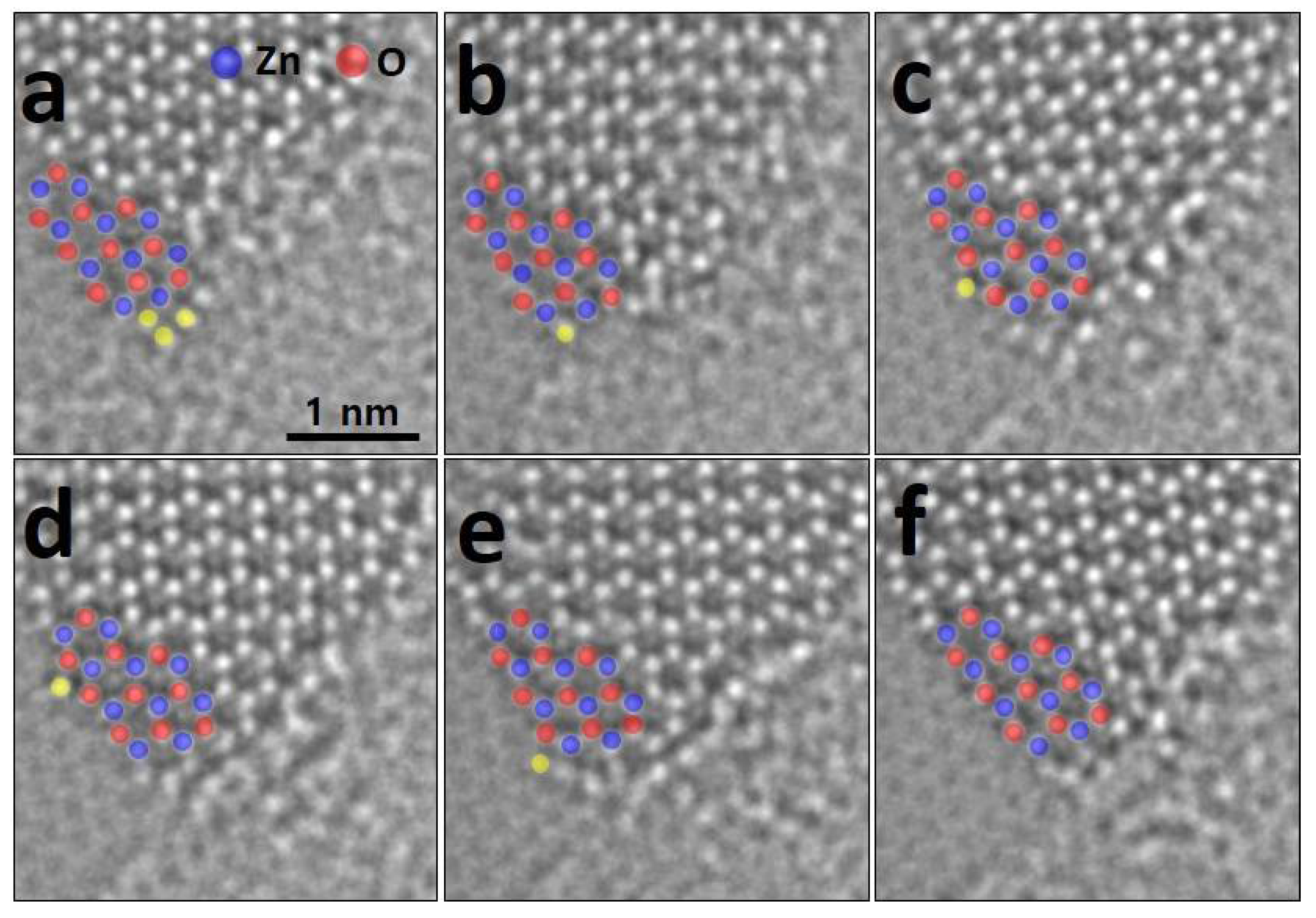
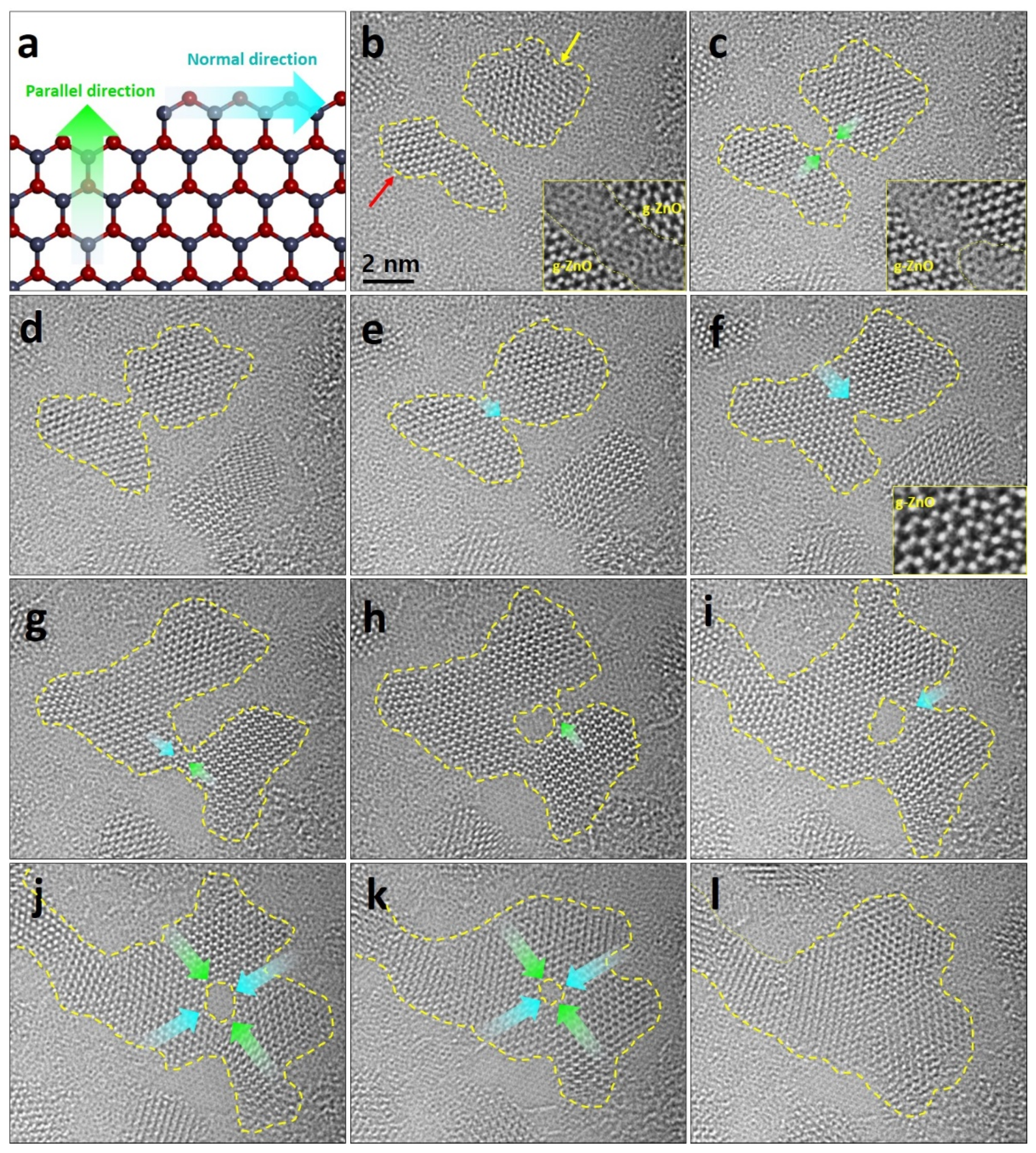
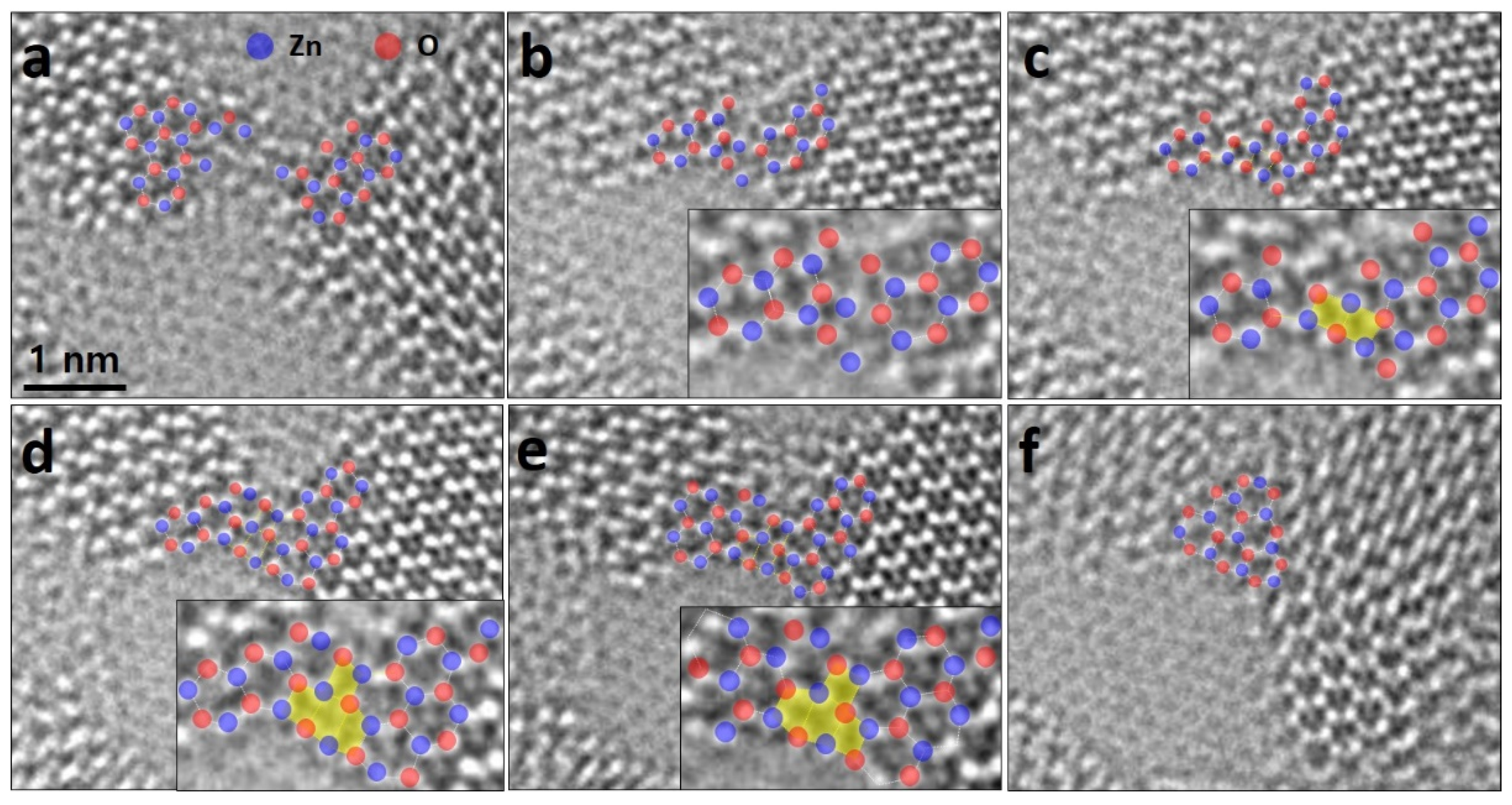
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morkoc, H.; Ozgur, U. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Choi, D.; Choi, M.Y.; Choi, W.M.; Shin, H.J.; Park, H.K.; Seo, J.S.; Park, J.; Yoon, S.M.; Chae, S.J.; Lee, Y.H.; et al. Fully Rollable Transparent Nanogenerators Based on Graphene Electrodes. Adv. Mater. 2010, 22, 2187–2192. [Google Scholar] [CrossRef]
- Chung, K.; Lee, C.H.; Yi, G.C. Transferable GaN Layers Grown on ZnO-Coated Graphene Layers for Optoelectronic Devices. Science 2010, 330, 655–657. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, Y.J.; Hong, Y.J.; Jeon, S.R.; Bae, S.; Hong, B.H.; Yi, G.C. Flexible Inorganic Nanostructure Light-Emitting Diodes Fabricated on Graphene Films. Adv. Mater. 2011, 23, 4614–4619. [Google Scholar] [CrossRef]
- Fan, Z.; Lu, J.G. Zinc Oxide Nanostructures: Synthesis and Properties. J. Nanosci. Nanotechnol. 2005, 5, 1561–1573. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.L. Zinc Oxide Nanostructures: Growth, Properties and Applications. J. Phys. Condens. Matter 2004, 16, 829–858. [Google Scholar] [CrossRef]
- Ta, H.Q.; Bachmatiuk, A.; Dianat, A.; Ortmann, F.; Zhao, J.; Warner, J.H.; Eckert, J.; Cunniberti, G.; Rümmeli, M.H. In-Situ Observations of Freestanding Graphene-Like Mono- and Bi-layer ZnO Membranes. ACS Nano 2015, 9, 11408–11413. [Google Scholar]
- Hong, H.-K.; Jo, J.; Hwang, D.; Lee, J.; Kim, N.Y.; Son, S.; Kim, J.H.; Jin, M.-J.; Jun, Y.C.; Erni, R.; et al. Atomic Scale Study on Growth and Heteroepitaxy of ZnO Monolayer on Graphene. Nano Lett. 2017, 17, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, S.; Cho, Y.; Hong, H.-K.; Lee, J.; Kim, J.H.; Kim, K.; Lee, Y.; Yoon, A.; Shin, H.-J.; Lee, Z. Spontaneous Formation of a ZnO Monolayer by the Redox Reaction of Zn on Graphene Oxide. ACS Appl. Mater. Interfaces 2020, 12, 54222–54229. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.; Nayak, S.K.; Chelliah, P.; Rath, M.K.; Prida, B. Observations of Two-Dimensional Monolayer Zinc Oxide. Mater. Res. Bull. 2016, 75, 134–138. [Google Scholar] [CrossRef]
- Tusche, C.; Meyerheim, H.L.; Kirschner, J. Observation of Depolarized ZnO(0001) Mono-Layers: Formation of Unreconstructed Planar Sheets. Phys. Rev. Lett. 2007, 99, 026102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, M.; Shen, Z. A Review on Mechanical Exfoliation for the Scalable Production of Graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Huo, C.; Yan, Z.; Song, X.; Zeng, H. 2D Materials via Liquid Exfoliation: A Review on Fabrication and Applications. Sci. Bull. 2015, 60, 1994–2008. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Ambrosi, A.; Sofer, Z.; Šimek, P.; Pumera, M. Electrochemistry of Transition Metal Dichalcogenides: Strong Dependence on the Metal-to-Chalcogen Composition and Exfoliation Method. ACS Nano 2014, 8, 12185–12198. [Google Scholar] [CrossRef]
- Chhowalla, M.; Liu, Z.; Zhang, H. Two-Dimensional Transition Metal Dichalcogenide (TMD) Nanosheets. Chem. Soc. Rev. 2015, 44, 2584–2586. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.Q.; Zhao, L.; Pohi, D.; Pang, J.; Trzebicka, B.; Rellinghaus, B.; Pribat, D.; Gemming, T.; Liu, Z.; Bachmatiuk, A.; et al. Graphene-Like ZnO: A Mini Review. Crystals 2016, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- Topsakal, M.; Cahangirov, S.; Bekaroglu, E.; Ciraci, S. First-Principles Study of Zinc Oxide Honeycomb Structures. Phys. Rev. B 2009, 80, 235119. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.C. First-Principles Study on Physical Properties of a Single ZnO Mono-Layer with Graphene-Like structure. J. Comput. Theor. Nanosci. 2010, 7, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
- Claeyssens, F.; Freeman, C.L.; Allan, N.L.; Sun, Y.; Ashfolda, M.N.R.; Harding, J.H. Growth of ZnO Thin Films-Experiment and Theory. J. Mater. Chem. 2005, 15, 139–148. [Google Scholar] [CrossRef]
- Tu, Z.C.; Hu, X. Elasticity and Piezoelectricity of Zinc Oxide Crystals, Single Layers, and Possible Single-Walled Nanotubes. Phys. Rev. B 2006, 74, 035434. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Sorescu, D.C.; Deng, X.Y. Tunable Lattice Constant and Band Gap of Single- and Few-Layer ZnO. J. Phys. Rev. Lett. 2016, 7, 1335–1340. [Google Scholar] [CrossRef]
- Demel, J.; Plestil, J.; Bezdicka, P.; Janda, P.; Klementova, M.; Lang, K. Few-Layer ZnO Nanosheets: Preparation, Properties, and Films with Exposed {001} Facets. J. Phys. Chem. C 2011, 115, 24702–24706. [Google Scholar] [CrossRef]
- Liu, G.; Debnath, B.; Pope, T.R.; Salguero, T.T.; Lake, R.K.; Balandin, A.A. A Charge-Density-Wave Oscillator Based on an Integrated Tantalum Disulfide-Boron Nitride-Graphene Device Operating at Room Temperature. Nat. Nanotechnol. 2016, 11, 845–850. [Google Scholar] [CrossRef]
- Wang, F.; Yin, X.; Wang, X. Morphological Control in the Adaptive Ionic Layer Epitaxy of ZnO Nanosheets. Extrem. Mech. Lett. 2016, 7, 64–70. [Google Scholar] [CrossRef]
- Wang, F.; Seo, J.-H.; Luo, G.; Starr, M.B.; Li, Z.; Geng, D.; Yin, X.; Wang, S.; Fraser, D.G.; Morgan, D.; et al. Nanometre-Thick Single-Crystalline Nanosheets Grown at the Water–Air Interface. Nat. Commun. 2016, 7, 10444. [Google Scholar] [CrossRef]
- Chai, G.L.; Lin, C.S.; Cheng, W.D. First-Principles Study of ZnO Cluster-Decorated Carbon Nanotubes. Nanotechnology 2011, 22, 445705. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.K.; Yadav, P.S.; Agrawal, S.; Agrawal, B.K. Structural and Electronic Properties of ZnO Nanoclusters: A B3LYP DFT Study. Adv. Mater. Res. 2013, 650, 29–33. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Suzuki, Y.; Watanabe, K.; Iwama, S.; Watanabe, S.; Ichinose, H. A HRTEM and EELS Study of Pd/ZnO Polar Interfaces. Philos. Mag. 2008, 88, 1493–1509. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.C.; Lee, Z.; Ryu, G.H. Atomic Arrangements of Graphene-like ZnO. Nanomaterials 2021, 11, 1833. https://doi.org/10.3390/nano11071833
Yoon JC, Lee Z, Ryu GH. Atomic Arrangements of Graphene-like ZnO. Nanomaterials. 2021; 11(7):1833. https://doi.org/10.3390/nano11071833
Chicago/Turabian StyleYoon, Jong Chan, Zonghoon Lee, and Gyeong Hee Ryu. 2021. "Atomic Arrangements of Graphene-like ZnO" Nanomaterials 11, no. 7: 1833. https://doi.org/10.3390/nano11071833
APA StyleYoon, J. C., Lee, Z., & Ryu, G. H. (2021). Atomic Arrangements of Graphene-like ZnO. Nanomaterials, 11(7), 1833. https://doi.org/10.3390/nano11071833






