Microstructure Evaluation and Impurities in La Containing Silicon Oxynitrides
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
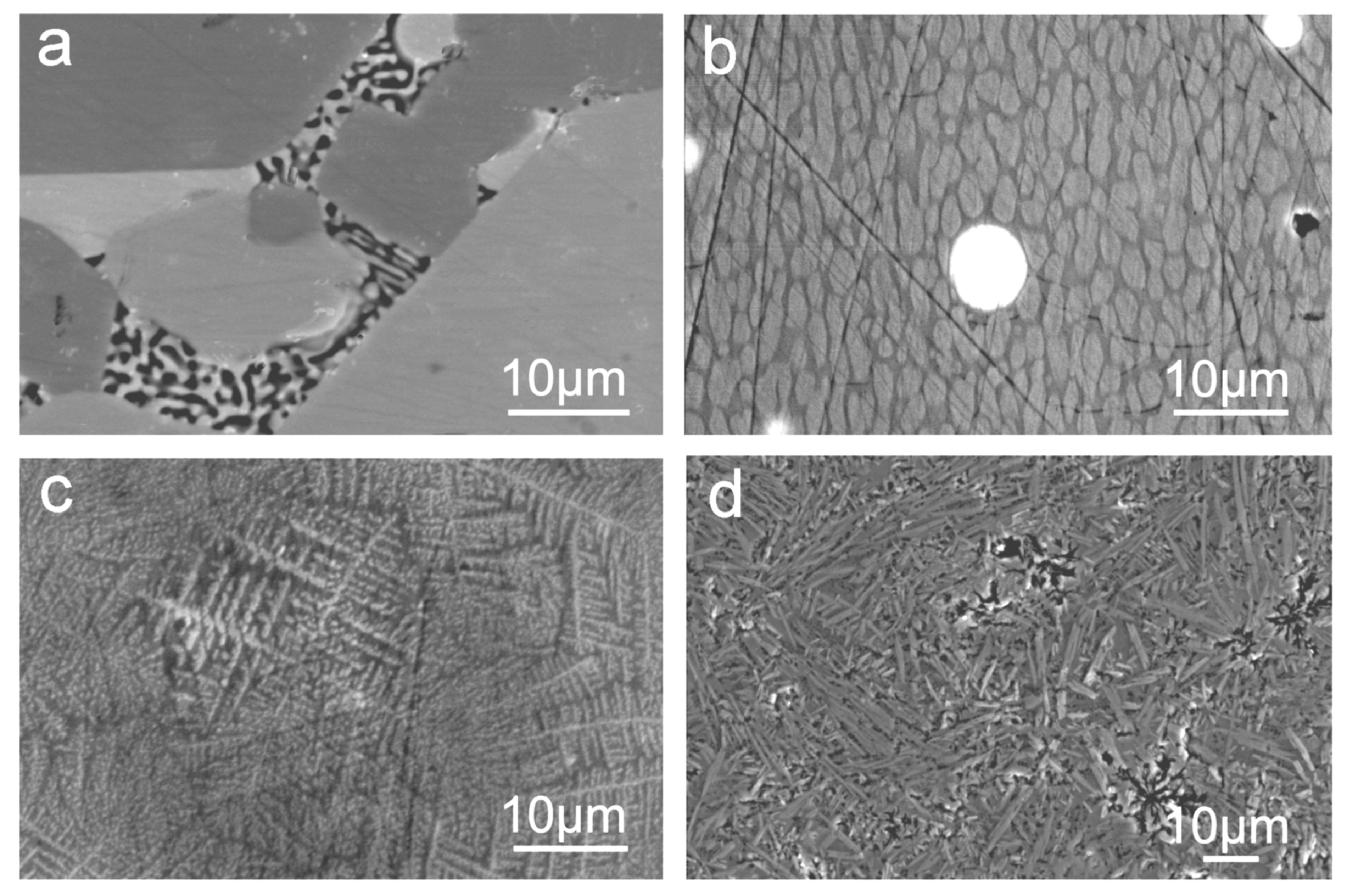
3.1. Influence of Time and Temperature
3.2. Influence of Raw Materials and Nitrogen Pressure
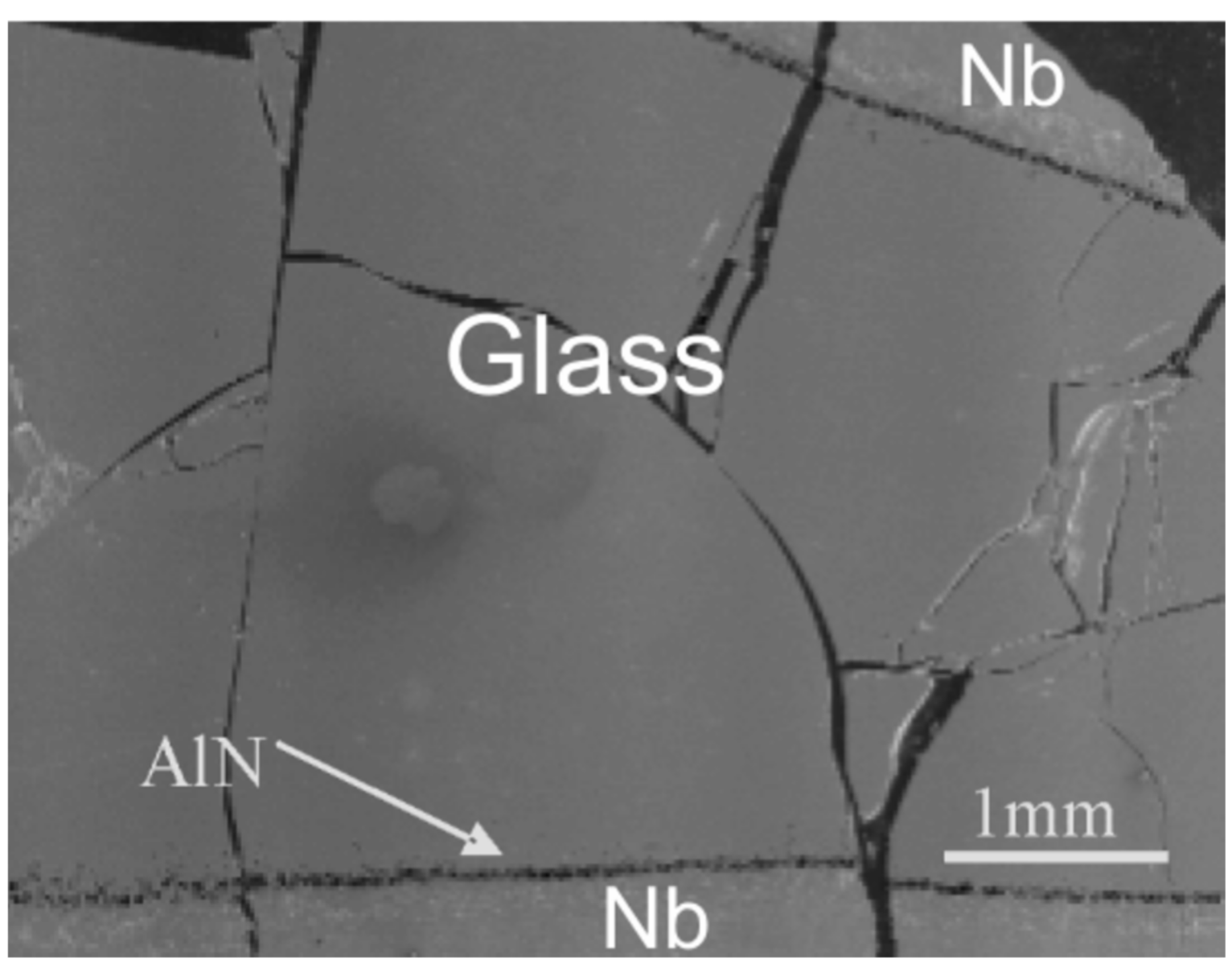
3.3. Observation of Interface between Glass and Niobium Crucible
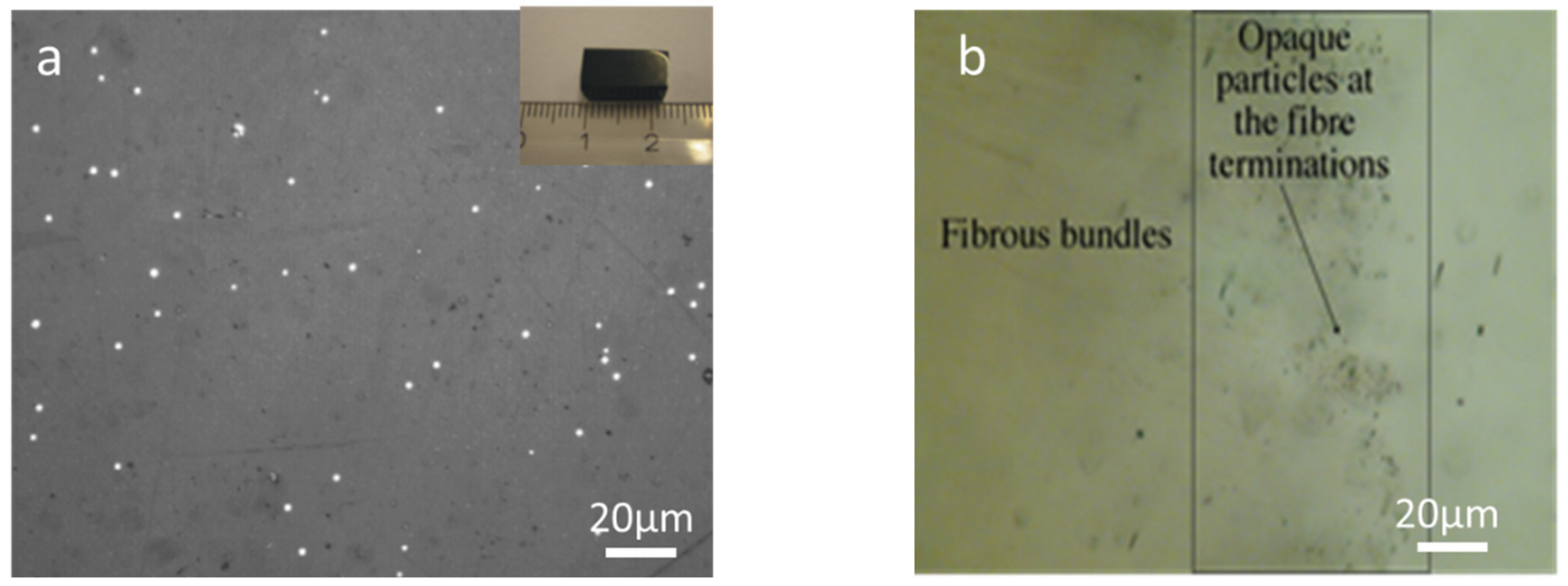
3.4. Optical Microscopy Observations
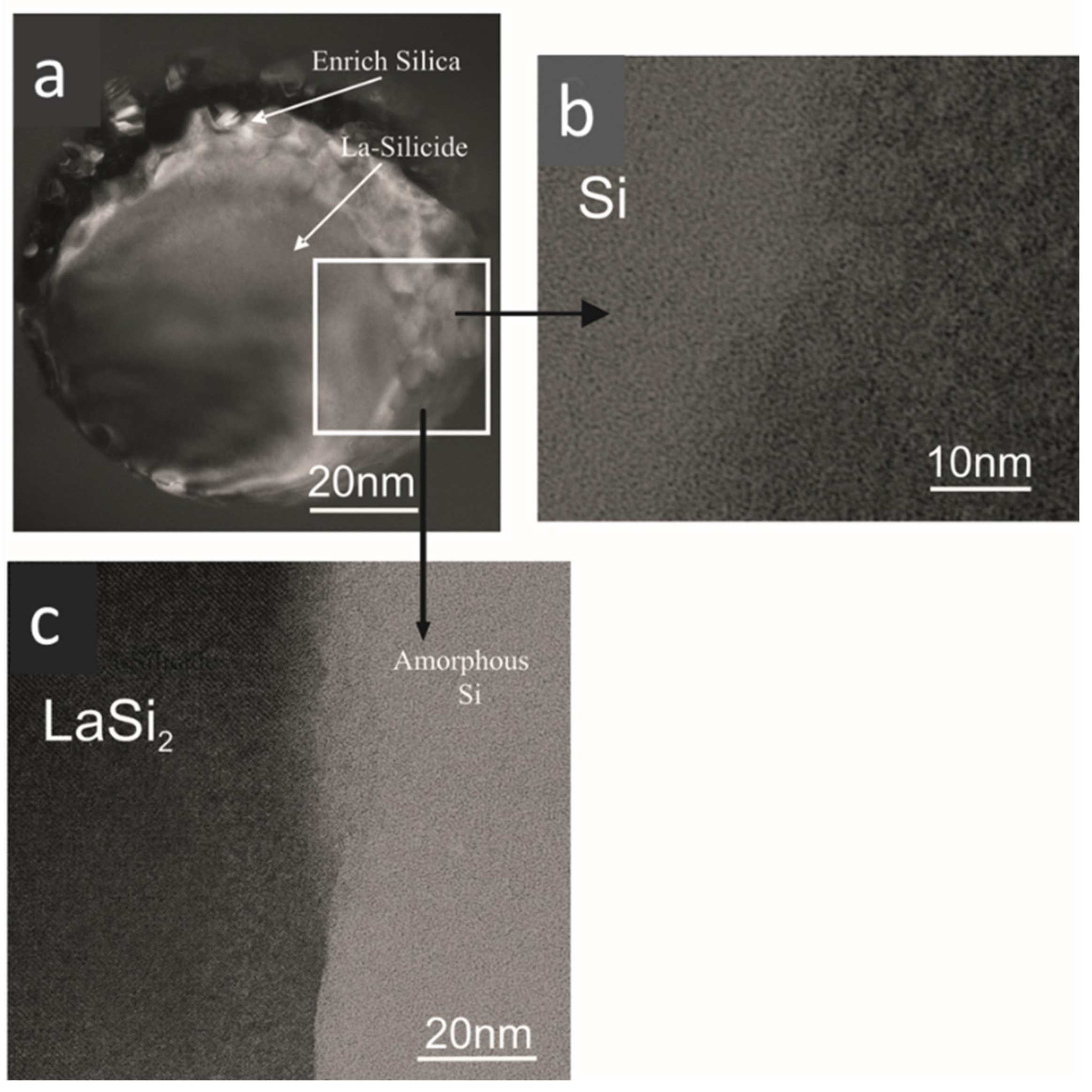
3.5. Scanning Electron Microscopy
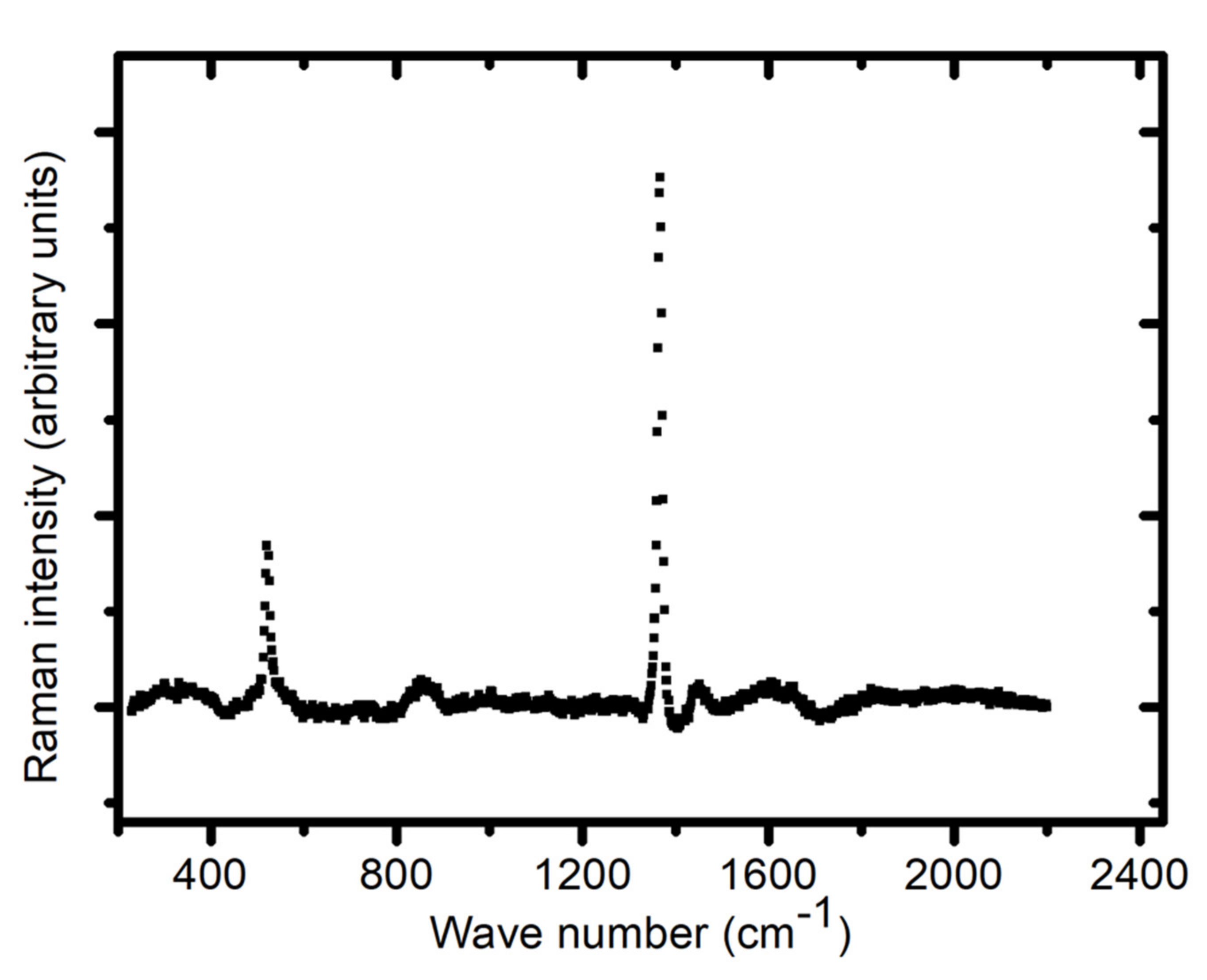
3.6. Raman Spectroscopy
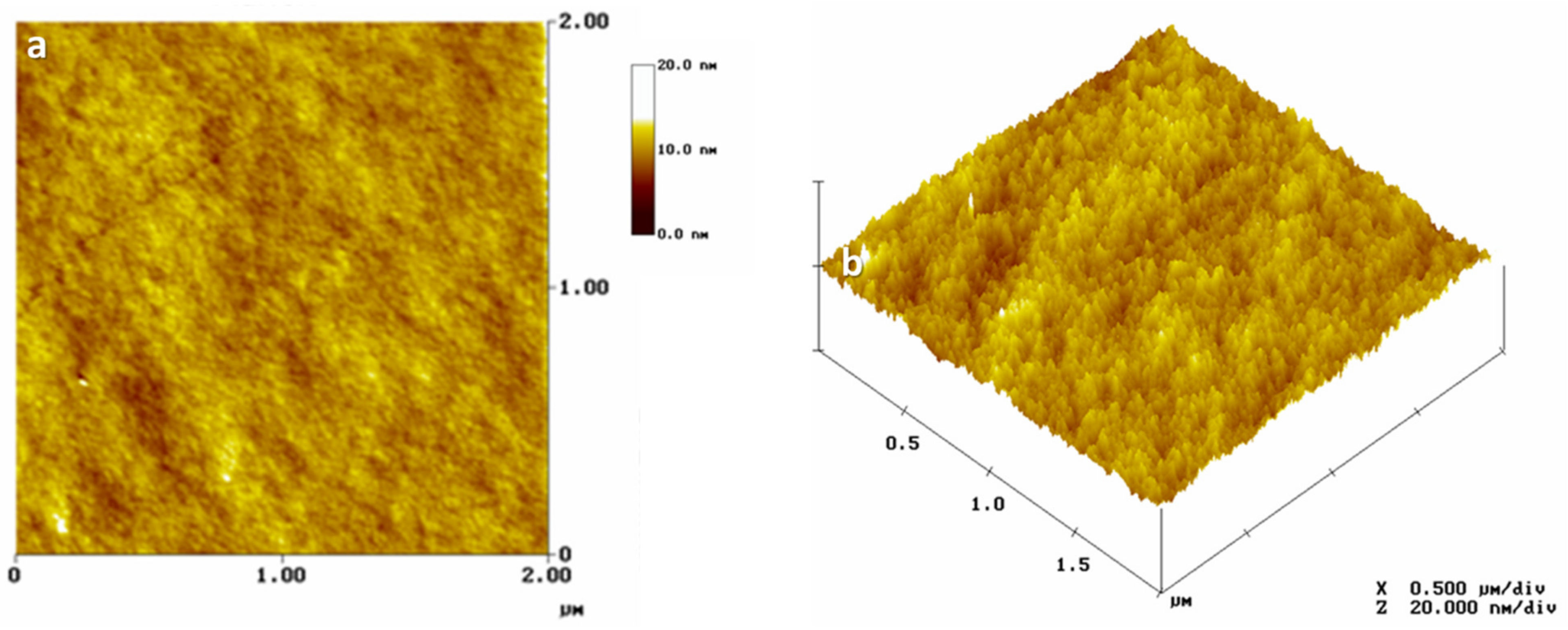
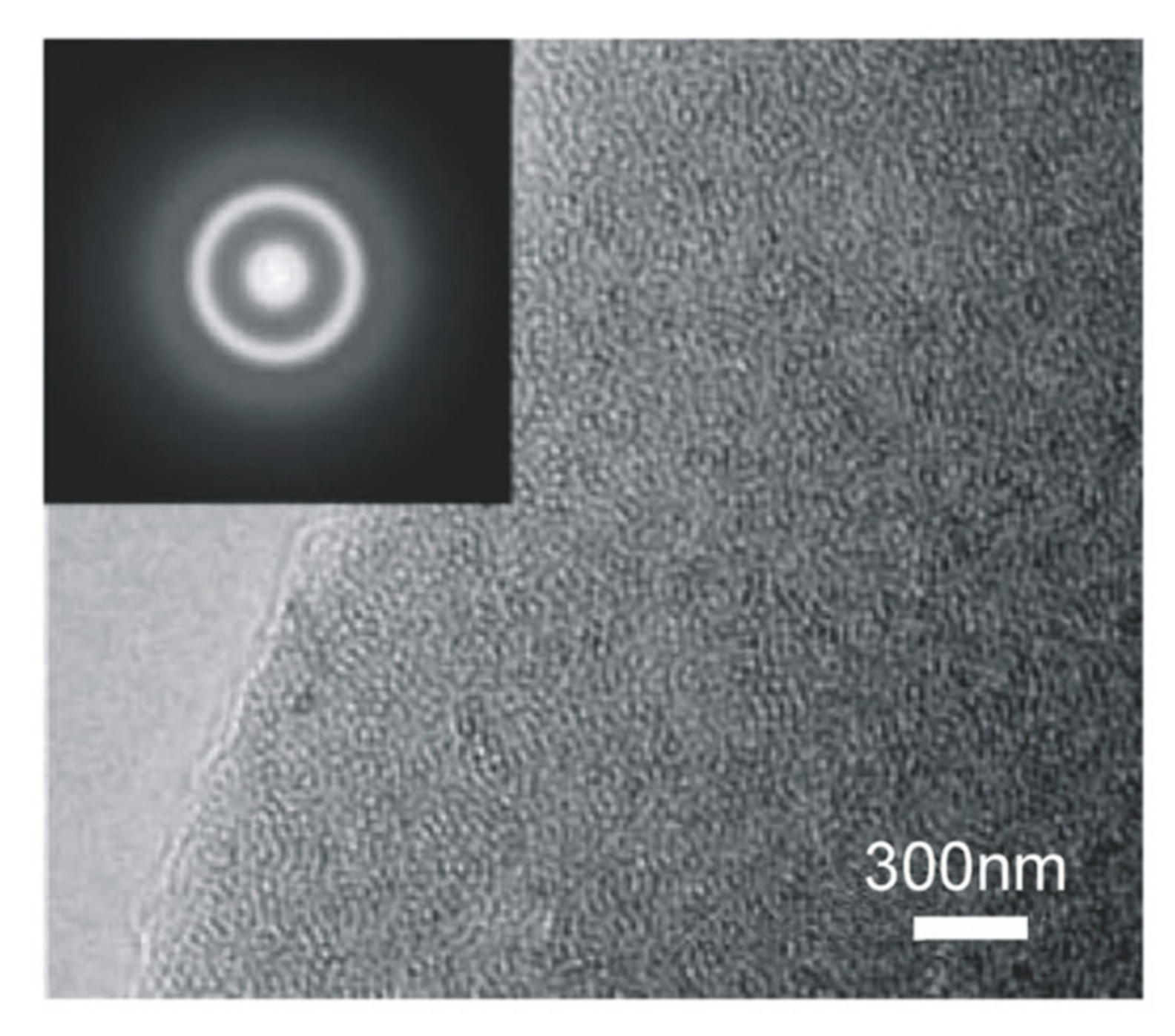
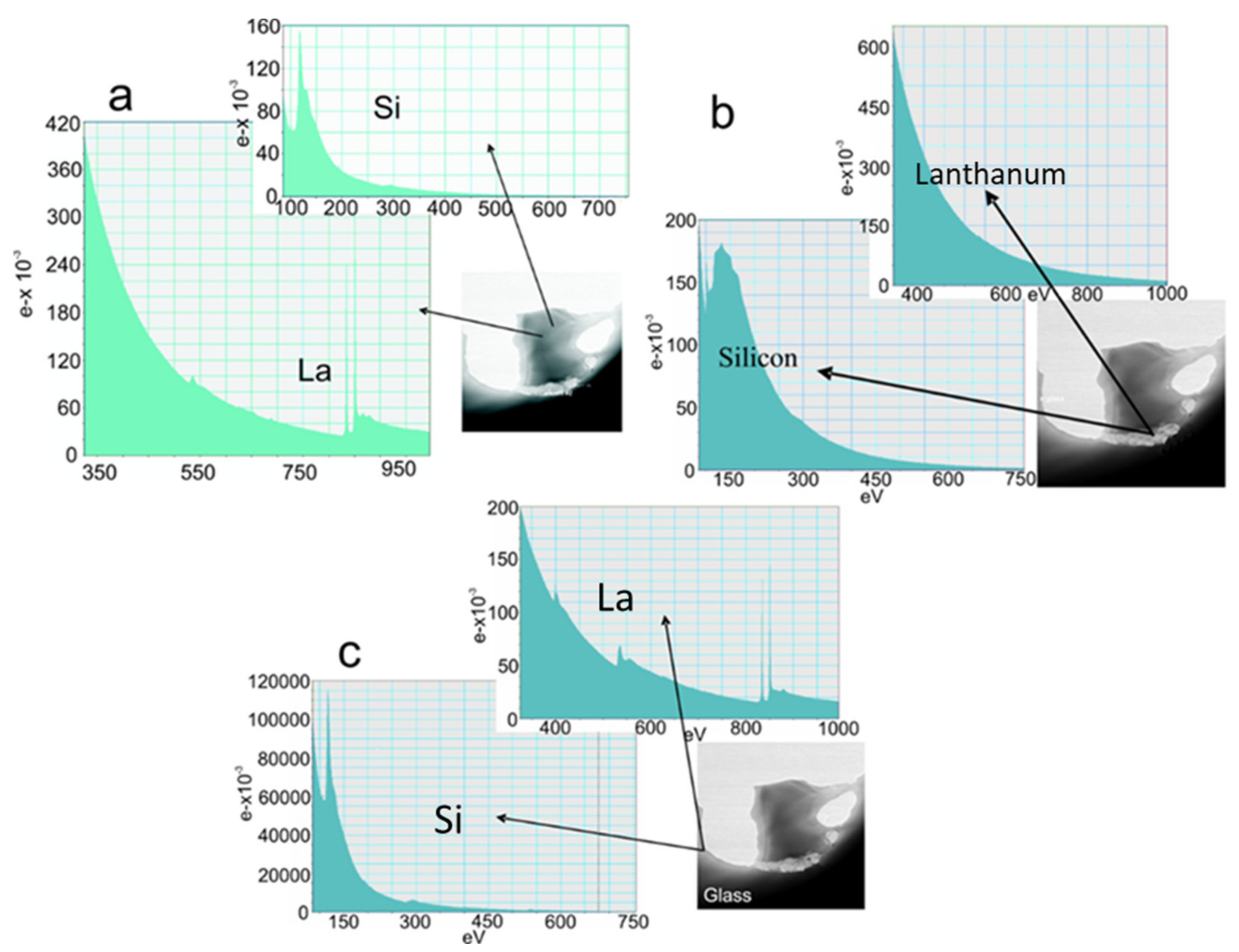
3.7. Atomic Force Microscopy (AFM), Transmission Electron Microscopy (TEM) and Electron Energy-Loss Spectroscopy (EELS) Analysis
3.8. Comparison with La−Si−O−N Thin Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hakeem, A.S.; Grins, J.; Esmaeilzadeh, S. La−Si−O−N glasses–Part, I. Extension of the glass forming region. J. Eur. Ceram. Soc. 2007, 27, 4773–4781. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Grins, J.; Esmaeilzadeh, S. La−Si−O−N glasses–Part II: Vickers hardness and refractive index. J. Eur. Ceram. Soc. 2007, 27, 4783–4787. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Glass-forming region in the Ca–Si–O–N system using CaH2 as Ca source. J. Eur. Ceram. Soc. 2008, 28, 2659–2664. [Google Scholar] [CrossRef]
- Sharafat, A.; Forslund, B.; Grins, J.; Esmaeilzadeh, S. Formation and properties of nitrogen rich strontium silicon oxynitride glasses. J. Mater. Sci. 2009, 44, 664–670. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Hardness and refractive index of Ca−Si−O−N glasses. J. Non Cryst. Solids 2009, 355, 301–304. [Google Scholar] [CrossRef]
- Leonova, E.; Hakeem, A.S.; Jansson, K.; Stevensson, B.; Shen, Z.; Grins, J.; Esmaeilzadeh, S.; Edén, M. Nitrogen rich La-Si-Al-O-N oxynitride glass structures probed by solid state NMR. J. Non-Cryst. Solids 2008, 354, 49–60. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Daucé, R.; Leonova, E.; Edén, M.; Shen, Z.; Grins, J.; Esmaeilzadeh, S. Silicate Glasses with Unprecedented High Nitrogen and Electropositive Metal Contents Obtained by Using Metals as Precursors. Adv. Mater. 2005, 17, 2214–2216. [Google Scholar] [CrossRef]
- Hakeem, A.S.; Ali, S.; Jonson, B. Preparation and properties of mixed La–Pr silicate oxynitride glasses. J. Non Cryst. Solids 2013, 368, 93–97. [Google Scholar] [CrossRef]
- Hakeem, A.S. Novel Route of Oxynitride Glass Synthesis and Characterisation of Glasses in the Ln−Si−O−N and Ln−Si−Al−O−N Systems. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2007. [Google Scholar]
- Höche, T.; Deckwerth, M.; Rüssel, C. Partial Stabilization of Tetragonal Zirconia in Oxynitride Glass-Ceramics. J. Am. Ceram. Soc. 1998, 81, 2029–2036. [Google Scholar] [CrossRef]
- Rouxel, T.; Piriou, B. Free silicon and crystallization in silicon nitride based ceramics and in oxynitride glasses. J. Appl. Phys. 1996, 79, 9074–9079. [Google Scholar] [CrossRef]
- Korgul, P.; Thompson, D.P. The transparency of oxynitride glasses. J. Mater. Sci. 1993, 28, 506–512. [Google Scholar] [CrossRef]
- Messier, D.R.; Deguire, E.J. Thermal-Decomposition in the System Si−Y−Al−O−N. J. Am. Ceram. Soc. 1984, 67, 602–605. [Google Scholar] [CrossRef]
- Murakami, Y.; Yamamoto, H. Properties of Oxynitride Glasses in the Ln−Si−Al−O−N Systems (Ln = Rare-Earth). Nippon Seramikkusu Kyokai Gakujutsu Ronbunshi-J. Ceram. Soc. Jpn. 1994, 102, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Hakeem, A.S.; Khan, M.; Ahmed, B.A.; Al Ghanim, A.; Patel, F.; Ehsan, M.A.; Ali, S.; Laoui, T.; Ali, S. Synthesis and characterization of alkaline earth and rare earth doped sialon ceramics by spark plasma sintering. Intern. J. Refract. Metal. and Hard Mater. 2021, 97, 1–12. [Google Scholar] [CrossRef]
- Jack, K.H. The Role of Additives in the Densification of Nitrogen Ceramics. Final Tech Rept, European Research Office, US Army, Grant No DAERO-76-G-067. 1979. Available online: https://apps.dtic.mil/sti/citations/ADA116581 (accessed on 15 June 2021).
- Zhong, J.S.; Gao, H.B.; Yuan, Y.J.; Chen, L.F.; Chen, D.Q.; Ji, Z.G. Eu3+-doped double perovskite-based phosphor-in-glass color converter for high-power warm w-LEDs. J. Alloy. Compd. 2018, 735, 2303–2310. [Google Scholar] [CrossRef]
- Muñoz, F.; Jiménez-Riobóo, R.J.; Balda, R. Chemical and structural heterogeneities in Nd-doped oxynitride phosphate laser glasses. J. Alloy. Compd. 2020, 816, 152657. [Google Scholar] [CrossRef]
- Navrotsky, A. Thermochemical studies of nitrides and oxynitrides by oxidative oxide melt calorimetry. J. Alloy. Compd. 2001, 321, 300–306. [Google Scholar] [CrossRef]
- Messier, D.R.; Broz, A. Microhardness and Elastic-Moduli of Si-Y-Al-O-N Glasses. J. Am. Ceram. Soc. 1982, 65, C123. [Google Scholar] [CrossRef]
- Greskovich, C.; Prochazka, S. Stability of Si3N4 and Liquid Phase(S) during Sintering. J. Am. Ceram. Soc. 1981, 64, C96–C97. [Google Scholar] [CrossRef]
- Sharafat, A.; Grins, J.; Esmaeilzadeh, S. Properties of high nitrogen content mixed alkali earth oxynitride glasses (AE(x)Ca(1-x))(1.2(1))SiO1.9(1)N0.86(6), AE = Mg, Sr, Ba. J. Non Cryst. Solids 2009, 355, 1259–1263. [Google Scholar] [CrossRef]
- Sharafat, A.; Paul, B.; Magnusson, R.; Greczynski, G.; Broitman, E.; Jonson, B.; Eklund, P.; Birch, J. Novel transparent MgSiON thin films with high hardness and refractive index. Vacuum 2016, 131, 1–4. [Google Scholar]
- Ali, S.; Paul, B.; Magnusson, R.; Broitman, E.; Jonson, B.; Eklund, P.; Birch, J. Synthesis and characterization of the mechanical and optical properties of Ca−Si−O−N thin films deposited by RF magnetron sputtering. Surf. Coat. Technol. 2017, 315, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Sharafat, A.; Paul, B.; Magnusson, R.; Ekström, E.; Pallier, C.; Jonson, B.; Eklund, P.; Birch, J. Optical and mechanical properties of amorphous Mg−Si−O−N thin films deposited by reactive magnetron sputtering. Surf. Coat. Technol. 2019, 372, 9–15. [Google Scholar]
- Höche, T.; Gerlach, J.W.; Petsch, T. Static-Charging Mitigation and Contamination Avoidance by Selective Carbon Coating of TEM Samples. Ultramicroscopy 2006, 106, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Lofaj, F.; Hvizdos, P.; Dorcakova, F.; Satet, R.; Hoffmann, M.J.; de Arellano-Lopez, A.R. Indentation moduli and microhardness of RE-Si-Mg-O-N glasses (RE = Sc, Y, La, Sm, Yb and Lu) with different nitrogen content. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2003, 357, 181–187. [Google Scholar] [CrossRef]
- Zhang, E.; Liddell, K.; Thompson, D.P. Glass forming regions and thermal expansion of some Ln−Si−Al−O−N glasses (Ln=La,Nd). Brit. Ceram. T. 1996, 95, 169–172. [Google Scholar]
- Weldon, L.M.; Pomeroy, M.J.; Hampshire, S. Glasses in the rare-earth sialon system. Key Eng. Mat. 1996, 118, 241–248. [Google Scholar] [CrossRef]
- Ohashi, M.; Nakamura, K.; Hirao, K.; Kanzaki, S.; Hampshire, S. Formation and Properties of Ln−Si−O−N Glasses (Ln=Lanthanides or Y). J. Am. Ceram. Soc. 1995, 78, 71–76. [Google Scholar] [CrossRef]
- Makishima, A.; Mitomo, M.; Ii, N.; Tsutsumi, M. Microhardness and Transparency of an La−Si−O−N Oxynitride Glass. J. Am. Ceram. Soc. 1983, 66, C55–C56. [Google Scholar] [CrossRef]
- Pasto, A.E. Causes and Effects of Fe-Bearing Inclusions in Sintered Si3N4. J. Am. Ceram. Soc. 1984, 67, C178–C180. [Google Scholar] [CrossRef]
- Ali, S.; Jonson, B. Glasses in the Ba−Si−O−N System. J. Am. Ceram. Soc. 2011, 94, 2912–2917. [Google Scholar] [CrossRef]
- Wójcik, N.A.; Jonson, B.; Möncke, D.; Palles, D.; Kamitsos, E.I.; Ghassemali, E.; Seifedine, S.; Eriksson, M.; Ali, S. Influence of synthesis conditions on glass formation, structure and thermal properties in the Na2O−CaO−P2O5 system doped with Si3N4 and Mg. J. Non Cryst. Solids. 2018, 494, 66–77. [Google Scholar] [CrossRef]
- Bulanova, M.V.; Zheltov, P.N.; Meleshevich, K.A.; Saltykov, P.A.; Effenberg, G.; Tedenac, J.C. Lanthanum–silicon system. J. Alloy. Compd. 2001, 329, 214–223. [Google Scholar] [CrossRef]
- Greaves, G.N.; Fontaine, A.; Lagarde, P.; Raoux, D.; Gurman, S.J. Local structure of silicate glasses. Nature 1981, 293, 611–616. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakeem, A.S.; Ali, S.; Höche, T.; Drmosh, Q.A.; Khan, A.A.; Jonson, B. Microstructure Evaluation and Impurities in La Containing Silicon Oxynitrides. Nanomaterials 2021, 11, 1896. https://doi.org/10.3390/nano11081896
Hakeem AS, Ali S, Höche T, Drmosh QA, Khan AA, Jonson B. Microstructure Evaluation and Impurities in La Containing Silicon Oxynitrides. Nanomaterials. 2021; 11(8):1896. https://doi.org/10.3390/nano11081896
Chicago/Turabian StyleHakeem, Abbas Saeed, Sharafat Ali, Thomas Höche, Qasem Ahmed Drmosh, Amir Azam Khan, and Bo Jonson. 2021. "Microstructure Evaluation and Impurities in La Containing Silicon Oxynitrides" Nanomaterials 11, no. 8: 1896. https://doi.org/10.3390/nano11081896
APA StyleHakeem, A. S., Ali, S., Höche, T., Drmosh, Q. A., Khan, A. A., & Jonson, B. (2021). Microstructure Evaluation and Impurities in La Containing Silicon Oxynitrides. Nanomaterials, 11(8), 1896. https://doi.org/10.3390/nano11081896






